Abstract
Redox properties of humic substances (HS) control important biogeochemical processes. Thus, accurate estimation of redox properties of HS is essential. However, there is no general consensus regarding the best available measurement method of HS redox properties. In this study, we compared several common HS redox property measurement methods using anthraquinone-2,6-disulfonate (AQDS) as model compound, and standard Elliot soil humic acid (1S102H, ESHA), reference Pahokee peat (1R103H, PPHA), and Suwannee River natural organic matter (1R101N, SRNOM) as representative HS. We found that the H2/Pd reduction method followed by incubation with ferric citrate (FeCit) reagent was incomplete, and the H2/Pd reduction method followed by incubation with potassium ferricyanide (K3Fe(CN)6) was insensitive. Stannous chloride (SnCl2) reduction followed by titration of excess stannous (Sn2+) by potassium dichromate (K2Cr2O7) was found to be most accurate. These findings will help in future investigations on detailed characterizations of functional groups of HS responsible for oxidation/reduction reactions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Redox properties of humic substances (HS) have manifold implications in biogeochemical processes in the environment (Peretyazhko and Sposito 2006; Macalady and Walton-Day 2011; Sposito 2011; Pipenbrock et al. 2014). A few examples are enhancement of Fe(III) bioreduction by electron shuttling (Nevin and Lovley 2000; Kappler et al. 2004; Rakshit et al. 2009; Pipenbrock et al. 2014), increasing the reduction rate of substituted nitrobenzene (Dunnivant et al. 1992; Hernandez-Monotoya et al. 2012), dechlorination of aliphatic chlorinated pollutants (Kappler and Haderlein 2003), and oxidation/reduction of arsenic (Jiang et al. 2009). All the above processes are known to occur because of the ability of HS to donate and accept electrons easily or in other words the reversibility of the functional groups of HS to get oxidized and reduced (Weber et al. 2006). Quinone and other aromatic moieties of HS have been identified as sole contributors of electron transfer processes (Scott et al. 1998; Chen et al. 2003; Ratasuk and Nanny 2007; Hernandez-Monotoya et al. 2012).
Quantification of redox properties of HS has recently gained enormous importance (Adhikari et al. 2016; de Melo et al. 2016; Yang et al. 2016; Pipenbrock et al. 2014; Klüpfel et al. 2014; Borch et al. 2010). Several operational procedures coupled with spectroscopic tools have been used for this purpose (Aeschbacher et al. 2010; Ratasuk and Nanny 2007; Kappler et al. 2004; Matthiessen 1995). However, owing to the complexity in structure and functional groups of HS, a general consensus regarding the most applicable methods to characterize redox properties of HS has not yet been reached. While discussing redox properties of HS, it should be noted that we will use the terminology of Peretyazhko and Sposito (2006). They defined native reducing capacity (NRC), chemical reducing capacity (CRC), and microbial reducing capacity (MRC) of HS as reducing capacity of HS that did not have any reduction pretreatment, chemically reduced HS that have undergone chemical pretreatment in the laboratory (usually by H2 gas in the presence of Pd catalyst at pH = 7), and microbially reduced HS that have been reduced by microbes, respectively. There is generally a good agreement between CRC and MRC. Thus, CRC became a standard surrogate for assessing electron transfer capacities of HS in the environment (Peretyazhko and Sposito 2006).
