Abstract
Fe3O4/multi-walled carbon nanotubes were prepared, characterized and used as a nanocatalyst for ozonation of p-hydroxybenzoic acid. The stability and reusability of the catalyst was evaluated. Characterization techniques including X-ray diffraction, Fourier transform infrared absorption spectroscopy, scanning electron microscope, high-resolution transmission electron microscopy and physical property measurement were used to analyze the reason for the decrease in catalyst activity. The addition of t-butanol and bicarbonate were used to explore the different process between hydroxyl radicals and ozone. The experimental results showed that the catalytic ozonation could significantly increase the degradation and mineralization of p-hydroxybenzoic acid. The initial pH value was a crucial factor influencing ozone decomposition and the surface property of catalyst or organic pollutant. The degradation of p-hydroxybenzoic acid increased by 32 % in catalyzed ozonation compared to single ozonation after 5 min reaction with unadjusted pH (about 5.4). In batch experiments, the removal efficiency of p-hydroxybenzoic acid and total organic carbon decreased 36.1 and 6.8 % after six run times. Bicarbonate significantly inhibited the mineralization of p-HBA, but it had almost no influence on the catalytic degradation of p-hydroxybenzoic acid. A possible pathway for p-hydroxybenzoic acid degradation was tentatively proposed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Parabens are a series of substances commonly used as preservatives, mainly in pharmaceuticals and personal care products (PPCPs). Their endocrine-disrupting potentials and the possible link with breast cancer have been widely discussed recently (Giri et al. 2010). Parabens are produced by the esterification of p-hydroxybenzoic acid (p-HBA) with an alkyl (methyl, ethyl, propyl, butyl or heptyl) or benzyl group under certain conditions (Jonkers et al. 2010). p-HBA has always been used as a model compound. The treatment processes include adsorption (Mittal et al. 2009), biodegradation and oxidation (Gupta et al. 2012). Although they are biodegradable, they always appear at low concentration levels in effluents of wastewater treatment plants (Haman et al. 2015).
The ozonation technique has been used worldwide because of its strong oxidizing capacity, environmentally friendly to achieve the degradation of many contaminants during wastewater treatment (Gharbani et al. 2008; Lee et al. 2011). However, the application of ozonation is still limited due to its selective reactions with organic pollutants (Beltrán et al. 2003). In order to overcome this limitation, a lot of improved ozonation was developed (Guo et al. 2015; Lee et al. 2011). The heterogeneous catalytic ozonation process has been developed as an emerging technology for advanced wastewater treatment. As one kind of advanced oxidation processes (Wang and Xu 2012), the production of hydroxyl radical can oxidize the organic pollution nonselective (Gupta et al. 2012).
The catalyst plays a significant role in heterogeneous catalytic ozonation. Rare earth oxides (Faria et al. 2009; Dai et al. 2014), transition metal oxides (Ichikawa et al. 2014; Turkay et al. 2014), carbon materials (Gupta and Saleh 2013; Saleh and Gupta 2014; Wang 2015) and molecular sieve (Li et al. 2014) are frequently used as catalysts. Among them, carbon-based materials, especially multi-walled carbon nanotubes (MWCNTs), have received increasing attention (Saleh and Gupta 2012a; Gupta et al. 2013) owing to its excellent characteristics, for example, (1) the high mesoporous area is favorable to the fluidity and mass transfer of reactants on catalyst surface; (2) the functional groups on MWCNTs surface are beneficial to their dispersion in water; (3) the resistance to abrasion and acidic/basic environments makes them suitable for intense oxidation circumstances, MWCNTs have been widely used as catalyst or catalyst support in wastewater treatment (Saleh and Gupta 2011, 2012b; Zhang et al. 2013).
However, the effective separation and further reuse of MWCNTs based materials are still a problem. Magnetic separation using magnetic materials and external magnetic field can solve this problem. Therefore, combining MWCNTs with magnetic materials is a promising approach. A number of materials, for instance, Fe3O4, γ- Fe2O3, FeCo, CoFe2O4 and NiFe2O4, have magnetic properties (Hosseini et al. 2014). Among these materials, Fe3O4 is in possession of unique electric properties, it can be used as catalyst based on electron transfer of Fe2+ and Fe3+ ions in the octahedral sites, which can accelerate the transformation of ozone into ·OH radicals. Furthermore, the high catalytic activity of nanoparticles has attracted more attention due to their uniform pore size distribution and high surface area (Saleh and Gupta 2012c). MWCNTs and Fe3O4 have been successfully used for wastewater treatment, including adsorption (Gupta et al. 2011a, b), catalytic ozonation system (Fan et al. 2014), and Fenton-like system (Deng et al. 2012), but few studies using Fe3O4–MWCNTs as catalyst in heterogeneous catalytic ozonation system.
