Abstract
The decomposition of phenol and trichlorophenol (TCP) by using granular ferric hydroxide (GFH) as a photo-Fenton catalyst was investigated and compared with homogeneous photo-Fenton process. Experiments were conducted in a batch mode, duplicate for the degradation of phenol and TCP in the presence of solar light for both the processes. The effect of operating variables for heterogeneous photo-Fenton process like pH, peroxide concentration and GFH concentration on the degradation of the model compounds was optimized by univariate approach. The optimum conditions for the degradation of phenol and TCP were pH 3.0 ± 0.2, peroxide concentration 29.4 mM for phenol and 14.7 mM for TCP at GFH concentration of 0.5 g/500 mL. At optimum conditions, the mineralization efficiency of phenol and TCP by heterogeneous process was compared with homogeneous process. The mineralization efficiency for phenol and TCP was 96 and 86 %, respectively, for heterogeneous photo-Fenton process, while almost complete mineralization (~96 %) was observed for homogeneous process. In heterogeneous photo-Fenton process, longer reaction time was witnessed for complete mineralization of the compounds studied. Low molecular weight aliphatic acids like oxalic acid, acetic acid and inorganic chloride ion (in case of TCP) were observed during both the processes. In these processes, the reaction proceeds by hydroxyl radical (·OH) abstraction of the model compound studied. The mineralization of phenol and TCP obeys pseudo-first-order kinetics irrespective of the processes studied. The results indicate that GFH can be an effective heterogeneous photo-Fenton catalyst for the degradation of phenol and TCP.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Advanced oxidation processes (AOPs) have been widely applied in the treatment of refractory, recalcitrant organic compounds in water and wastewater treatment. AOPs produce highly oxidative, non-selective transient hydroxyl radical (·OH) as the primary oxidant for the destruction and mineralization of organic compounds (Vilhunen and Sillanpaa 2010) in aqueous media. Among different AOPs, Fenton process employs ferrous ion and hydrogen peroxide which are capable of generating ·OH radical at room conditions. The efficacy of Fenton process is accelerated by applying solar/UV light, regarded as homogeneous photo-Fenton process (since ferrous ion is dissolved in aqueous solution) for degrading wide range of contaminants in water and wastewater (Babuponnusami and Muthukumar 2014). However, this process suffers from serious limitations in the removal of iron sludge formed during the treatment process. The reusability of iron sludge for treatment of phenol (Kavitha and Palanivelu 2004), leachate (Bolobajev et al. 2014) and dye (Li et al. 2007) has been reported. Moreover, the concentration of iron in the treated water by homogeneous photo-Fenton process exceeded the discharge limit of 1 mg/L prescribed by pollution control boards and European Union Water Frame work Directive (EU Directive 2000/60/EC). In order to comply with the regulations of statutory agencies, many attempts have been made by several researchers to develop a newer, efficient catalyst like immobilization of iron onto solid support like Nafion (Sabhi and Kiwi 2001), zeolite (Bossmann et al. 2001), laponite clay-based iron nanocomposite (Feng et al. 2003), Fe-loaded mesoporous silica SBA-15 (Shukla et al. 2010), Al–Fe pillared interlayered clays (Catrinescu et al. 2012) or by utilizing natural iron minerals like goethite (Lu et al. 2002; Andreozzi et al. 2002), iron oxides (Pouran et al. 2014; Gupta et al.2011; Teel et al. 2001; Valentine and Wang 1998). In these processes, iron is not dissolved in aqueous media, and hence, it is regarded as heterogeneous photo-Fenton process. The synthetic heterogeneous catalysts are expensive in nature and contribute to meagre mineralization. It has been reported by Huang et al. (2001) that some of the iron oxides like granular ferrihydrite and goethite are inactive towards degradation of chlorophenols compared to haematite. Bandara et al. (2001) have accounted that α-Fe2O3 catalysed the degradation of chlorophenol and α-FeOOH degraded only 2,4-dichlorophenol, while other chlorophenols cannot be degraded by iron oxides.