There are various methods available to assess CRC of HS. One of the most common methods is reduction of HS dissolved in phosphate buffer at pH 7 using H2/Pd (Visser 1964; Kappler et al. 2004) followed by incubation with ferric citrate for 15 min. Then, the suspension is extracted by 0.5 M HCl, and Fe(II) is measured colorimetrically by UV–Vis spectrophotometer at 562 nm by complexation with ferrozine reagent (Stookey 1970). There are a few assumptions made here. First, H2/Pd will cause complete reduction of all functional groups of HS responsible for electron transfer, and second, ferric citrate will react with all reduced functional groups of HS within 15 min. These assumptions have been questioned by Ratasuk and Nanny (2007) and Aeschbacher et al. (2010). Ratasuk and Nanny (2007) found that depending on pH values and the presence of neighboring functional groups (i.e., either electron donating or electron withdrawing) near the quinone groups of HS, the H2/Pd reduction method can destroy the quinone moieties in HS. They supported their hypothesis using several model compounds such as anthraquinone-2,6-disulfonate (AQDS), anthraquinone-2-carboxylate (AQC), 2,6-dihydroxyanthraquinone (AQOH), which have both electron-donating and electron-withdrawing neighboring groups close to quinone moieties. Aeschbacher et al. (2010) indicated that 15 min incubation by ferric citrate was not sufficient to measure all the reducible functional groups of HS. Therefore, both assumptions one and two may underestimate the CRC measured by this method. However, although the kinetic limitation of the second step of the process was demonstrated clearly by Aeschbacher et al. (2010), the limitation of H2/Pd reduction method, indicated by Ratasun and Nanny (2008), at variable pH, and the presence of different functional groups near quinone moieties were not obvious from the UV–visible and IR spectroscopic evidences. This is because the quinone peaks are known to shift in the blue or red regions in the presence of both electron-withdrawing and electron-donating neighboring groups at different pH values (Gautrot et al. 2007; Diaz 1990; Peters and Sumner 1953). Therefore, consideration of 290-nm peak as quinone for all the model compounds such as AQOH, AQC, 1,4-naphthoquinone-2-sulfonate (NQS), plumbagin, juglone, and 1,4-naphthoquinone (NQ) under different chemical treatments is questionable.
An alternative to the second step of the above procedure is to use a different oxidant such as potassium ferricyanide [K3Fe(CN)6] (Matthiessen 1995). The procedure relies on the ability of reduced HS to oxidize [K3Fe(CN)6] to [K4Fe(CN)6] and the loss of absorbance measured in UV–Vis at 420 nm. The incubation time for this procedure is 24 h, which is much longer than ferric citrate (i.e., 15 min). However, no efforts have been made till date to compare these two incubation methods resulting in different reported CRC and MRC values for same HS and model compounds.
Schnitzer and Riffaldi (1972) modified a method from Vasilyevskaya et al. (1971) to determine quinone contents of HS and model compounds and found very good agreement with the theoretical quinone values for model compounds. This procedure uses Sn2+ as a reductant for HS. The quinone content is determined via potentiometric titration of excess Sn2+ using potassium dichromate (K2Cr2O7). None of the recent studies used this method to investigate the relative reliability of quinone content measurements of HS. We decided to apply this method to assess its applicability in regard to redox property measurements of HS and compare with other common methods. Our main objective of this study was therefore to compare and contrast redox property measurements of HS by Schnitzer and Riffaldi (1972), ferric citrate incubation, and [K3Fe(CN)6] incubation methods using AQDS as a model compound, and standard Elliot soil humic acid (1S102H, ESHA), reference Pahokee peat (1R103H, PPHA), and Suwannee River natural organic matter (1R101N, SRNOM) as representative HS.
Materials and methods
Humic acids and model compounds
Anthraquinone-2,6-disulfonate (AQDS) was purchased from Fluka and used as received. Standard Elliot soil humic acid (1S102H, ESHA), reference Pahokee peat (1R103H, PPHA), and Suwannee River natural organic matter (1R101N, SRNOM) were purchased from International Humic Substances Society and used as received. The properties of these HS and AQDS can be found in Table 1 of Rakshit et al. (2009).
Reduction pretreatment
An aliquot of unreduced HS taken from stock aqueous suspension (200 mg L−1) in phosphate buffer at pH 7 was reduced with N2/H2 gas (95:5%; Visser 1964; Kappler et al. 2004) in the presence of Pd catalyst (5% Pd on activated charcoal 100 mg L−1; Acros Organics) for 24 h. The reduction pretreatment was performed in amber-colored sealed serum vials under the hood. After the reduction pretreatment, headspace was flushed with Ar gas to remove excess H2 and transferred to anaerobic glovebox for further analysis. A similar reduction pretreatment was conducted on AQDS with concentration of 2 g L−1.