The objective of this study was to prepare Fe3O4/MWCNTs for the catalytic ozonation of p-HBA. The effects of catalyst dosage, pH values and ozone concentration on the degradation of p-HBA were investigated. The stability and reusability of the catalyst were evaluated in term of the catalytic activity during the degradation of p-hydroxybenzoic acid (p-HBA), the physicochemical and morphological properties of the catalyst were determined using X-ray diffraction (XRD), Fourier transform infrared absorption spectroscopy (FT-IR), scanning electron microscope (SEM), and high-resolution transmission electron microscopy (HRTEM). This work was conducted in 2014–2015, in the Laboratory of Environmental Technology, Tsinghua University, Beijing, China.
Materials and methods
Materials
p-HBA was obtained from TCI (Japan). MWCNTs were purchased from Chengdu Organic Chemistry Co., Ltd, Chinese Academy of Sciences. Their specifications are as follows: purity: >95 wt%, outer diameter, 8–15 nm; inner diameter, 3–5 nm; length, 10–0 um; average specific surface area, >140 m2/g; bulk density: 0.15 g/cm3; true density ~2.1 g/cm3. The reagents used for the experiments including FeSO4·7H2O, Fe2(SO4)3, NaOH, t-butanol, NaHCO3 and Na2S2O3 were of analytical grade obtained from Beijing Chemical Plant (China). Bicarbonate (HCO3 −) was provided in form of NaHCO3. All solutions used in the experiments were prepared with deionized water without further purification.
Synthesis of Fe3O4/MWCNTs
Nanocatalyst, Fe3O4/MWCNTs were synthesized through the co-precipitation method. The whole reaction process was occurred at a four-necked flask. Firstly, 100 ml NaOH (0.2 M) solution and a quantity of carbon nanotubes were added into it. Before use, MWCNTs were washed in boiled deionized water for 2 h and dried at 80 °C under vacuum for 12 h. Then, FeSO4·7H2O (0.28 g), Fe2(SO4)3 (0.41 g) and H2SO4 (0.2 mL) were dissolved in 100 mL of deionized water under ultrasonic, the mixture solution was added drop-wise into the flask. The weight ratio of Fe3O4 and MWCNTs was 1:1, the whole reaction process was stirred vigorously under an argon gas protection at 80 °C. After 1.5 h reaction, resulting products were deposited and washed with deionized water two times and finally dried in a vacuum freeze dryer overnight.
Characterization of catalysts
In order to determine the complex phase, XRD measurements were taken at room temperature on an XRD diffractometer (D8-Advance, Bruker) with Cu Kα radiation at 40 kV and 40 mA. The morphologies were observed by a Hitachi S-4800 SEM at 80 kV and HRTEM (TecnaiG2 F20 S-Twin). Magnetic measurements were taken at room temperature with a physical property measurement system (PPMS, 730T, Lakeshore, USA). Infrared spectra were recorded using a FTIR spectrometer (V70 Hyper1000) at room temperature in the range of 8000–350 cm−1. The leaching of iron was quantified by flame atomic absorption spectrophotometer (ZA3000, Hitachi).
Catalytic ozonation of p-HBA
Batch experiments were conducted with a 1.2-L cylindrical reactor. The concentrations of ozone in inlet and outlet were monitored by an ozone analyzer (BMT 963 Germany). In catalytic ozonation experiments, 1 L of p-HBA solution (20 mg/L) and catalyst were added into the reactor firstly, the solution pH was adjusted using 1 M H2SO4 and NaOH solutions. Ozone gas was generated by an ozonizer (3S-A3 Tonglin Technology, China) and continuously fed into the solution through a porous aeration device at the bottom of the reactor. At a time intervals, samples were collected and filtered through a PTFE filter (pore size 0.22 mm) for analysis. An aliquot of 0.1 M Na2S2O3 was subsequently added to the sample in order to remove any residual ozone. In order to study whether the free radical was involved in catalytic reaction, radical scavengers including 200 mg/L t-Butanol or bicarbonate were added into the reactor when needed. Fe3O4/MWCNTs had strong dispersion ability, so the whole process of the experiment was not stirred.
