Abstract
The functionalized nano-clay composite adsorbent was prepared, and its properties were characterized using FT-IR, XRD and SEM techniques. The synthesized nano-clay composite was studied with regard to its capacity to remove ibuprofen under different adsorption conditions such as varying pH levels (5–9), initial ibuprofen concentrations (3, 5 and 10 mg L−1), contact time, and the amount of adsorbent (0.125, 0.25, 0.5 and 1 g). In order to evaluate the nanocomposite adsorption capacity, the adsorption results were assessed using nine isotherm models. The results showed that the optimum adsorption pH was 6 and that an increase or decrease in the pH reduced the adsorption capacity. The adsorption process was fast and reached equilibrium after 120 min. The maximum efficacy of ibuprofen removal was approximately 95.2%, with 1 g of adsorbent, 10 mg L−1 initial concentration of ibuprofen, 120 min contact time and pH = 6. The optimal adsorption isotherm models were the Freundlich, Fritz–Schlunder, Redlich–Peterson, Radke–Prausnitz, Sip, Toth and Khan models. In addition, four adsorption kinetic models were employed for adsorption system evaluation under a variety of experimental conditions. The kinetic data illustrated that the process is very fast, and the reaction followed the Elovich kinetic model. Therefore, this nano-clay composite can be used as an effective adsorbent for the removal of ibuprofen from aqueous solutions, such as water and wastewater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ibuprofen is a common nonsteroidal anti-inflammatory agent, which acts via the inhibition of prostaglandin and is used in the treatment of pain and fever (Caldwell et al. 2015; Petrie et al. 2015; Söderström et al. 2009). There are numerous concerns with regard to the indiscriminate use of anti-inflammatory agents, and their contamination of water, as they are not completely metabolized, and enter the environment through wastewater treatment facilities (Klavarioti et al. 2009; Birch et al. 2015). One particular study showed that 85% of ingested ibuprofen is excreted via urine (Rainsford 2009; Kolpin et al. 2002) reported that in 9.5% of streams examined in the USA, the amount of ibuprofen present was approximately 0.018–1.0 μg L−1. Further, Heberer (2002), found around 0.22 μg L−1 of ibuprofen in sewage effluents. In another study, Richardson and Ternes reported the presence of 0.90–2.11 μg L−1 ibuprofen in raw water and recycled resources (Richardson and Ternes 2014).
Ibuprofen, which metabolizes as hydroxyl and carboxy-ibuprofen in aqueous solutions, has an accumulative effect and results in great changes in the ecosystem (Nikolaou et al. 2007; Mompelat et al. 2009). In addition, the presence of ibuprofen in the environment and its effects on the organisms in aquatic ecosystems are the cause of some concern. As a result, the removal of this contaminant from sewage is essential. Since conventional wastewater treatment processes remove only 30–40% of drugs, advanced treatment systems should be used for this task (Deblondea et al. 2011). Several method and technologies were used for removal of pharmaceuticals from environments including sedimentation, advanced oxidation processes (AOP), photocatalytic degradation and adsorption (Yan et al. 2013; Dong et al. 2013; Feng et al. 2013; Fernández et al. 2014; Mahvi et al. 2008; Kakavand et al. 2014 ; Zazouli et al. 2014).
Tertiary or advanced wastewater treatment systems must be used to eliminate these drugs from wastewater. Studies indicate that tertiary treatment including physical treatment by microfiltration (MF), nanofiltration (NF) and reverse osmosis (RO) can be effective in reducing these drugs (Rostamian et al. 2011; Shu et al. 2016). Adsorption is one of the most promising techniques among the advanced treatment techniques used in eliminating and destroying nonsteroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen from aqueous solutions (Rafati et al. 2010).
Recently, surface functionalization of clays has garnered intense interest for use as solid supports due to its large surface area; fast adsorption kinetics and controllable pore size and pore arrangement in comparison with other conventional adsorbents. Although modified nano-clay has several advantages over simple clay adsorbents, it seems to be less suitable for some processes in water treatment such as column methods, due to smaller interlayer distances and inclusion complex formation abilities.