Determination of reducing equivalent upon reduction pretreatment
Two procedures were used to determine the extent of reduction. The first one was to incubate the reduced AQDS and HS with ferric citrate for 15 min and subsequent 0.5 M HCl extraction of the suspension followed by measurement in UV–Vis at 562 nm wavelength (Lovley and Blunt-Harris 1999). The second procedure was incubation by K3Fe(CN)6 and determination of loss of absorbance at 420 nm in UV–Vis spectrophotometer. Details of the procedure are in Matthiessen (1995). Only AQDS was measured by this method.
Measurement of quinone content using potassium dichromate and stannous chloride
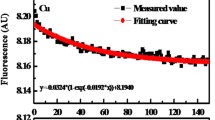
Procedure described in Schnitzer and Riffaldi (1972) was used for quinone measurements. All concentrations were kept exactly same as that of Schnitzer and Riffaldi (1972). However, for few experiments, a K2Cr2O7 concentration of 0.025 or 0.005 N was used instead of 0.05 N to increase precision. Here N of K2Cr2O7 was related to its M as N = M/6 considering the recommendation of IUPAC (Irving 1978) and assuming a six-electron transfer reaction between K2Cr2O7 and Sn2+. Ten milliliters of 0.05 N SnCl2, 2H2O was always used. Three types of titration data are reported here: first, titration of either HS or AQDS by dichromate in the presence of SnCl2 (called Sn(II)-reduced, which is essentially the original quinone titration by Schnitzer and Riffaldi (1972)); second, titration of H2/Pd-reduced HS or AQDS by dichromate in the absence of SnCl2 (called control, a required experiment to check potential undesirable reaction between reduced AQDS/HS and K2Cr2O7); and third, titration of H2/Pd-reduced HS/AQDS in the presence of SnCl2 (called H2-Sn(II)-reduced, which was a necessary experiment to evaluate completeness of the H2/Pd reduction pretreatment). All these titrations were accompanied by a blank, in which 10 mL 0.05 N SnCl2 was titrated with 0.05 N K2Cr2O7 using the methods of Schnitzer and Riffaldi (1972). For a majority of the titrations, two replicates of samples and blanks were used. In some cases, where all three titrations, i.e., two replicate samples and one blank, were performed on the same day, only one replicate of blank was reported.
Electrode calibration
pH electrodes were calibrated using pH 4 and 7 buffers (Fisher). Platinum redox electrodes (Orion Model 97-98) were calibrated with redox buffers containing either 0.1 M potassium ferrocyanide and 0.05 M potassium ferricyanide or 0.01 M potassium ferrocyanide, and 0.36 M potassium fluoride. The measured cell electromotive force values were 238 ± 7 and 304 ± 5 mV, respectively, as expected for the two buffers.
Results and discussion
AQDS
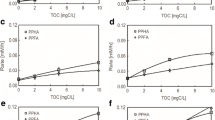
Charge concentrations of AQDS are listed in Table 1 and Fig. 1. The one-way analysis of variance was significant, F(3, 8) = 68.35, p < 0.05, in which total charge concentration was significantly different among four methods evaluated in this study (Fisher’s LSD, p < 0.05). It is evident from the data that the K2Cr2O7 titration by Schnitzer and Riffaldi (1972) resulted in the most accurate value of theoretical AQDS charge concentrations arising from quinone moieties (i.e., a theoretical quinone charge of 5.46 mol kg−1 AQDS). Titration of H2/Pd-reduced AQDS in the absence of SnCl2 (termed control in Fig. 1) suggests that the reduced AQDS did not react with K2Cr2O7. Experiment with reduced AQDS in the presence of SnCl2 was carried out to assess whether H2/Pd reduction method was incomplete (Fig. 2). A quinone charge of 1.88 mol kg−1 of AQDS was determined, indicating that H2/Pd reduction method did not reduce all quinone moieties of AQDS (Fig. 2). Incubation by K3Fe(CN)6 resulted in higher AQDS charge concentrations compared to the theoretical value. Additionally, precision was poor as reflected by the large standard error values. Due to very small absorbance change (see standard curves in “Appendix” Figs. 9, 10) with concentration in case of K3Fe(CN)6 compared to that of ferrozine method (Stookey 1970), the K3Fe(CN)6 method cannot be used. For example, K3Fe(CN)6 changed absorbance by only 0.46 U for a concentration change of 500 μM. In comparison, for ferrozine, absorbance changed by 2.44 U for a concentration difference of 100 μM. Hence, K3Fe(CN)6 incubation method is not sensitive enough to be used for measurements of small differences in charge concentrations.
