Abstract
The disposal of wastewater sludge generated during the treatment of the various municipal and industrial wastewaters is a major environmental problem. In this study the thermophilic bacterium Bacillus licheniformis, which enhances the efficiency of sludge reduction, was isolated from waste activated sludge acclimated to 55 °C. The resulting suspended solids’ degradation was 12 % and chemical oxygen demand solubilization was 18 %. To further enhance the sludge reduction potential, extra polymeric substances, which play a major role in the formation of flocs, were removed. A chemical extractant, ethylenediaminetetraacetate that is also a cation binding agent, was used to remove the extra polymeric substances. After the removal of extra polymeric substances, the suspended solids’ degradation increased from 12 to 23 % and the chemical oxygen demand solubilization increased from 18 to 25 %. These observations confirm that Bacillus licheniformis enhanced sludge reduction in non-flocculated sludge (with the removal of extra polymeric substances) as compared to flocculated sludge (without the removal of extra polymeric substances).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Excess sludge produced by treatment plants is an environmental menace. Conventional sludge disposal methods such as landfill, incineration and dumping in the sea are increasingly prohibited by regulation and encounter strong public opposition (Chul-Hwi et al. 2010). Due to such regulatory restrictions, prior to final disposal, excess sludge is first stabilized and then treated to reduce pathogens, apart from elimination of offensive odors and to inhibit potential putrefaction (Victoria and Deokjin 2004). Thus sludge problem has been a vexatious ongoing concern all over the world. Therefore an advanced and environmentally friendly way of reducing sludge is required (Yang et al. 2010). Among several methods available for sludge reduction, the thermophilic digestion process is advantageous as it reduces pathogens and produces an effluent that is almost free of pathogenic agents (Daniel et al. 2008). The spectrum of microflora occurring during thermophilic aerobic digestion may include bacteria that produce thermostable proteases which can break cell walls (Kim et al. 2002). Of all proteases, alkaline proteases produced by Bacillus species are of great importance due to their high thermostability and pH stability (Krishna and Kodidhela 2005). Bacteria in the activated sludge often degraded the complex organic matter (polymeric substrates such as proteins, lipids and carbohydrates) into low molecular weight intermediates by the action of extracellular hydrolases (Nybroe et al. 1992). These low molecular weight compounds are in turn assimilated by the cells and used as sources of energy and carbon (Burgess and Pletschke 2008).
Bacterial flocculation in sludge is due to the extracellular polymeric substances (EPS). EPS are complex mixture of high molecular polymers (M w > 10,000) excreted by micro-organisms, lysis and hydrolysis products and organic matter adsorbed from the substrate (Morgan et al. 1990). EPS include a wide variety of materials including polysaccharides, proteins (Leroy et al. 2008), nucleic acid, uronic acid and humic substances (Orgaz et al. 2006). EPS are further differentiated into extractable EPS (the EPS fraction bound tightly to solid surfaces), and soluble EPS (also called slime polymers) (Rosenberger and Kraume 2003). There is a correlation between the amount of loosely bound EPS and the flocculation and sedimentation features of activated sludge (Li and Yang 2007). In the light of the above knowledge, in this study, slime EPS were removed, which are responsible for flocculation. EPS are typically known to aid in the formation of a gel-like network that helps bacteria to aggregate in biofilms, causes adherence of biofilms to surfaces, and protect bacteria against noxious environmental conditions (Wingender et al. 1999). Hence these flocs that were disrupted by the removal of EPS, released the trapped or bound cells from the sludge structure leading to the enhancement of the solubilizing efficiency of Bacillus licheniformis. EPS could thus be removed by both physical and chemical methods. Physical extraction methods included centrifugation, ultrasonication and heating, whereas chemical extraction methods included the use of alkali, ethylenediaminetetraacetate (EDTA) and cation exchange resin. In the present study EDTA was used to remove EPS from sludge. It was deduced from the literature that EDTA was more effective in extracting EPS (Xiangliang et al. 2010) and relatively low concentrations of EDTA would not cause any environmental problems (William and David 2000). EDTA forms complexes with calcium and magnesium ions during the process of EPS removal. These complexes are further degraded by bacteria (Henneken et al. 1995) and hence EDTA is not retained after removing the EPS from sludge. After removing EPS, the sludge was subjected to the action of Bacillus licheniformis to study the sludge reduction potential. The research was carried out from January 2011 to March 2011 in the Department of Civil Engineering, Anna University of Technology Tirunelveli, South India.
Materials and methods
Collection of sample
Waste activated sludge was collected from municipal sewage treatment plant at the Medical College, Trivandrum. The collected samples were aerated continuously at room temperature to avoid settling of the solids and to avoid the changes in the biological sludge. These samples served as the inoculum for further studies. The initial characteristic of the waste activated sludge are given in Table 1.
Acclimatization of sludge
To isolate thermophilic bacteria, a part of the sludge was acclimated to a high temperature (55 °C) in an incubator shaker. Activated sludge autoclaved for 30 min at 121 °C was used as a feed to supply the nutrient needs of the thermophilic bacteria in aerobic cultivation. Then, 30 ml of waste activated sludge was replaced daily with 30 ml of autoclaved sludge which served as a fresh substrate for the micro-organisms in the waste activated sludge.