The aims of the present study were: (i) synthesize a functionalized nano-clay composite adsorbent; (ii) investigate the efficacy of a prepared nanocomposite for removal of ibuprofen from aqueous solutions in a batch system, as well as to evaluate the effects of pH, adsorbent dose, contact time and initial ibuprofen concentration; and (iii) examine equilibrium isotherm and kinetic modeling of the ibuprofen adsorption process.
Materials and methods
Materials
Cloisite 15A from Neutrinos Co., ibuprofen pure from pharmaceutical company Alborz bulk (Iran) as a gift and all other regent and chemicals from Merck (Germany) with analytical grade were prepared. All chemicals were used without further purification. The standard solution of ibuprofen was prepared by dissolving certain amounts of pure ibuprofen in double distilled water. All diluted solutions (3, 5 and 10 mg L−1) were prepared from the stock solution by dilution.
Methods
Preparation of adsorbent
Amino-functionalized nano-clay was prepared according to the method described by Najafi et al. (2012) and Whilton et al. (1998) with minor modifications. Briefly, 0.5 g of Cloisite 15A, 2.5 mol of 3-aminopropyltriethoxysilane (APTS) and 25 ml of toluene were refluxed for a minimum of 2 h. After that, the white slurry obtained was stirred overnight, and then the precipitate was collected by centrifugation. The precipitate was washed thoroughly with distilled water several times and dried in an air oven at 150 °C for 2 h.
Also, mono-tosyl β-cyclodextrin (β-CD) was prepared by the method reported in the literature (Tripodo et al. 2013). Eighteen grams of β-CD was dissolved in 100 ml of dry pyridine, and then 2.5 g of p-toluenesulfonyl chloride (12 mmol) was added to the solution. The reaction mixture was refluxed at 2–4° for 8 h and then kept at room temperature for 2 days. The product (mono-tosyl β-CD) was separated by precipitation in diethyl ether and drying in a vacuum oven. Afterward, the resulting white precipitate was washed with acetone and then dried under vacuum conditions at 60 °C.
For functionalization of nano-clay with β-CD, a solution of 0.25 g of Ts-β-CD in 10 ml DMF was mixed with a suspension of 2 g amino-functionalized nano-clay in 50 ml dimethyl formamide (DMF). The mixture was stirred for 7 h under nitrogen atmosphere. Afterward, the solution was filtered and washed with acetone and DMF in order to remove any unreacted compound, and then dried in air oven at 60 °C for 24 h in order to remove the additional solvent.
Finally, the adsorbent was prepared by dropwise adding a suspension containing 0.5 g of CD@Clay in 15 ml of toluene to the solution of 0.05 g of PVP in 15 ml toluene assisted by ultrasonication for 1 h. The resulting mixture was kept at room temperature for 1 day. The obtained compound was washed with toluene three times and dried overnight in an oven at 60 °C. The designed synthesis strategy is presented in Scheme 1.
Adsorbent characterization
FT-IR spectra of each fabricated materials were conducted on a Perkin–Elmer FT-IR spectrophotometer (model Spectrum GX). A KBr pellet was used for analysis. The spectra were recorded with width ranging from 400 to 4000 cm−1. Scanning electron microscopy (SEM) analysis was carried out using a MIRA3 TESCAN HV: 20.0 kV from the Czech Republic. X-ray diffraction (XRD) patterns were recorded on XRD spectrometer; ITAL structures model APD2000, using Cu Kα radiation at a scanning speed of 3° min−1 over 2 h from 0° to 60°.