Potentiometric titration of H2-Sn(II)-reduced PPHA by 0.05 N potassium dichromate in the presence of 10 mL 0.05 N SnCl2. PPHAR1 and PPHAR2 represent two replicated experiments where H2/Pd-reduced PPHA was titrated with dichromate in the presence of 10 mL 0.05 N SnCl2. Blank represents where no reduced PPHA added
Potentiometric titration of H2-Sn(II)-reduced SRNOM by 0.025 N potassium dichromate in the presence of 10 mL 0.05 N SnCl2. Reduced_SRNOMR1 and Reduced_SRNOMR2 represent two replicated experiments where H2/Pd-reduced SRNOM were titrated with dichromate in the presence of 10 mL 0.05 N SnCl2. Blank represents where no reduced SRNOM added
Ferric citrate incubation resulted in lower charge concentrations of AQDS arising from quinone moieties compared to theoretical value (Table 1). However, the precision was very good, supported by our reasoning that this method is very sensitive. The lower value of charge concentration resulted from incomplete reduction of AQDS by H2/Pd (evidenced in Fig. 2) and the short incubation time of 15 min, as suggested by Aeschbacher et al. (2010). Therefore, the method of Schnitzer and Riffaldi (1972) provides accurate charge concentrations for AQDS.
Humic substances
From the titration results of H2/Pd-reduced HS (ESHA, PPHA, SRNOM) in the absence of Sn(II), it is clear that K2Cr2O7 does not react with H2/Pd-reduced HS and does not interfere with the titration method (control in Figs. 3, 5, 7). For ESHA and PPHA, there is little reduction in redox potential at the time of titration; however, the interference occurs at a lot lower redox ladder than the actual reaction between excess Sn2+ with K2Cr2O7. Therefore, it is unlikely that H2/Pd-reduced HS will react with K2Cr2O7 to cause erroneous titration values. Hence, we can safely rule out the possibility of overestimation of charge concentrations during titration due to reaction between H2/Pd-reduced HS and K2Cr2O7 (Figs. 4, 6).
The next logical step was to analyze the conditions where H2-Sn2+-reduced HS had been reacted with K2Cr2O7. These charge concentration values would represent the reducing equivalents in HS that are not reduced by H2/Pd. Table 2 and Figs. 4 and 6 show that there remains considerable amount of reducible charge concentrations for ESHA and PPHA that cannot be reduced by H2/Pd treatment. Therefore, the hypothesis of Ratasuk and Nanny (2007), which assumed that H2/Pd treatment may destroy quinone moieties, could not be validated. This is because if quinone moieties were destroyed by H2/Pd reduction treatment at pH 7, the titration method would not produce any residual charge concentrations. Hence, we conclude that H2/Pd reduction method at pH 7 is incomplete for ESHA and PPHA. For SRNOM, we did not detect any charge concentrations after H2/Pd reduction followed by titration with K2Cr2O7 in the presence of SnCl2 (Table 2). This is most likely due to the fact that H2/Pd reduction completely reduced all the reducible moieties of SRNOM. The charge concentration values resulting from Schnitzer and Riffaldi (1972) method (or Sn2+-reduced method) are also listed in Table 2. The little difference in values, i.e., 0.25 compared to 0.205 mol charge kg−1, may arise from experimental variability. In this context, it should be mentioned that the titration method cannot distinguish between small differences in charge concentrations in the range of 0.03–0.05 mol charge kg−1 HS. For example, if one single drop from the buret changes 0.1 mL volume reading for either 0.05 or 0.025 N K2Cr2O7, the calculated charge concentrations for 0.1 mL would then be in the range of 0.03–0.05 mol charge kg−1 HS. This argument supports our conclusion that for SRNOM, H2/Pd reduction was also complete. Hence, we conclude that charge concentration determination of HS by the method of Schnitzer and Riffaldi (1972) is most accurate in comparison with the other tested methods. In fact, for ESHA and PPHA, the charge conc. values by quinone titration were 1.38 and 1.25 mol charge/kg, respectively. These values were close to the values reported by Aeschbacher et al. (2010), in which the reported charge conc. of ESHA and PPHA were 1.96 and 1.62 mol charge/kg, respectively (Fig. 8).