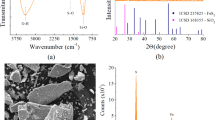
In this paper, the potential feasibility of using granular ferric hydroxide (GFH) as a photo-Fenton catalyst towards degradation of phenol and 2,4,6-TCP was studied. Several researchers have focused on GFH by exploiting its application in wastewater treatment as a sorbent for removing arsenic (Chen et al. 2015), antimony and vanadium (Kolbe et al. 2011), phosphate (Kartashevsky et al. 2015), phosphonate (Boels et al. 2012), or hydrogen sulphide (Sun et al. 2014). GFH used in the present study is a β-FeOOH or akaganeite type possessing high specific area and is porous in nature (Cornell and Schwertmann 2003). The choice of selecting phenol and 2,4,6-trichlorophenol (TCP) as a model compound for the present study is due to their toxic, carcinogenic nature, and it has been regarded as ‘priority pollutants’ listed by USEPA. The operating variables in the process like pH, catalyst concentration and peroxide concentration were optimized together with the stability of the catalyst in order to assess the efficiency of the process. The mineralization efficiency of the process was compared with homogeneous photo-Fenton process, and the formation of stable intermediates was also monitored in order to elucidate the possible reaction mechanism. The kinetics of both these processes was explored to examine the efficacy of the process.
Date and location of the research were July, 2012, and Centre for Environmental studies, Anna University, Chennai (Tamil Nadu), respectively.
Materials and methods
Chemicals
Analytical-grade reagents, phenol and 2,4,6-TCP were purchased from SD Fine Chemicals and Fluka, respectively. All chemicals were used as received without any purification. Ferrous sulphate heptahydrate (AR, CDH) was prepared at a concentration of 90 mM Fe2+ in sulphuric acid. Hydrogen peroxide was prepared at a concentration of 6.7–6.8 N and stored at acidic pH. GFH (GEH® 102) containing ~52–57 % of β-FeOOH and Fe(OH)3 with a grain size of 0.32–2 mm was purchased from Wasserchemie Gmbh & Co. KG (Germany) and used as a source of iron in heterogeneous photo-Fenton process. Its specific area was 250–300 m2/g as given by manufacturer specification. The point of zero charge (p pzc) for GFH was found to be 4.6 ± 0.1 as described in Noh and Schwarz (1989). The solar intensity and luminance during the experimental run were in the range of 700–792 W/m2 and 90–96 Klx, respectively.
Homogeneous photo-Fenton process
Experiments were conducted in duplicate, batch mode and in shallow glass troughs (23.5 × 14 cm) using phenol and TCP as model compounds. The initial concentration of the phenolic solution during the experimental run was 200 mg/L, and the total volume of the reacting solution is 500 mL. The glass trough containing the acidic phenolic solution, ferrous ion and peroxide was exposed to solar light and aerated using air bubbler for 2 h.
Heterogeneous solar-Fenton process
Batch studies were conducted in shallow glass troughs as described above using phenol and TCP as model compounds. The glass trough containing 500 mL of acidic phenolic solution is at a depth of 2.2 cm in order to ensure maximum solar radiation penetrates through the upper open surface of the trough. A known amount of GFH was added as a source of iron to the phenolic solution containing peroxide. The solution was aerated using air bubbler and exposed to solar light for a maximum of 4 h.
Heterogeneous UV-Fenton process
Experiments were performed in immersion-type photoreactor (Heraeus, TQ150) of 500 mL capacity. A medium-pressure mercury lamp of 150 W was used as an UV source, which emits characteristic wavelength of 254 nm. The reacting solution containing the phenolic solution and GFH surrounds the UV lamp. It is surrounded by another Pyrex glass vessel. The double surface of immersion type permits the circulation of cooling water in order to reduce the heat generated by UV light. The whole assembly was mounted on a magnetic stirrer. The lamp was switched on with the simultaneous addition of peroxide which marks the beginning of the experiment.