Batch adsorption study
In this research, the functionalized nano-clay composite adsorbent was prepared and used for removal of ibuprofen in the batch system study at different conditions such as the effects of pH (5–9), initial concentration of ibuprofen (3, 5 and 10 mg L−1), contact time (5–120 min) and various amounts of adsorbent (0.125, 0.25, 0.5 and 1 g). All experiments were performed at room temperature. The final ibuprofen concentration in the effluent was determined by the UV–Vis spectrophotometric method using UV–Vis spectrophotometer (PG-Instruments Ltd) at 225 nm. Finally, the kinetic models, adsorption isotherms and nonlinear error function analysis were performed. We analyzed laboratory and instrumental blanks throughout the experiment and found that there was no sign of interference or contamination in the samples. Spiked matrixes recoveries were computed, and they were greater than 90%.
The ibuprofen removal percentage was obtained by the following equation:
where C a and C 0 are the amount of adsorbed (the difference between ibuprofen concentration before and after sorption) and the initial concentration of ibuprofen, respectively.
The ibuprofen adsorption uptake of nanocomposite adsorbent (q t ) was calculated from the following equation:
where C 0 and C t are the initial concentration and concentration at a time of (t) of ibuprofen in terms of mg L−1, respectively. Also, V is the volume of the solution (L) and m is the amount of adsorbent (g).
Adsorption isotherm models were applied for data analysis by using nine chosen models. Also, the data were fitted to some kinetic models such as pseudo-first-order (PFO), pseudo-second-order (PSO), Elovich and intraparticle models. Finally, error function analysis was investigated for the best fitting of experimental data.
Result and discussion
Characterization of CD@Clay-PVP
The FT-IR spectra of the clay (a), amino-functionalized clay (b), CD-Clay (c), PVP (d) and CD@Clay-PVP (e) were recorded between 400 and 4000 cm−1 as shown in Fig. 1. The nanocomposite (CD@Clay-PVP) IR spectrum showed bands which could be recognized on β-CD, PVP and Cloisite 15A spectra. For example, the band at 1058 cm−1 on Cloisite 15A (Si–O stretching vibration) was absent on β-CD and PVP spectrum but present on nanocomposite. Cloisite 15A clay silylated resulting from Si–O group has a distinctive peak in 1058, 529, and 467 cm−1. The peaks at 1696 cm−1 (C=O stretching vibration) on PVP are not seen on clay and β-CD, but are present in the nanocomposite. Further, clay has an IR peak at 3645 cm−1 which is related to the free O–H group. The peaks at 2932 and 2861 cm−1 are as a result of the existence of organic modifying alkyl chain in clay. Meanwhile, the nanocomposite has the following IR peaks: characteristic peaks of CH2 (2932, 2861 cm−1) and Si–O (1058, 529 and 467 cm−1), which are related to the polymer and clay, respectively.
The SEM images in Fig. 2 confirm that the surface morphologies of final adsorbent are different in comparing with precursor materials. The greater interlayer spaces can be seen in Fig. 2b suggesting the filling of polymer among the layers of nano-clay.
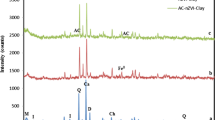
The wide-angle X-ray diffraction patterns of nano-powder clay and CD@Clay-PVP are demonstrated in Fig. 3. The characteristic peaks at 2θ = 7.3° and 2.9° are attributed to the clay crystal structure, and the distance of layer clay is equal to 1.21 nm. From the XRD patterns of adsorbent, it can be seen that the distance of clay layer was increased to 3.04 due to intercalation of PVP between clay layers. Also, the adsorbent, CD@Clay-PVP showed a peak shift toward the refraction angle (2θ) which is lower than that of pure Cloisite 15A, implying an increased interlayer distance of silicate layers and filling between layers out of polymer chains.