The incompleteness of H2/Pd followed by FeCit incubation (i.e., CRC method) can be corrected using the charge concentration values from H2-Sn2+-reduced HS. For example, in Table 2, for ESHA and PPHA, if one adds the charge concentration values from the CRC method and H2-Sn2+ methods, it will be in good agreement with charge concentrations obtained from Schnitzer and Riffaldi (1972) (or Sn2+ reduced) methods.
In summary, K3Fe(CN)6 incubation method should not be used because of its low sensitivity. FeCit incubation for 15 min may underestimate charge concentrations of HS. H2/Pd reduction method results in incomplete reduction pretreatment that does not destroy all quinone moieties. Quinone determination method of Schnitzer and Riffaldi (1972) results in accurate estimate of quinones for model compounds (AQDS) and estimates quinone charge concentrations of HS, which can be used for characterizing the redox properties of HS. Sensitivity of Schnitzer and Riffaldi (1972) method can be enhanced by using lower concentrations of K2Cr2O7.
References
Adhikari D, Poulson SR, Sumalia S, Dynes JJ, McBeth JM, Yang Y (2016) Asynchronous reductive release of iron and organic carbon from hematite-humic acid complexes. Chem Geol 430:13–20
Aeschbacher M, Sander M, Schwarzenbach RP (2010) Novel electrochemical approach to assess the redox properties of humic substances. Environ Sci Technol 44:87–93
Borch T, Kretzschmar R, Kappler A, Van Cappellen P, Ginder-Vogel M, Voegelin A, Campbell KM (2010) Biogeochemical redox processes and their impact on contaminant dynamics. Environ Sci Technol 44:15–23
Chen J, Gu B, Royer RA, Burgos WD (2003) The roles of natural organic matter in chemical and microbial reduction of ferric iron. Sci Total Environ 307:167–178
de Melo BAG, Motta FL, Santana MHA (2016) Humic acids: structural properties, and multiple functionalities for novel technological developments. Mater Sci Eng C 62:967–974
Diaz AN (1990) Absorption and emission spectroscopy and photochemistry of 1,10-anthraquinone derivatives: a review. J Photochem Photobiol A 53:141–167
Dunnivant FM, Schwarzenbach RP, Macalady DL (1992) Reduction of substituted nitrobenzenes in aqueous solutions containing natural organic matter. Environ Sci Technol 26:2133–2141
Gautrot JE, Hodge P, Cupertino D, Helliwell M (2007) 2, 6-Diaryl-9,10-anthraquinones as models for electron-accepting polymers. New J Chem 31:1585–1593
Hernandez-Monotoya V, Alvarez LH, Montes-Moran MA, Cervantes FJ (2012) Reduction of quinone and non-quinone redox functional groups in different humic acid samples by Geobacter sulfurreducens. Geoderma 183–184:25–31
Irving HMNH (1978) Recommendations on the usage of the terms ‘Equivalent’ and ‘Normal’. Pure and Appl Chem 50:325–338
Jiang J, Bauer I, Paul A, Kappler A (2009) Arsenic redox change by microbially and chemically formed semiquinone radicals and hydroquinones in a humic substance model quinone. Environ Sci Technol 43:3639–3645
Kappler A, Haderlein SB (2003) Natural organic matter as reductant for chlorinated aliphatic pollutants. Environ Sci Technol 37:2714–2719
Kappler A, Benz M, Schink B, Brune A (2004) Electron shuttling via humic acids in microbial iron(II) reduction in freshwater sediment. FEMS Microbiol Ecol 47:85–92