In the reuse experiments, the reusability tests were done for six times. The catalyst and wastewater were separated by a strong magnet. Considering the possibility of practical application, the catalyst was directly used for the next reaction without washing. The experiment conditions was similar except the reaction was stopped after 5 min reaction, the samples were collected at that time. After six times used, catalyst was separated and dried for further characterization. Adsorption experiments were carried out in the same reactor with oxygen instead of ozone bubbling into the reactor.
The concentration of p-HBA was measured with high-performance liquid chromatography (HPLC) (Agilent 1200 Series, Agilent, USA) equipped with a diode array detector (DAD) with a detection wavelength of 255 nm and using an XDB-C18 (4.6 × 150 mm) reversed-phase column at flow rate was 1.0 mL/min. The column temperature was 30 °C and the injection volume was 10 mL. Separation was carried out in isocratic mode using a mobile phase composed of water: methanol: acetic acid mixture (88:10:2 in volume). The total organic carbon (TOC) of the solution were analyzed by a Multi TOC/TN Analyzer (2100, Analytik Jena AG Corporation). All experiments were carried out at least twice.
Results and discussion
Characterization of catalyst
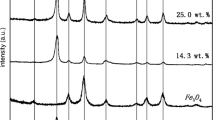
The XRD patterns for Fe3O4/MWCNTs composites are shown in Fig. 1. It can be seen that the XRD patterns of Fe3O4 with a cubic spinel structure were dominant with space group Fd-3m (227), corresponding to the standard card of Fe3O4 (JCPDS No. 19-0629) assigned to the (220), (311), (400), (511), and (440) planes. This was consistent with our previous studies and confirmed the existence of Fe2+ and Fe3+ ions by X-ray photoelectron spectroscopy (Xu and Wang 2012a, b). The diffraction peaks at 2θ = 26.3 can be indexed to the (002) reflection of MWCNTs (Saleh et al. 2011). Deduced by Scherrer’s equation, the average size of the new and used Fe3O4/MWCNTs were 7 and 9 nm by analyzing the most intense peak (311), respectively. The increased particle size may be due to the agglomeration or dissolution of the catalysts. Compared new with used Fe3O4/MWCNTs, the intensity of MWCNTs peak became weak, which may be due to the loss of MWCNTs.
Figure 2 shows the typical SEM and HRTEM graphs of new catalyst. From Fig. 2a, the distribution of Fe3O4 granules on the surface of MWCNTs was not uniform, globular Fe3O4 clusters were agglomerated together. This may be caused by the inadequate mixing or the fast drop of mixture solution under synthesis of Fe3O4/MWCNTs. The HRTEM shows the diameters of the Fe3O4 clusters were mainly spherical with 8–10 nm, the MWCNTs with diameter range of 3–7 nm. Since the nano-Fe3O4 particles have magnetic properties, the particles exhibit a certain mutual attraction. Similar observations were reported (Gupta and Nayak 2012). This agglomeration and non-uniform would decrease the interaction between Fe3O4 and MWCNTs, which further caused the loss of MWCNTs during separate them from water.
The magnetic properties of the Fe3O4/MWCNTs are shown in Fig. 3. The saturation magnetization values of used catalyst significantly increased from 9.18 to 20.44 emu/g compared with new Fe3O4/MWCNTs, which may be due to the enlarged particle size or the surface-related effects (Salado et al. 2008). The catalyst can be easily separated and recovered from solution by an external magnetic field. In addition, after six times reuse, the loss of MWCNTs increased the relative content of Fe3O4, which was also a reason for the increase in the saturation magnetization values after reuse.