Analytical methods
Samples were withdrawn from the batch process and analysed immediately without any storage for chemical oxygen demand (COD), dissolved organic carbon (DOC), dissolved iron (Fe2+ and Fe3+) and also for their stable intermediates formed during the reaction. The degradation efficiency was studied by the reduction in COD. For COD measurements, the samples were pretreated with 6 N NaOH to quench the reaction, heated at 40 °C to remove any residual H2O2 (Jones 1999) and determined by dichromate titrimetric method as described in APHA (2000). The mineralization efficiency was estimated by the decrease in the DOC content of the reacting solution. The DOC concentration was measured using TOC analyzer, Analytik Jena Model 1997 equipped with liquid autosampler ALS-C-104. The degradation efficiency or mineralization efficiency was calculated by the following Eq. (1)
where C 0 is the initial COD or DOC of the phenolic solution in mg/L, C t is the COD or DOC of the phenolic solution in mg/L at time t (min).
The concentration of dissolved iron (Fe2+ and Fe3+) in the treated solution was determined by Fe(II)/1,10 phenanthroline complex at 510 nm (APHA 2000) using Spectronic Genesys-20 spectrophotometer. Aliphatic acids like oxalic acid and acetic acid formed during the processes were quantitatively determined in ion chromatographic system (Dionex DX120) provided with an Ion Pac AS-14 column. The mobile phase consisted of 3.5 mM Na2CO3 and 1.0 mM NaHCO3 operated at isocratic mode and at a flow rate of 1.2 mL/min. The peaks were detected at 2.68 ± 0.3, 3.10 ± 0.3 and 7.84 ± 0.3 min for acetic acid, chloride and oxalic acid, respectively.
Results and discussion
Homogeneous photo-Fenton process
Fenton’s reagent is a mixture of ferrous ion and peroxide and generates a highly reactive non-selective hydroxyl radical (·OH) in situ according to Eq. (2)
When peroxide is added to an aqueous phenolic solution containing ferrous ion in the presence of solar light, the colour of the solution changed from colourless to brown and turbid during the initial stages of the reaction. As the reaction proceeded, the dark colour changed to colourless and clear within 1 h of the reaction. The pH of the treated solution was reduced to 2.5 ± 0.1 during the reaction. The parameters involved in homogeneous Fenton process are H2O2 and Fe2+ which are varied from 10 to 60 and 0.1 to 0.6 mM, respectively, at pH 3.0. The optimum conditions obtained for the degradation of phenol and TCP are pH 3.0 ± 0.2, H2O2 of 29.4 mM [phenol], 14.7 mM [TCP] and ferrous ion of 0.4 mM [phenol], 0.12 mM [TCP], respectively.
Heterogeneous photo-Fenton process
When GFH was added to acidic phenolic solution at room conditions or by exposing at UV 254 nm, neither adsorption/degradation of phenol nor dissolution of GFH takes place up to 24 h. Experiments were performed in the presence of peroxide at room conditions and in the presence of UV up to 5 h. During the experimental study, there was no detectable change in the reacting media or no change in DOC was observed. The results indicate that GFH is less efficient in absorbing UV 254 nm. Mazellier and Bolte (2000) have reported that the disappearance of 2,6 dimethylphenol takes place upon irradiation of goethite at 546 nm which is consistent with the results observed. Hence, an aqueous suspension of aerated phenol-GFH was exposed to solar light in the presence and absence of peroxide. On irradiation with GFH alone, there was no change in DOC or dissolution of iron. However, in the presence of peroxide, the phenolic solution changes to brown within 1 h, then to yellow and finally to colourless at the end of 4 h together with reduction in DOC. The degradation exhibited by β-FeOOH in the presence of solar light is due to its low band gap energy of 2.2 eV (Mohapatra et al. 2010) which corresponds to the visible portion (560 nm) of the spectrum. In order to assess the photo-catalytic behaviour of GFH, experiments were conducted with H2O2 alone (in the absence of GFH); phenol removal was observed, but there is negligible removal in DOC. The parameters involved in heterogeneous system are varied by univariate method.