Batch adsorption study
Effect of pH
The effect of pH on the adsorption efficiency was studied by changing the initial pH (5–9). The amount of adsorbent dose was selected at 0.01 g, the initial concentration of ibuprofen 3 mg L−1 and the contact time 120 min. Figure 4a shows the results of the effects of pH on the adsorption efficiency. It can be observed that the adsorption efficiency passes through a maximum at pH = 6 by varying the pH from 5 to 9. Also the removal percentage was decreased from 90% (at pH = 6) to 66% (at pH = 9). It is well known that the pH has an effect on the electrical charge of adsorbent’s surface, and by decreasing pH, the ibuprofen removal increases due to the interaction between the surface charge of adsorbent and ionic charge of ibuprofen molecules (Sari and Tuzen 2009). According to the earlier studies, the most removal percentage of pollutants by adsorbents takes place under acidic condition (Chen et al. 2002).
Effect of contact time
The effect of time on the adsorption efficiency was studied by changing the contact time from 5 to 120 min at pH 6. The adsorbent dose and the initial concentration of ibuprofen were selected at 0.125 g and 10 mg L−1, respectively. Figure 4b depicts the effect of contact time on the removal efficiency. As illustrated in this figure, the ibuprofen removal percentage was increased from 36.7 to 84.4% with increasing the contact time from 5 to 120 min. By increasing the contact time, the curve becomes closer to the equilibrium state. This curve shows a slow slope that it can be due to filling pores on the adsorbent by ibuprofen (Dönmez and Aksu 2002). In fact, the nature of adsorption in the initial step is the adsorption on the surface binding sites, while the adsorption occurred in internal and pore sites with adsorption progress. This process was continuing until the saturation of adsorbent (nano-clay composite) (Razmovski and Sciban 2008).
Effect of initial concentration
The effect of initial concentration on the adsorption efficiency was investigated by changing the initial concentration of ibuprofen (3, 5 and 10 mg L−1) at pH 6. The adsorbent dose and the contact time of ibuprofen were selected at 0.25 g and 120 min, respectively. Figure 5a shows the effect of initial concentration on the ibuprofen removal efficiency. As illustrated in this figure, the ibuprofen removal is increased by increasing the initial concentration. Therefore, according to the obtained results, it is indicated that the adsorption capacity of nano-clay composite adsorbent depends on the initial concentration of ibuprofen. The initial concentration is a considerable driving force for overcoming all mass transfer resistance between aqueous and solid phases (Dönmez and Aksu 2002).
Effect of adsorbent dose
The effect of the amount of functionalized nano-clay composite adsorbent on the adsorption efficiency in the batch system was studied using 0.125, 0.25, 0.5 and 1 g of functionalized nano-clay composite adsorbent at pH 6, contact time 120 min and initial concentration of ibuprofen 10 mg L−1. Figure 5b illustrates this effect on the ibuprofen removal efficiency. As can be seen from Fig. 5b, increasing the amount of adsorbent from 0.125 to 1 g increases the ibuprofen removal efficiency from 84.38 to 95.2%. This behavior can be explained based on the increases of adsorption sites and surface area of the adsorbent with increasing of adsorbent dosage (Bayramoglu et al. 2009). In lower amounts of adsorbent, the adsorbent’s surface is quickly saturated with ibuprofen molecules and the amount of ibuprofen remained in the solution is high. Since the increasing of adsorbent dose raises available surface area, so the adsorption of ibuprofen molecules was increased (Razmovski and Sciban 2008). At the amount of 0.125 g of adsorbent dose, the removal is very low, and the equilibrium occurs quickly (Sari and Tuzen 2009).
Equilibrium study
Adsorption isotherm is one of the fundamental tools for the evaluation of the adsorption ability of an adsorbent. To evaluate the performance of nanocomposite adsorbent, batch adsorption studies were carried out to examine its adsorption capacity for ibuprofen. Some isotherm models are used to analyzing the experimental data and describe the equilibrium in the adsorption process. In order to describe the removal of ibuprofen by nanocomposite adsorbent, the isotherms data were analyzed using nine models which have been tabulated in Table 1.