FTIR spectra of new catalyst and used catalyst are shown in Fig. 4. It can be observed that plentiful chemical group existed on the surface of Fe3O4/MWCNTs. This main peak at 3434 cm−1 indicated the generation of –OH. Spectra showed a band at 2916 cm−1 resulting from an asymmetric and symmetric stretching of C-H. Bands at 1630 cm−1 and 1585 cm−1 are due to C=O stretching band and stretching vibrations of isolated C=C double bonds, respectively. Feeble peak at 1099 cm−1 is attributed by C–O and C–O–C stretching. Fe–O bending was shown at 576 cm−1 on the spectra (Gupta et al. 2011a, b; Saleh et al. 2011; Saleh and Gupta 2011; Moussavi et al. 2014). Comparing the FTIR spectra between new catalyst and used catalyst, an increase intensity of –OH band can be observed, which suggested the generation of hydroxyl radical during the ozonation reaction. Moreover, the C=O stretching band was only occurred at used catalyst, which could be explained by the adsorption of p-HBA or residual intermediates product.
Based on the existence of Fe–O, C=O and –OH bans on the surface of Fe3O4/MWCNTs, the hydrogen bond should be formed by the aligned of oxygen atom of Fe3O4 and hydrogen atom of hydroxyl group of MWCNTs (Gupta et al. 2011a, b), the attractive van der Waals forces is the main power for their union. Moreover, the acid sites of Fe3O4 and the basic sites of MWCNTs were the main binding sites.
Effect of catalysts on p-HBA ozonation
Figure 5 displays the degradation of p-HBA with different catalysts. It can be seen that P-HBA was completely degraded within 20 min by ozonation alone. The addition of Fe3O4, MWCNTs or Fe3O4/MWCNTs could increase the removal efficiency of p-HBA in different degrees. It was noteworthy that Fe3O4/MWCNTs exhibited the highest catalytic activity, the removal efficiency of p-HBA reached 100 % within 10 min. Moreover, the catalytic activity of MWCNTs was slightly higher than that of Fe3O4. Meanwhile, the results of adsorption tests indicated that only 18.83 % (MWCNTs), 1.44 % (Fe3O4), and 8.91 % (Fe3O4/MWCNTs) of p-HBA was adsorbed after 30 min. Hence, we concluded that the removal efficiency increased was mainly due to the catalytic ozonation rather than adsorption by the catalysts. In addition, the results also indicated that the adsorption of p-HBA and ozone on the surface of the catalyst was important step for catalytic ozonation. Previous research (Dai et al. 2014) also revealed that the utilization efficiency of ozone can be improved when adding the catalyst, this improvement also accelerated the degradation of p-HBA.
Effect of pH on p-HBA ozonation
The influence of initial pH on p-HBA, removal, including adsorption, ozonation and catalyzed ozonation was investigated, and the results are shown in Fig. 6. It can be seen that p-HBA was slightly removed by adsorption at all tested pH conditions, and the absorption capacity decreased with increase in pH. The basic sites (Xing et al. 2014) and charged surface (Yang et al. 2014) have influence on chemisorption, and the charged surface can be determined by the pH of solution and pHpzc of catalyst (Parfiti 1976; Usharani et al. 2012). According to our research, the pHpzc of prepared Fe3O4/MWCNTs was in acid range (3.43). It was deprotonated and negatively charged in aqueous solution when pH > 3.43. In addition, pKa of organic pollutants should be taken into account when discussing the process of catalytic ozonation (Kasprzyk-Hordern 2003). p-HBA positively charged in aqueous solution at its pka (4.57). Consequently, the strong electrostatic adsorption happened at pH = 3.5. As pH increased, the strength of electrostatic repulsions between deprotonated p-HBA and negatively charged Fe3O4/MWCNTs surface increased.
In ozonation system, p-HBA degradation was due to both direct oxidation by ozone molecules and indirect oxidation by hydroxyl radical generated from ozone self-decomposition (Valdés and Zaror 2006). The degradation rate of p-HBA was stable (from 52.4 to 59.3 %) at acid conditions, but it increased rapidly (91.2 %) when pH was 9.5, because OH− is a major initiator for aqueous ozone decomposition to form hydroxyl radicals. In catalyzed ozonation system, degradation of p-HBA was almost independent on pH. When the initial pH was not adjusted (about 5.4), p-HBA removal reached 91.3 % at 5 min, and p-HBA removal increased by 32 % in catalyzed ozonation compared with ozonation alone. This results indicated that the catalyst did not act as an absorbent only, the positive effect should be contributed by the electron transfer of Fe2+ and Fe3+ (Tanaka and Abe 1997) ions, the reaction between iron ions and ozone can produce hydroxyl radicals from water (Eqs. 1–5). Moreover, the active sites (acid sites and basic sites) existing on Fe3O4/MWCNTs surface could accelerate the decomposition of ozone and enhance the generation of hydroxyl radicals (Kasprzyk-Hordern 2003; Yang et al. 2010).