Isolation and identification of protease secreting bacteria
One milliliter of acclimated sludge enriched with a mixture of aerobic thermophilic bacteria was mixed with 9 ml of sterile distilled water and diluted up to 10−7 to obtain isolated colonies. After thorough mixing, 0.1 ml (100 μl) of the diluted sample was spread plated onto the skimmed milk agar medium and incubated at 55 °C for 24 h. The composition of the skimmed milk agar medium is shown in Table 2.
Colonies that hydrolyzed the casein in the skim milk formed a clear halo zone. These colonies were subcultured to obtain pure culture. The colony which formed a more effective halo zone was identified using 16S rRNA sequencing (Greg 2010).
Removal of EPS
EDTA exhibits no environmental toxicity (Henneken et al. 1995) and hence can be used safely to remove EPS. Thirty ml of sludge was inoculated with 30 ml of 0.1, 0.5, 1, 1.5 and 2 % of EDTA. The mixtures were incubated at 4 °C for 3 h with constant agitation to insure proper mixing (Sponza 2004). After incubation, the mixtures were centrifuged, respectively, at 7,800 rpm for 15 min to obtain the soluble EPS in the supernatant (Liu and Fang 2002; Zhang et al. 1999, 2002). The supernatant was filtered through 0.45 μm cellulose acetate membranes (Guo-Ping et al. 2004).
Characterization of EPS
The EPS in the filtrate were chemically analyzed and quantified in the following manner. The carbohydrate content in the EPS was determined by the anthrone method (Tapia et al. 2009), using glucose as a standard and measuring absorbance at 660 nm. The protein content was measured using the Lowry method (Takahashi et al. 2009), with bovine serum albumin as the standard and a wavelength of 750 nm. The DNA content in the EPS was measured by the diphenylamine colorimetric method (Sun et al. 1999) using herring sperm DNA as the standard.
Dry weight of EPS
The filtrate obtained after centrifugation was precipitated with 2.2 volumes of absolute chilled ethanol (Subramanian et al. 2008) by incubating overnight. After incubation, the precipitate was centrifuged at 7,800 rpm for 20 min to obtain the soluble EPS in the pellet. Dry weight of the EPS was measured by drying at 105 °C to a constant weight (American Public Health Association 2005).
Sludge solubilization using Bacillus licheniformis
Sludge solubilization was performed for 3 days in a 250 ml reactor with a working volume of 150 ml. An optimum temperature of 55 °C was maintained and the sample was agitated at 100 rpm in an incubator shaker. The strain which formed an effective zone of hydrolysis in skimmed milk agar medium was used as an inoculate. The inoculum was added in a concentration of 77 × 106 bacterial cells/mg of suspended solids (SS). The following two combinations were studied (a) mixture of flocculated sludge (without removal of EPS) and bacteria and (b) mixture of non-flocculated sludge (with the removal of EPS) and bacteria.
Optimization of pH and temperature for the growth of Bacillus licheniformis
The effect of pH on the growth of Bacillus licheniformis was studied at different pH values such as from 4 to 9 adjusted using 0.1 N NaOH and 0.1 N HCl. The growth was monitored at a time interval of 24 h for 3 days in a constant temperature of about 55 °C and was observed by measuring the optical density of the medium at a wavelength of 550 nm (Kashid and Ghosh 2010).
Analytical methods
Parameters such as total chemical oxygen demand (TCOD), soluble chemical oxygen demand (SCOD), and suspended solids (SS) were measured according to the standard methods (APHA 2005) to determine the reduction efficiency of each reactor. SCOD was measured after the centrifugation of the sample at 10,000 rpm for 10 min. The degree of COD solubilization was calculated using Eq. 1 (Jeongsik et al. 2003).
Results and discussion
Isolation and identification of protease secreting bacteria
Skim milk agar is opaque, and the casein in the medium is hydrolyzed upon the action of protease secreted by the bacteria. Therefore, a clear area or, halo is produced around each colony. Thermostable proteases are advantageous as higher processing temperatures can be employed, resulting in faster reaction rates, increase in solubility of non-gaseous reactants and products and reduced incidences of microbial contamination by mesophilic organisms (Olajuyigbe and Ajene 2005). Thus six protease secreting bacteria (B1–B6) were isolated from the sludge acclimated to 55 °C. The thermophilic bacteria with greater relative halo size percentage were isolated (Table 3).
Relative halo size was determined using Eq. 2:
where D halo is the diameter of the halo formed around the colony (mm) and, D colony is the diameter of the colony (mm).
DNA homology analysis of the 16S rRNA regions of isolated colonies of bacterial culture indicated that bacteria with greater relative halo size displayed the greatest similarity with Bacillus licheniformis (97 %), NCIM 2042, ATCC 14580, genome accession number CP000002. The corresponding bacterial colony was used further for the solubilization studies.