Effect of pH
Degradation studies were carried out by varying the pH of the solution from 2.0 to 6.0 in the presence of peroxide and solar light as shown in Fig. 1. Maximum degradation efficiency is achieved at pH 3.0 which is in accordance with Neppolian and Choi (2004), He et al. (2002) and Yaping et al. (2010). The increase in photo-catalytic degradation at acidic pH can be explained in terms of point of zero charge (p zpc). The p zpc of GFH was found to be 4.6 ± 0.3. The effect of pH plays an important role in the adsorption of phenolic compounds onto GFH surface. The adsorption is considered to be a prominent step for photo-degradation reaction (Saleh and Gupta 2011; Gupta et al. 2012). In acidic pH (below p zpc), the surface of GFH is positively charged due to prevalent forms of Fe-OH2 (Andreozzi et al. 2002). The phenolic compounds remained in its undissociated form (at acidic pH) is adsorbed onto the surface of the catalyst due to electrostatic attraction between the dipole of phenol and positively charged GFH surface (Saleh and Gupta 2012). As the pH is increased (above p zpc), the surface of GFH is reversed, and there is a repulsion of phenolic anion and GFH surface which contributes to reduction in degradation efficiency. The pH of the treated solution is acidic (irrespective of the initial pH), indicating the presence of acidic components in the reaction media.
Effect of catalyst loading
A series of experiments were performed by varying the amounts of GFH catalyst from 0.25 to 1 g/500 mL to aerated phenolic solutions containing peroxide at pH 3.0 ± 0.2 and are illustrated in Fig. 2. The degradation reaction proceeds in the presence of GFH indicating its vital role as photo-catalyst. As the concentration of GFH was increased from 0.25 to 0.5 g/500 mL, the efficiency also increases due to large number of active sites available for the adsorption of phenol and TCP with concomitant increase in degradation. On further increasing the concentration from 0.75 to 1 g, the degradation efficiency remains unaltered that may be due to the screening of solar light by GFH. Thus, a catalyst loading of 0.5 g/500 mL was used for further studies.
Effect of peroxide
Experiments that were conducted by aerating the phenolic solution containing varying concentrations of peroxide at pH 3.0 ± 0.2 in the presence of GFH are depicted in Fig. 3. In the absence of peroxide (i.e. by aeration alone), the recombination of electron–hole takes place rapidly leading to meagre degradation efficiency at the conditions employed. The peroxide plays an important role as ‘electron acceptor’ which suppress the electron–hole recombination and facilitates the generation of ·OH as shown in Eq. 3, necessary for complete degradation of the phenolic compound studied
The degradation efficiency increases with increase in peroxide concentration. Above the optimum conditions, further addition of peroxide did not improve the degradation efficiency which may be due to
- 1.
-
2.
The ·OH radical reacts with excess peroxide to form hydroperoxyl radical \(({\text{HO}}_{ 2}^{ \cdot } )\) as in Eq. 5 which has lower oxidation potential than ·OH radical towards phenol degradation. Hence, further experiments were conducted by aerating and adding optimum peroxide concentration.
Stability and reusability of the catalyst
In order to assess the stability and efficiency of GFH catalyst, experiments were conducted at optimum conditions for five times using phenol as a model compound. The catalyst was separated from the reacting media by filtration and used as such without any post-treatment. The mineralization efficiency remained almost same during the experimental runs, and the results are given in Fig. 4. Neither Fe3+ nor Fe2+ ions were detected during the experimental run from I to V which suggests both photo-dissolution and leaching of iron at pH 3.0 do not take place which is in accordance with the results observed by Mazellier and Bolte (2000). In the first run, the mineralization efficiency was 96.7 %. The GFH catalyst was recovered and used in subsequent runs. In the second, third, fourth and fifth run, the efficiency was decreased and found to be 94.2, 93.4, 92.1 and 90.8 %, respectively. The reaction proceeds with minor retardation in its initial stages of the reaction in all the runs. The slight decrease in efficiency may be due to reduction in specific catalyst site which promotes the oxidation process. The results indicate that GFH can be an efficient photo-Fenton catalyst which is stable up to five cycles of process.