Effect of ozone and catalysts dosage on p-HBA ozonation
As a powerful oxidizing agent, ozone dosage is a very important factor in catalytic ozonation process. Figure 7a showed that increase in ozone dosage remarkably accelerated the removal of p-HBA and TOC. After 5 min reaction, p-HBA removal increased from 84.2 to 98.1 % when ozone dosage increased from 6 to 12 mg/min. Moreover, the removal of TOC increased from 25.5 to 35.9 % when ozone dosage increased from 6 to 9 mg/min. However, further increase in ozone dosage resulted in slight increase in TOC removal (to 39.1 %). These results may be explained by the interactions between hydroxyl radicals displayed in Eqs. (6, 7) (Kasprzyk-Hordern 2003). Furthermore, the residence time of reactive oxygen species was reduced by increasing the speed of ozone gas. This effect of increasing ozone dosage on the reaction was consistent with the previous studies (Yang et al. 2014), which could be due to the increase in the concentration of dissolved ozone and more hydroxyl radicals produced.
Figure 7b shows that the increase in catalyst dosage was advantageous for p-HBA removal. At the oxidation time of 5 min, p-HBA removal in the presence of 0.2 g/L catalyst was 83.6 %, whereas in the presence of 0.8 g/L catalyst the removal was 91.2 %. The degree of mineralization also increased from 34.1 to 48.8 % in 30 min. These results were consistent with the previous studies (Yang et al. 2014; Huang et al. 2015). Higher dosage of Fe3O4/MWCNTs resulted in more active sites for oxidation reaction, and higher active sites induced more hydroxyl radicals, which accounted for the obvious enhancement of p-HBA and TOC removal.
The stability and reusability of Fe3O4/MWCNTs
The reusability of the prepared catalysts in catalytic ozonation was examined and shown in Fig. 8. Compared to the fresh catalysts, the catalytic activity decreased gradually for the recovered and reused catalysts, and the removal efficiency of TOC and p-HBA decreased 6.8 and 36.1 % after the sixth run. However, leaching of irons from catalysts to the liquid can be ignored because it was lower than 20 μg/L even after the sixth time run. Consequently, this loss of activity could be ascribed to the decay of active catalytic sites caused by small amount of MWCNTs loss, corresponding to the observation results from XRD (Fig. 1) and SEM (Fig. 2). Similar results were also observed by other researchers in catalytic ozonation or Fenton-like reaction, the catalyst deactivation was attributed to several factors, including the poisoning of the active catalytic sites by absorbed organic species, and the decrease in the catalyst specific area (Chen et al. 2012). In addition, the decrease in the activity was also influenced by the adsorption of intermediates. Because the catalyst was not washed before further reuse, the p-HBA and its degradation intermediates remained on the surface of catalyst may occupy the reaction sites and hinder the reaction.
Possible pathway of p-HBA degradation
The influence of radical scavengers on pollutants removal is shown in Fig. 9. It can be seen that t-Butanol and HCO3 − are strong radical scavengers, their reaction rate constant with hydroxyl radicals was 6 × 108 M−1 S−1 and 1.5 × 107 M−1 S−1, but they do not react with ozone (Buxton et al. 1988). Thus, t-butanol and HCO3 − were considered as the indicator and quencher for the radical type reaction. As shown in Fig. 9, the addition of HCO3 − significantly inhibited the degradation of TOC, but it had nearly no effect on the catalytic ozonation of p-HBA. This result suggested that p-HBA can be degraded by ozone and hydroxyl radicals, but the intermediates were difficult to degrade by single ozone. Catalytic ozonation system can produce more hydroxyl radicals, which played an important role in the degradation of organic intermediates.