Figure 6a shows the experimental equilibrium adsorption uptake data of the adsorbent as well as fitting curves obtained from different isotherm models. According to the results obtained from isotherm equations (Fig. 6a; Table 1), it can be concluded that the equilibrium data of ibuprofen adsorption process on the functionalized nano-clay composite adsorbent follow the most studied isotherms.
Our method of synthesis which is based on the intercalation of PVP and functionalization of clay surface with β-CD can be improved and specify the adsorption process in comparison with previous reported researches based on clay adsorbent and some other adsorbents (Table 2).
Adsorption kinetic models
In the adsorption systems, several kinetic models have been established in order to understand of the adsorption kinetics of ibuprofen on clay nanocomposite. The common models employed in this study are pseudo-first-order (PFO), pseudo-second-order (PSO), Elovich and intraparticle diffusion models that their equations have been shown in Table 3. Also, Fig. 6b shows fitting curves derived from four different models. As can be seen, the Elovich model is the best model for fitting experimental data with the kinetic model. All the parameters obtained by the kinetic models are presented in Table 3.
Nonlinear error function analysis
In the adsorption systems, to verify models (kinetics or isotherms models), the model parameters were evaluated by nonlinear regression method and the best fit can be selected by an error function analysis. In the present research, eight error functions including residual root-mean-square error (RMSE), the coefficient of determination (R 2), correlation coefficient (r), the sum of the square of the error (SSE), the sum of the absolute error (SAE), average relative error (ARE), nonlinear Chi-square (χ 2) and the average relative standard error (ARS) were used to measure the goodness of fit. As we know, the smallest amount of error function shows the best curve fitting. Table 4 illustrates the comparison of errors for adsorption isotherms. As can be seen, the most correlation and the smallest error were achieved for Freundlich, Fritz–Schlunder, Redlich–Peterson, Radke–Prausnitz, Sip, Toth and khan models.
Conclusion
In this research, a functionalized nano-clay composite adsorbent was used for the removal of ibuprofen from aqueous solutions. Effective conditions for this process were studied, and an optimum condition was obtained for maximum efficiency of ibuprofen removal. The results showed that ibuprofen removal rate increases via the increase of initial ibuprofen concentration and the amount of adsorbent. In addition, the maximum ibuprofen adsorption capacity (1.1 mg/g) was obtained using 0.1 g of adsorbent, a contact time of 120 min and a pH of 6. The experimental data for the adsorption equilibrium were in accordance with all isotherm models, with the exception of the Tempkin and Langmuir models. Further, investigation of the kinetic models of adsorption showed a high correlation with the Elovich model. This study showed that the adsorbent nano-clay composite has a high adsorption capacity with regard to ibuprofen removal from aqueous solution.
References
Baccar R, Sarrà M, Bouzid J, Feki M, Blánquez P (2012) Removal of pharmaceutical compounds by activated carbon prepared from agricultural by-product. Chem Eng J 211–212:310–317
Bayramoglu G, Gursel I, Tunali Y, Yakup Arica M (2009) Biosorption of phenol and 2-chlorophenol by Funaliatrogii pellets. Bioresour Technol 100(10):2685–2691. doi:10.1016/j.biortech.2008.12.042