Comparison between homogeneous and heterogeneous photo-Fenton process
The mineralization efficiency of phenol and TCP at optimum conditions by homogeneous and heterogeneous photo-Fenton process is represented in Fig. 5. During homogeneous process, almost complete mineralization of phenolics (~95 %) was achieved within 120 min of the reaction time, while in heterogeneous system, around 95 % mineralization was observed in 4 h. Almost 70 % of the organic compound was mineralized within 15 min in homogeneous system, whereas negligible decrease in DOC is witnessed in heterogeneous system during this time. In homogeneous system, almost complete mineralization was observed in a relatively short time, due to the attack of highly oxidative ·OH radical. The ·OH radical generated as in Eq. 2 attacks the organic compound by abstracting the π electron cloud of the aromatic ring leading to mineralization. The mineralization efficiency is increased by photo-reduction in hydroxylated ferric ion as in Eq. 6, together with photolysis of hydrogen peroxide as in Eq. 7
In this process, four ·OH radicals are produced as per Eqs. 2, 6 and 7 which accelerates the mineralization process.
In heterogeneous process, longer reaction time was witnessed due to low ·OH radical concentration. The photo-catalytic activity of GFH was attributed to chemical nature of iron. Irradiation of solar light on GFH results in the formation of electrons and holes. The electrons react with electron acceptor, H2O2, according to Eq. 3. The holes react with organic adsorbed on the surface of GFH along with H2O2 or with ·OH radical as follows, and the reaction scheme is represented in Fig. 6
During the process, neither Fe2+/Fe3+ ion was detected, indicating the absence of photo-dissolution of iron which is in contrary to iron mineral system using goethite (Lu et al. 2002) and ferrihydrite (Valentine and Wang 1998). The result also indicates that the decomposition of phenolic compounds does not take place in aqueous phase but on the surface of GFH catalyst as witnessed by Mazellier and Bolte (2000), Huang et al. (2001) and Andreozzi et al. (2002). Lower mineralization efficiency of TCP compared to phenol is encountered due to steric hinderance of two chlorine atoms at ortho-position resulting in their resistant nature towards heterogeneous photo-Fenton system. The mineralization efficiency increases slowly as the reaction proceeds which indicates the conversion of phenolic compound to organic intermediates and finally to CO2.
During the reaction, low molecular weight organic compounds like acetic acid and oxalic acids are produced and their concentration profiles are presented in Fig. 7. The presence of oxalic acid was reported by Kavitha and Palanivelu (2005) during the degradation of cresol by Fenton oxidation process. The concentration of oxalic acid and acetic acid increases during the initial stages of the reaction and then decreases due to photolysis reactions in both homogeneous and heterogeneous process. Both these acids form stable photo-active complexes with ferric ions in the aqueous media at pH 2–4. These complexes undergo a series of photochemical reactions in UV–visible range of 250–480 nm as shown in Eqs. 10–13 through ligand to metal charge transfer reactions (LCMT), thereby generating Fe2+ ion and ·OH radical with high quantum yield of (\(\phi_{\text{Fe}}^{2 + }\) = 1.11–1.26).
Intermediates formed during the degradation of phenol and TCP by homogeneous and heterogeneous photo-Fenton processes (conditions were same as in Fig. 5)
The peroxide produced in the above reaction reacts with Fe2+ ion producing additional ·OH radical. These acids were removed with concomitant increase in mineralization efficiency. In homogeneous process, complete removal of oxalic acid takes place within 60 min for both phenol and TCP, while acetic acid remains in meagre concentration of 0.016 and 0.13 mM for phenol and TCP, respectively. Hence, complete mineralization (100 %) was not achieved in this process. The presence of acetic acid contributes to residual DOC resulting in 94 and 96 % mineralization for phenol and TCP, respectively.
In heterogeneous process, the concentration of acetic acid varies from 0.0433 to 0.09166 and 0.00725 to 0.03366 mM for phenol and TCP, respectively. The concentration of oxalic acid varies from 0.00855 to 0.04533 and 0.004 to 0.026 mM for phenol and TCP, respectively. Both these acids were completely removed at the end of the reaction which contributes to increase in mineralization efficiency. Minor amount of organic compounds still remain in reacting media which hinder complete mineralization of organic compound studied.