According to the above results, different oxidation mechanisms existed for p-HBA removal by ozone and hydroxyl radicals, which was proposed and shown in Fig. 10. The electrophilic attack by hydroxyl radical on p-HBA initially proceeded through addition of the hydroxyl to the aromatic ring and forming 3, 4-dihydroxybenzoic acid (Duesterberg and Waite 2007). Then, 3, 4-dihydroxybenzoic acid was broken down, resulting in the formation of a wide range of cleavage compounds. Moreover, p-HBA occurred in the decarboxylation reaction by the oxidation of ozone and turned into phenol (Triki et al. 2011). Phenol was degraded through further hydroxylation of the aromatic ring to hydroquinone and catechol, after that the aromatic ring was broken down to form small molecule acids. Finally, the cleavage compounds and small molecule acids were mineralized.
Conclusion
Fe3O4/MWCNTs were synthesized, characterized and used as a heterogeneous ozonation catalyst for degradation of p-HBA. Fe3O4/MWCNTs catalyst could significantly enhance the degradation and mineralization of p-HBA. Solution pH, ozone dosage and catalyst dosage had important influence on the catalytic ozonation. p-HBA was completely degraded within 10 min, and TOC removal efficiency was more than 35 % within 30 min. The use of radical scavengers proved the existence of hydroxyl radical and suggested the different mechanism for p-HBA oxidation by radicals and ozone molecules. In batch experiments, the removal efficiency of p-HBA and total organic carbon (TOC) decreased 36.1 and 6.8 % after six run times, indicating that the catalyst was relatively stable and can be reused.
References
Beltrán FJ, Rivas FJ, Montero-de-Espinosa R (2003) Ozone-enhanced oxidation of oxalic acid in water with cobalt catalysts. 2. Heterogeneous catalytic ozonation. Ind Eng Chem Res 42(14):3218–3224
Buxton GV, Greenstock CL, Ross WPH, Ross AB (1988) Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/·O–) in aqueous solution. J Phys Chem Ref Data 17(2):513–886
Chen Y, Ai Z, Zhang L (2012) Enhanced decomposition of dimethyl phthalate via molecular oxygen activated by Fe@Fe2O3/AC under microwave irradiation. J Hazard Mater 235–236:92–100
Dai Q, Wang J, Yu J, Chen J, Chen J (2014) Catalytic ozonation for the degradation of acetylsalicylic acid in aqueous solution by magnetic CeO2 nanometer catalyst particles. Appl Catal B Environ 144:686–693
Deng J, Wen X, Wang Q (2012) Solvothermal in situ synthesis of Fe3O4-multi-walled carbon nanotubes with enhanced heterogeneous Fenton-like activity. Mater Res Bull 47(11):3369–3376
Duesterberg CK, Waite TD (2007) Kinetic modeling of the oxidation of p-hydroxybenzoic acid by Fenton’s reagent: implications of the role of quinones in the redox cycling of iron. Environ Sci Technol 41(11):4103–4110
Fan X, Restivo J, Órfão JJM, Pereira MFR, Lapkin AA (2014) The role of multiwalled carbon nanotubes (MWCNTs) in the catalytic ozonation of atrazine. Chem Eng J 241:66–76
Faria PCC, Monteiro DCM, Órfão JJM, Pereira MFR (2009) Cerium, manganese and cobalt oxides as catalysts for the ozonation of selected organic compounds. Chemosphere 74(6):818–824
Gharbani P, Tabatabaii SM, Mehrizad A (2008) Removal of Congo red from textile wastewater by ozonation. Int J Environ Sci Technol 4(5):495–500
Giri RR, Ozaki H, Ota S, Takanami R, Taniguchi S (2010) Degradation of common pharmaceuticals and personal care products in mixed solutions by advanced oxidation techniques. Int J Environ Sci Technol 7(2):251–260
Guo W, Yin R, Zhou X, Du J, Cao H, Yang S, Ren N (2015) Sulfamethoxazole degradation by ultrasound/ozone oxidation process in water: kinetics, mechanisms, and pathways. Ultrason Sonochem 22:182–187