Birch GF, Drage DS, Thompson K, Eaglesham G, Mueller JF (2015) Emerging contaminants (pharmaceutical, personal care products, a food additive and pesticides) in waters of Sidney estuary, Australia. Mar Pollut Bull 97(1–2):56–66. doi:10.1016/j.marpolbul.2015.06.038
Caldwell DJ, Mertens B, Kappler K, Senac T, Journel R, Wilson P, Meyerhoff RD, Parke NJ, Mastrocco F, Mattson B, Murray-Smith R, Dolan DG, Straub JO, Wiedemann M, Hartmann A, Finan DS (2015) A risk-based approach to managing active pharmaceutical ingredients in manufacturing effluent. Environ Toxicol Chem 35(4):813–822. doi:10.1002/etc.3163
Chen P, Xiong Z, Luo J, Lin J, Tan KL (2002) Interaction of hydrogen with metal nitrides and imides. Nature 420:302–304. doi:10.1038/nature01210
Deblondea T, Cossu-Leguille C, Hartemann P (2011) Emerging pollutants in wastewater: a review of the literature. Int J Hyg Environ Health 214(6):442–448. doi:10.1016/j.ijheh.2011.08.002
Dong J, Xia X, Zhai Y (2013) Investigating particle concentration effects of polycyclic aromatic hydrocarbon (PAH) sorption on sediment considering the freely dissolved concentrations of PAHs. J Soils Sediments 13(8):1469–1477. doi:10.1007/s11368-013-0736-9
Dönmez G, Aksu Z (2002) Removal of chromium (VI) from saline wastewaters by Dunaliella species. Process Biochem 38(5):751–762. doi:10.1016/S0032-9592(02)00204-2
Feng L, van Hullebusch ED, Rodrigo MA, Esposito G, Oturan MA (2013) Removal of residual anti-inflammatory and analgesic pharmaceuticals from aqueous systems by electrochemical advanced oxidation processes. A review. Chem Eng J 228:944–964. doi:10.1016/j.cej.2013.05.061
Fernández RL, McDonald JA, Khan SJ, Le-Clech P (2014) Removal of pharmaceuticals and endocrine disrupting chemicals by a submerged membrane photocatalysis reactor (MPR). Sep Purif Technol 127(30):131–139. doi:10.1016/j.seppur.2014.02.031
Heberer T (2002) Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: a review of recent research data. Toxicol Lett 131(1–2):5–17. doi:10.1016/S0378-4274(02)00041-3
Kakavandi B, Kalantary RR, Farzadkia M, Mahvi AH, Esrafili A, Azari A, Yari AR, Javid AB (2014) Enhanced chromium (VI) removal using activated carbon modified by zero valent iron and silver bimetallic nanoparticles. J Environ Health Sci Eng 12(1):115. doi:10.1186/s40201-014-0115-5
Khazri H, Ghorbel-Abid I, Kalfat R, Trabelsi-Ayadi M (2016) Removal of ibuprofen, naproxen and carbamazepine in aqueous solution onto natural clay: equilibrium, kinetics, and thermodynamic study. Appl Water Sci. doi:10.1007/s13201-016-0414-3
Klavarioti M, Mantzavinos D, Kassinos D (2009) Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ Intern 35(2):402–417. doi:10.1016/j.envint.2008.07.009
Kolpin DW, Furlong ET, Meyer MT, Michael Thurman E, Zaugg SD, Barber LB, Buxton HT (2002) Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999–2000: a national reconnaissance. Environ Sci Technol 36(6):1202–1211. doi:10.1021/es011055j
Madikizela LM, Chimuka L (2016) Synthesis, adsorption and selectivity studies of a polymer imprinted with naproxen, ibuprofen and diclofenac. J Environ Chem Res Eng 4(4):4029–4037
Mahvi AH, Gholami F, Nazmara S (2008) Cadmium biosorption from wastewater by Ulmus leaves and their ash. Eur J Sci Res 23:197–203
Martin DF, Martin JM, Ward TA (2016) Removal of selected NSAIDs (nonsteroidal anti-inflammatory drugs) in aqueous samples by Octolig®. J Environ Sci Health A Tox Hazard Subst Environ Eng 51(2):186–191
Mompelat S, Le Bot B, Thomas O (2009) Occurrence and fate of pharmaceutical products and by-products, from resource to drinking water. Environ Int 35(5):803–814. doi:10.1016/j.envint.2008.10.008