The presence of inorganic chloride ions during the process is a clear indication of TCP decomposition which is also observed by Teel et al. (2001) during the treatment of trichloroethylene by iron oxides. The concentration profile of inorganic chloride is presented in Fig. 8. In homogeneous Fenton process, more than 50 % of chloride ion is observed during 1 min of the reaction, suggesting the possible attack of ·OH on chlorine at the second and sixth position of carbon atom. The organic bound chlorine is removed as inorganic chloride in an aqueous system. As the reaction proceeds, the concentration of chloride increases and remains constant up to 45 min. Near-stoichiometric chloride ions was accounted for TCP degradation. In heterogeneous Fenton process, the chloride ion concentration increases as the reaction proceeds and the concentration reaches up to 2.552 mM. During the reaction, nearly 84 % of stoichiometric organic bound chlorine was accounted as inorganic chloride. The remaining stoichiometric chlorine is present as oxidized chlorinated intermediate compounds which support the presence of residual DOC in the reacting media. The formation of acetic acid, oxalic acid and chloride ion confirms the photo-catalytic nature of GFH towards phenol and TCP decomposition.
Kinetics of phenolic degradation
The kinetics of phenolic compound degradation by homogeneous and heterogeneous photo-Fenton processes is modeled to pseudo-first-order kinetics,
where C 0 and C t are the initial concentration of phenolic compounds at time ‘0’ and ‘t’ min, respectively, and k is the pseudo-first-order rate constant. During the processes, the phenolic compounds are removed immediately from the reacting solution. Hence, it is appropriate to monitor the concentration of phenolics in terms of residual DOC. Thus, the above equation becomes
where DOC0 and DOC t are the initial DOC and the residual DOC at time ‘t’ min. Pseudo-first-order rate constant (k) can be obtained through a linear least-square fit for phenolic data for the above equation, and the kinetic plots for both the processes are shown in Fig. 9. In heterogeneous kinetic plot, the mineralization efficiency up to 60 min was not considered since there was no appreciable change in DOC, while in homogeneous process, the data from initial stages till the end of the reaction were accounted for calculation.
Pseudo-first-order kinetic plots for the degradation of phenol and TCP by homogeneous and heterogeneous photo-Fenton processes (conditions were same as in Fig. 5)
The pseudo-rate constant (k) and the corresponding half-life (τ 1/2) of phenolics for homogeneous and heterogeneous photo-Fenton process are represented in Table 1. The \(k_{\text{Homo}}^{\prime }\) and \(k_{\text{Hetero}}^{\prime }\) represent the rate constant for homogeneous and heterogeneous photo-Fenton processes. The rate constant for heterogeneous process is low compared to homogeneous process as expected, indicating the slow reaction of phenolic compounds towards GFH catalyst. The efficiency of homogeneous process is increased by a factor of 1.5 for phenol and 3.3 for TCP compared to heterogeneous process which also suggests the recalcitrant nature of TCP.
Conclusion
The following implications were obtained from the above experimental study:
-
The mineralization efficiency of phenol and TCP is 94 and 96 %, respectively, for homogeneous photo-Fenton process, while 94 and 86 % are achieved for heterogeneous process. Even though both these processes have almost same mineralization efficiency, homogeneous process is more effective to degrade the compounds in a relatively short time.
-
Low molecular weight aliphatic acids like oxalic acid and acetic acid were detected in both homogeneous and heterogeneous photo-Fenton processes.
-
In TCP degradation, near-stoichiometric amount of inorganic chloride is accounted for homogeneous photo-Fenton process, while 84 % of organically bound chlorine is observed for heterogeneous process.
-
Both these processes follow pseudo-first-order kinetics for the removal of phenol and TCP with the rate constant of 0.0212 and 0.0269; 0.014 and 0.008 min−1 for homogeneous and heterogeneous photo-Fenton process.