Gupta VK, Nayak A (2012) Cadmium removal and recovery from aqueous solutions by novel adsorbents prepared from orange peel and Fe2O3 nanoparticles. Chem Eng J 180:81–90
Gupta VK, Saleh TA (2013) Sorption of pollutants by porous carbon, carbon nanotubes and fullerene- an overview. Environ Sci Pollut Res 20(5):2828–2843
Gupta VK, Agarwal S, Saleh TA (2011a) Chromium removal by combining the magnetic properties of iron oxide with adsorption properties of carbon nanotubes. Water Res 45(6):2207–2212
Gupta VK, Agarwal S, Saleh TA (2011b) Synthesis and characterization of alumina-coated carbon nanotubes and their application for lead removal. J Hazard Mater 185(1):17–23
Gupta VK, Ali I, Saleh TA, Nayak A, Agarwal S (2012a) Chemical treatment technologies for waste-water recycling—an overview. RSC Adv 2:6380–6388
Gupta VK, Jain R, Mittal A, Saleh TA, Nayak A, Agarwal S, Sikarwar S (2012b) Photo-catalytic degradation of toxic dye amaranth on TiO2/UV in aqueous suspensions. Mater Sci Eng, C 32(1):12–17
Gupta VK, Kumar R, Nayak A, Saleh TA, Barakat MA (2013) Adsorptive removal of dyes from aqueous solution onto carbon nanotubes: a review. Adv Colloid Interface 193–194:24–34
Haman C, Dauchy X, Rosin C, Munoz J (2015) Occurrence, fate and behavior of parabens in aquatic environments: a review. Water Res 68:1–11
Hosseini M, Memari Z, Ganjali MR, Khoobi M, Faridbod F, Shafiee A, Norouzi P, Shamsipur M, Hajinezhad A (2014) A novel mercury-sensitive fluorescent nano-chemosensor using new functionalized magnetic core-shell Fe3O4 @SiO2 nanoparticles. Int J Environ Res 8(4):861–870
Huang Y, Cui C, Zhang D, Li L, Pan D (2015) Heterogeneous catalytic ozonation of dibutyl phthalate in aqueous solution in the presence of iron-loaded activated carbon. Chemosphere 119:295–301
Ichikawa S, Mahardiani L, Kamiya Y (2014) Catalytic oxidation of ammonium ion in water with ozone over metal oxide catalysts. Catal Today 232:192–197
Jonkers N, Sousa A, Galante-Oliveira S, Barroso CM, Kohler HE, Giger W (2010) Occurrence and sources of selected phenolic endocrine disruptors in Ria de Aveiro, Portugal. Environ Sci Pollut Res 17(4):834–843
Kasprzyk-Hordern B (2003) Catalytic ozonation and methods of enhancing molecular ozone reactions in water treatment. Appl Catal B Environ 46(4):639–669
Lee E, Lee H, Kim YK, Sohn K, Lee K (2011) Hydrogen peroxide interference in chemical oxygen demand during ozone based advanced oxidation of anaerobically digested livestock wastewater. Int J Environ Sci Technol 8(2):288–381
Li J, Na H, Zeng X, Zhu T, Liu Z (2014) In situ DRIFTS investigation for the oxidation of toluene by ozone over Mn/HZSM-5, Ag/HZSM-5 and Mn–Ag/HZSM-5 catalysts. Appl Surf Sci 311:690–696
Mittal A, Kaur D, Malviya A, Mittal J, Gupta VK (2009) Adsorption studies on the removal of coloring agent phenol red from wastewater using waste materials as adsorbents. J Colloid Interface Sci 337(2):345–354
Moussavi G, Aghapour AA, Yaghmaeian K (2014) The degradation and mineralization of catechol using ozonation catalyzed with MgO/GAC composite in a fluidized bed reactor. Chem Eng J 249:302–310
Parfiti GD (1976) Surface chemistry of oxides. Pure Appl Chem 48:415–418
Salado J, Insausti M, Gil De Muro I, Lezama L, Rojo T (2008) Synthesis and magnetic properties of monodisperse Fe3O4 nanoparticles with controlled sizes. J Non-Cryst Solids 354(47–51):5207–5209
Saleh TA, Gupta VK (2011) Functionalization of tungsten oxide into MWCNT and its application for sunlight-induced degradation of rhodamine B. J Colloid Interface Sci 362(2):337–344
Saleh TA, Gupta VK (2012a) Photo-catalyzed degradation of hazardous dye methyl orange by use of a composite catalyst consisting of multi-walled carbon nanotubes and titanium dioxide. J Colloid Interface Sci 371(1):101–106