Klüpfel L, Pipenbrock A, Kappler A, Sander M (2014) Humic substances as fully regenerable electron acceptors in recurrently anoxic environments. Nat Geosci 7:195–2000
Lovley DR, Blunt-Harris EL (1999) Role of humic-bound iron as an electron transfer agent in dissimilatory Fe(III) reduction. Appl Environ Microbiol 65:4252–4254
Macalady DL, Walton-Day K (2011) Redox chemistry and natural organic matter (NOM): geochemists’ dream, analytical chemists’ nightmare. In: Tratnyek PG, Grundl TJ, Haderlein SB (eds). American Chemical Society, Washington, pp 85–111
Matthiessen A (1995) Determining the redox capacity of humic substances as a function of pH. Vom Wasser 84:229–233
Nevin KP, Lovley DR (2000) Potential for nonenzymatic reduction of Fe(III) via electron shuttling in subsurface sediments. Environ Sci Technol 34:2472–2478
Peretyazhko T, Sposito G (2006) Reducing capacity of terrestrial humic acids. Geoderma 137:140–146
Peters RH, Sumner HH (1953) Spectra of anthraquinone derivatives. J Chem Soc 2102–2110. doi:10.1039/JR9530002101
Pipenbrock A, Schröder C, Kappler A (2014) Elelctron transfer from humic substances to biogenic and abiogenic Fe(III) oxyhydroxy minerals. Environ Sci Technol 48:1656–1664
Rakshit S, Uchimiya M, Sposito G (2009) Iron(III) bioreduction in soil in the presence of added humic substances. Soil Sci Soc Am J 73:66–71
Ratasuk N, Nanny MA (2007) Characterization and quantification of reversible redox sites in humic substances. Environ Sci Technol 41:7844–7850
Schnitzer M, Riffaldi R (1972) The determination of quinone groups in humic substances. Soil Sci Soc Am Proc 36:772–777
Scott DT, McKnight DM, Blunt-Harris EL, Kolesar SE, Lovley DR (1998) Quinone moieties act as electron acceptors in the reduction of humic substances by humics-reducing microorganisms. Environ Sci Technol 32:2984–2989
Sposito G (2011) Electron shuttling by natural organic matter: twenty years after. In: Tratnyek PG, Grundl TJ, Haderlein SB (eds) American Chemical Society, Washington, pp 113–127
Stookey LL (1970) Ferrozine: a new spectrometric reagent for iron. Anal Chem 42:779–781
Vasilyevskaya NA, Glebko LI, Maximov OB (1971) Determination of quinoid groups in humic acids. Pochvovdenie 4:63–67 (in Russian)
Visser SA (1964) Oxidation-reduction potentials and capillary activities of humic acids. Nature 204:581
Weber KA, Achenbatch LA, Coates JD (2006) Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction. Nat Rev Microbiol 4:752–763
Yang Z, Du M, Jiang J (2016) Reducing capacities and redox potentials of humic substances extracted from sewage sludge. Chemosphere 144:902–908
Acknowledgements
This research was supported by the Evans-Allen Grant from USDA and NSF Grant DEB-0543558. The authors express gratitude to Professor Garrison Sposito at the University of California, Berkeley, for providing laboratory facilities to conduct experimental work for completing this manuscript. The authors also thank Mr. Andrew Yang for his help in the laboratory.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: R. Datta.
Rights and permissions
About this article
Cite this article
Rakshit, S., Sarkar, D. Assessing redox properties of standard humic substances. Int. J. Environ. Sci. Technol. 14, 1497–1504 (2017). https://doi.org/10.1007/s13762-017-1263-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-017-1263-9