-
The photo-catalyst, GFH, is stable and reused up to five cycles of treatment without any major loss in mineralization efficiency. It can therefore be suggested that GFH can be an alternative photo-Fenton catalyst in mineralizing organic compounds by utilizing solar light, especially for tropical countries like India, while sunlight is available throughout the year.
References
Andreozzi R, D’Apuzzo A, Marotta R (2002) Oxidation of aromatic substrates in water/goethite slurry by means of hydrogen peroxide. Water Res 36:4691–4698
APHA (2000) Standard methods for the examination of water and wastewater, 20th edn. APHA/AWWA/WEF, Washington, DC
Babuponnusami A, Muthukumar K (2014) A review on Fenton and improvements to the Fenton process for wastewater treatment. J Environ Chem Eng 2(1):557–572
Bandara J, Mielczarski JA, Lopez A, Kiwi J (2001) Sensitized degradation of chlorophenols on iron oxides induced by visible light comparison with titanium oxide. Appl Catal B Environ 34:321–333
Boels L, Keesman KJ, Witkamp G-J (2012) Adsorption of phosphonate antiscalant from reverse osmosis membrane concentrate onto Granular Ferric Hydroxide. Environ Sci Technol 46:9638–9645
Bolobajev J, Kattel E, Viisimaa M, Goi A, Trapido M, Tenno T, Dulova N (2014) Reuse of ferric sludge as an iron source for the Fenton-based process in wastewater treatment. Chem Eng J 255:8–13
Bossmann SM, Oliveros E, Gob S, Kantor M, Goppert A, Lei L, Yue PL, Braun AM (2001) Degradation of polyvinyl alcohol (PVA) by homogeneous and heterogeneous photocatalysis applied to photochemically enhanced Fenton reaction. Water Sci Technol 44:257–262
Catrinescu C, Arsene D, Apopei P, Teodosiu C (2012) Degradation of 4-chlorophenol from wastewater through heterogeneous Fenton and photo-Fenton process, catalyzed by Al–Fe PILC. Appl Clay Sci 58:96–101
Chen ASC, Sorg TJ, Wang L (2015) Regeneration of iron-based adsorptive media used for removing arsenic from groundwater. Water Res 77:85–97
Cornell RM, Schwertmann U (2003) The iron oxides: structure, properties, reactions, occurrences and uses, 2nd edn. Wiley-VCH, Weinheim, pp 1–339
Feng J, Hu X, Yue PL, Zhu HY, Gq Lu (2003) Discoloration and mineralization of reactive red HE-3B by heterogeneous photo-Fenton reaction. Water Res 37:3776–3784
Gupta VK, Shilpi A, Saleh TA (2011) Chromium removal by combining the magnetic properties of iron oxide with adsorption properties of carbon nanotubes. Water Res 45:2207–2212
Gupta VK, Jain R, Mittal A, Saleh TA, Nayak A, Agarwal S, Sikarwar S (2012) Photo-catalytic degradation of toxic dye amaranth on TiO2/UV in aqueous suspensions. Mater Sci Eng C 32:12–17
He J, Ma W, He J, Zhao J, Yu JC (2002) Photooxidation of azo dye in aqueous dispersions of H2O2/α-FeOOH. Appl Catal B Environ 39:211–214
Huang H-H, Lu M-C, Chen J-N (2001) Catalytic decomposition of hydrogen peroxide and 2-chlorophenol with iron oxides. Water Res 35:2291–2299
Jones CW (1999) Introduction to the preparation and properties of hydrogen peroxide. In: Clark JH (ed) Applications of hydrogen peroxide and derivatives. RSC, Cambridge, p 30
Kartashevsky M, Semiat R, Dosoretz CG (2015) Phosphate adsorption on granular ferric hydroxide to increase product water recovery in reverse osmosis-desalination of secondary effluents. Desalination 364:53–61
Kavitha V, Palanivelu K (2004) The role of ferrous ion in Fenton and photo-Fenton processes for the degradation of phenol. Chemosphere 55:1235–1243
Kavitha V, Palanivelu K (2005) Destruction of cresols by Fenton oxidation process. Water Res 39:3062–3072