Saleh TA, Gupta VK (2012b) Column with CNT/magnesium oxide composite for lead (II) removal from water. Environ Sci Pollut Res 19(4):1224–1228
Saleh TA, Gupta VK (2012c) Synthesis and characterization of alumina nano-particles polyamide membrane with enhanced flux rejection performance. Sep Purif Technol 89:245–251
Saleh TA, Gupta VK (2014) Processing methods, characteristics and adsorption behavior of tire derived carbons: a review. Adv Colloid Interface 211:93–101
Saleh TA, Agarwal S, Gupta VK (2011) Synthesis of MWCNT/MnO2 and their application for simultaneous oxidation of arsenite and sorption of arsenate. Appl Catal B Environ 106(1–2):46–53
Tanaka KA, Abe K (1997) Fe3+ and UV enhanced ozonation of chlorophenolic compounds in aqueous medium. Chemosphere 12(15):2837–2847
Triki M, Ksibi Z, Ghorbel A, Medina F (2011) Preparation and characterization of CeO2–Al2O3 aerogels supported ruthenium for catalytic wet air oxidation of p-hydroxybenzoic acid. J Sol–Gel Sci Technol 59(1):1–6
Turkay O, Inan H, Dimoglo A (2014) Experimental and theoretical investigations of CuO-catalyzed ozonation of humic acid. Sep Purif Technol 134:110–116
Usharani K, Muthukumar M, Kadirvelu K (2012) Effect of pH on the degradation of aqueous organophosphate (methylparathion) in wastewater by ozonation. Int J Environ Res 6(2):557–564
Valdés H, Zaror CA (2006) Heterogeneous and homogeneous catalytic ozonation of benzothiazole promoted by activated carbon: kinetic approach. Chemosphere 65(7):1131–1136
Wang Z (2015) Efficient adsorption of dibutyl phthalate from aqueous solution by activated carbon developed from phoenix leaves. Int J Environ Sci Technol 12(6):1923–1932
Wang JL, Xu LJ (2012) Advanced oxidation processes for wastewater treatment: formation of hydroxyl radical and application. Crit Rev Environ Sci Tecnol 42(3):251–325
Xing L, Xie Y, Cao H, Minakata D, Zhang Y, Crittenden JC (2014) Activated carbon-enhanced ozonation of oxalate attributed to HO oxidation in bulk solution and surface oxidation: effects of the type and number of basic sites. Chem Eng J 245:71–79
Xu L, Wang J (2012a) Fenton-like degradation of 2, 4-dichlorophenol using Fe3O4 magnetic nanoparticles. Appl Catal B 123–124(18):117–126
Xu L, Wang J (2012b) Magnetic nanoscaled Fe3O4/CeO2 composite as an efficient Fenton-like heterogeneous catalyst for degradation of 4-chlorophenol. Environ Sci Technol 46:10145–10153
Yang L, Hu C, Nie Y, Qu J (2010) Surface acidity and reactivity of β-FeOOH/Al2O3 for pharmaceuticals degradation with ozone: in situ ATR-FTIR studies. Appl Catal B Environ 97(3–4):340–346
Yang Y, Cao H, Peng P, Bo H (2014) Degradation and transformation of atrazine under catalyzed ozonation process with TiO2 as catalyst. J Hazard Mater 279:444–451
Zhang S, Wang D, Quan X, Zhou L, Zhang X (2013) Multi-walled carbon nanotubes immobilized on zero-valent iron plates (Fe0-CNTs) for catalytic ozonation of methylene blue as model compound in a bubbling reactor. Sep Purif Technol 116:351–359
Acknowledgments
The research was supported by the Program for Changjiang Scholars and Innovative Research Team in University (IRT-13026). The authors would also like to thank the financial support provided by the National Natural Science Foundation of China (Grant No. 51338005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bai, Z.Y., Yang, Q. & Wang, J.L. Fe3O4/multi-walled carbon nanotubes as an efficient catalyst for catalytic ozonation of p-hydroxybenzoic acid. Int. J. Environ. Sci. Technol. 13, 483–492 (2016). https://doi.org/10.1007/s13762-015-0881-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-015-0881-3