Najafi M, Yousefi Y, Rafati AA (2012) Synthesis, characterization and adsorption studies of several heavy metal ions on amino-functionalized silica nano hollow sphere and silica gel. Sep Purif Technol 85:193–205. doi:10.1016/j.seppur.2011.10.011
Nikolaou A, Meric S, Fatta D (2007) Occurence patterns of pharmaceuticals in water and wastewater environments. Anal Bioanal Chem 387(4):1225–1234. doi:10.1007/s00216-006-1035-8
Petrie B, Barden R, Kasprzyk-Hordern B (2015) A review on emerging contaminants in wastewaters and the environment: current knowledge, understudied areas and recommendations for future monitoring. Water Res 72:3–27. doi:10.1016/j.watres.2014.08.053
Rafati L, Mahvi AH, Asgari AR, Hosseini SS (2010) Removal of chromium (VI) from aqueous solutions using Lewatit FO36 nano ion exchange resin. Int J Environ Sci Technol 7(1):147–156. doi:10.1007/BF03326126
Rainsford KD (2009) Ibuprofen: pharmacology, efficacy and safety. Inflammopharmacology 17(6):275–342. doi:10.1007/s10787-009-0016-x
Razmovski R, Sciban M (2008) Biosorption of Cr (VI) and Cu (II) by waste tea fungal biomass. Ecol Eng 34(2):179–186. doi:10.1016/j.ecoleng.2008.07.020
Richardson SD, Ternes TA (2014) Water analysis: emerging contaminants and current issues. Anal Chem 77(6):2813–2848. doi:10.1021/ac500508t
Rostamian R, Najafi M, Rafati AA (2011) Synthesis and characterization of thiol-functionalized silica nano hollow sphere as a novel adsorbent for removal of poisonous heavy metal ions from water: kinetics, isotherms and error analysis. Chem Eng J 171(3):1004–1011. doi:10.1016/j.cej.2011.04.051
Sari A, Tuzen M (2009) Equilibrium, thermodynamic and kinetic studies on aluminum biosorption from aqueous solution by brown algae (Padinapavonica) biomass. J Hazard Mater 171(1–3):973–979. doi:10.1016/j.jhazmat.2009.06.101
Shu HY, Chang MC, Liu JJ (2016) Cation resin fixed-bed column for the recovery of valuable THAM reagent from the wastewater. Process Saf Environ 104:571–586. doi:10.1016/j.psep.2016.05.015
Söderström H, Lindberg RH, Fick J (2009) Strategies for monitoring the emerging polar organic contaminants in water with emphasis on integrative passive sampling. J Chromat A 1216(3):623–630. doi:10.1016/j.chroma.2008.08.030
Tripodo G, Wischke C, Neffe AT, Lendlein A (2013) Efficient synthesis of pure monotosylated beta-cyclodextrin and its dimers. Carbohydr Res 381:59–63. doi:10.1016/j.carres.2013.08.018
Whilton NT, Burkett SL, Mann S (1998) Hybrid lamellar nanocomposites based on organically functionalized magnesium phyllosilicate clays with interlayer reactivity. Mater Chem 8:1927–1932. doi:10.1039/A802120A
Yan X, Chai L, Li Q (2013) Effect of precipitant additives on the sludge settling and compacting performance for heavy metal wastewater treatment. Sep Sci Technol 48:1442–1449
Zazouli MA, Mahvi AH, Dobaradaran S, Barafrashtehpour M, Mahdavi Y, Balarak D (2014) Adsorption of fluoride from aqueous solution by modified Azolla filiculoides. Fluoride 47(4):349–358
Acknowledgements
The authors greatly acknowledge Shahid Sadoughi University of Medical Sciences for the financial support from the Grant Research Council.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: Zhenyao Shen.
Rights and permissions
About this article
Cite this article
Rafati, L., Ehrampoush, M.H., Rafati, A.A. et al. Removal of ibuprofen from aqueous solution by functionalized strong nano-clay composite adsorbent: kinetic and equilibrium isotherm studies. Int. J. Environ. Sci. Technol. 15, 513–524 (2018). https://doi.org/10.1007/s13762-017-1393-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-017-1393-0