Kolbe F, Weissa H, Wennrich R, Lorenzc WG, Dausa B (2011) Remobilization of pentavalent antimony and vanadium from granular iron hydroxide material—a comparative study of different leaching systems. Talanta 85:2089–2093
Li C-W, Chen Y-M, Chiou Y-C, Liu C-K (2007) Dye wastewater treated by Fenton process with ferrous ion electrolytically generated from iron-containing sludge. J Hazard Mater B 144:570–576
Lu M-C, Chen J-N, Huang HH (2002) Role of goethite dissolution in the oxidation of 2-chlorophenol with hydrogen peroxide. Chemosphere 46:131–136
Mazellier P, Bolte M (2000) Heterogeneous light-induced transformation of 2,6-dimethylphenol in aqueous suspensions containing goethite. J Photochem Photobiol A Chem 132:129–135
Mohapatra M, Mohapatra L, Anand S, Mishra BK (2010) In-situ one pot synthesis of high surface area nano akaganeite powder and its cation sorption behaviour. J Chem Eng Data 55:1486–1491
Neppolian JSP, Choi H (2004) Effect of Fenton-like oxidation on enhanced oxidative degradation of para-chlorobenzoic acid by ultrasonic irradiation. Ultrason Sonochem 11:273–279
Noh JS, Schwarz JA (1989) Estimation of the point of zero charge of simple oxides by mass titration. J Colloid Interface Sci 130:157–164
Pouran SR, Raman AAA, Daud WMAW (2014) Review on the application of modified iron oxides as heterogeneous catalysts in Fenton reactions. J Clean Prod 64:24–35
Sabhi S, Kiwi J (2001) Degradation of 2,4-dichlorophenol by immobilized iron catalysts. Water Res 35:1994–2002
Saleh TA, Gupta VK (2011) Functionalization of tungsten oxide into MWCNT and its application for sunlight-induced degradation of rhodamine. J Colloid Interface Sci 362:337–344
Saleh TA, Gupta VK (2012) Photo-catalyzed degradation of hazardous dye methyl orange by use of a composite catalyst consisting of multi-walled carbon nanotubes and titanium dioxide. J Colloid Interface Sci 371:101–106
Shukla P, Wang S, Sun H, Ang H-M, Tadé M (2010) Adsorption and heterogeneous advanced oxidation of phenolic contaminants using Fe loaded mesoporous SBA-15 and H2O2. Chem Eng J 164:255–260
Sun J, Zhou J, Shang C, Kikkert GA (2014) Removal of aqueous hydrogen sulfide by granular ferric hydroxide—kinetics, capacity and reuse. Chemosphere 117:324–329
Teel AL, Warberg CR, Atkinson DA, Watts RJ (2001) Comparison of mineral and soluble iron Fenton’s catalysts for the treatment of trichloroethylene. Water Res 35:977–984
Valentine RL, Wang HCA (1998) Iron oxide surface catalyzed oxidation of quinoline by hydrogen peroxide. J Environ Eng 124:31–38
Vilhunen S, Sillanpaa M (2010) Recent developments in photochemical and chemical AOPs in water treatment: a mini-review. Rev Environ Sci Biotechnol 9:323–330
Yaping Z, Jiangyong H, Chen H (2010) Estimation of estrogen and its estrogenicity by heterogeneous photo-Fenton catalyst β-FeOOH/resin. J Photochem Photobiol A Chem 212:94–100
Acknowledgments
The authors would like to express their sincere thanks to Department of Chemistry, Sathyabama University, and Centre for Environmental Studies, Anna University, for providing technical assistance to carry out the work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kavitha, V., Palanivelu, K. Degradation of phenol and trichlorophenol by heterogeneous photo-Fenton process using Granular Ferric Hydroxide®: comparison with homogeneous system. Int. J. Environ. Sci. Technol. 13, 927–936 (2016). https://doi.org/10.1007/s13762-015-0922-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-015-0922-y