Characterization of the extracted EPS
EDTA is more effective in extracting EPS (Xiangliang et al. 2010) as EDTA can increase the solubility of EPS and thus increase the yield (Comte et al. 2006). The amount of EPS extracted by EDTA may be overestimated since EDTA may bind with some components in EPS and form complexes that cannot be removed by dialysis (Liu and Fang 2002).Thus EDTA was used as a chemical extractant to remove EPS from the flocculated sludge. Proteins in EPS are bridged by divalent ions, including Ca2+ and Mg2+ and a small fraction of carbohydrate and nucleic acid is linked with these divalent ions. Under neutral conditions, the carboxyl of a protein would ionize and become negative. Through ion interaction, the divalent ions bridged the protein and cells. Thus, as EDTA removes these ions, the EPS are released from the bacteria (Guo-Ping et al. 2004). After centrifugation, the supernatant obtained contains the soluble EPS (Ramesh et al. 2006). Li and Yang (2007) identified a correlation between the amount of soluble EPS and flocculation. Hence soluble EPS are removed from sludge to make it non-flocculated. The soluble EPS in the supernatant are filtered through a cellulose filter and the filtrate obtained is biochemically characterized to observe the amount of EPS removed.
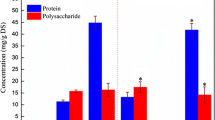
Protein, carbohydrate and nucleic acid content were determined in the filtrate (Fig 1). The carbohydrate concentration in EPS was higher than the protein content indicating that carbohydrates play a major role in sludge flocculation, due to their capacity to form bridged chelated structures between their negatively charged groups and the divalent cations available in sludge (Hirst et al. 2003). Proteins also contribute to flocs formation due to hydrophobic interactions and polyvalent cation bridging, both enhancing the stability of the biopolymer network (Jorand et al. 1998). Large quantities of nucleic acid materials can be expected to be released when harsh extraction induces cell destruction. Thus the EPS nucleic acid content acts as a good marker to estimate the EPS extraction method (Abzac et al. 2009). A stepwise increase in protein, carbohydrate, DNA and EPS release up to an EDTA dosage of 0.4 % was observed. At 0.4 % EDTA dosage their concentrations were 16, 22, 3.5 and 38 mg/l followed by a gradual decrease in the release of EPS with respect to the concentration of EDTA dosage and this effect was found to be stabilized at 0.5 % EDTA dosage. Based on these findings it can be concluded that there is significant release of EPS without cell lysis at an EDTA concentration of 0.4 %; further increases in EDTA concentration enhanced the cell lysis but not the EPS release.
Effect of pH on the growth of Bacillus licheniformis
The removal of EPS by EDTA decreases the pH to 3 which may affect the growth of Bacillus licheniformis. The microbial enzyme dynamics used by each species of micro-organisms for its characteristic metabolic processes have variable pH optima and of which can be tolerated by all extracellular enzymes and the micro-organisms involved (Burgess and Pletschke 2008). Due to this sporadic shift in the pH, the growth of Bacillus licheniformis was monitored at various pH levels at a time interval of 24 h, and was measured spectrophotometrically at a wavelength of 550 nm. From the results (Fig 2), it can be perceived that the growth of the bacterium was greatest at an optimum pH of 6. With the addition of cation binding agents, there was a change in pH that resulted in an unfavorable environment for the growth of Bacillus licheniformis. Hence the pH was maintained at 6 for further experiments.
Most biological reactions depend on the temperature of the medium. When there is a 10 °C difference in temperature, the reaction rate is halved or doubled, or the reaction is inhibited completely. Hence it is necessary to optimize the temperature to extract maximum biological activity from the bacteria. According to Chenel et al. (2008), 55 °C is optimal temperature for the growth of protease secreting Bacillus sp. Therefore further experiments were carried out at 55 °C.
Sludge reduction using Bacillus licheniformis
Flocculated sludge was disrupted using the chemical extractant EDTA. Reduction of the sludge with and without EPS removal by Bacillus licheniformis was observed by measuring SS reduction and COD solubilization.
The results (Fig 3) indicated that the SS reduction was greatest (about 23 %) in non-flocculated sludge (with EPS removal) and about 12 % in flocculated sludge (without EPS removal). The increase in SS reduction in the initial stage was due to protein hydrolysis (Bomio et al. 1989). The rapid decline in SS after 24 h may be either due to the solubilized sludge on the one hand, being utilized by thermophilic micro-organisms for the growth while, on the other hand, the high temperature contributed mainly to the death of micro-organism and released the intracellular contents to the liquid phase (Xuesong et al. 2009).
Further data (Fig. 4) demonstrated maximum solubilization (about 25 %) in the EPS removed sludge compared to the flocculated sludge (about 18 %). This may be due to the availability of greater surface area for the action of the bacteria and the subsequent release of entrapped organic matter from the flocs. As a result of all the above changes, the EDTA liberated the trapped or bound enzymes from the sludge structure. Other studies showed that the increased availability of enzyme stimulated a more efficient release of organic matter from the sludge (Burgess and Pletschke 2008) thereby enhancing the solubilization. Solubilization was maximum within 24 h and after that there was a gradual decrease. This may be because the organic matters present in the liquid phase were utilized as carbon energy substrates by Bacillus licheniformis and the soluble matter eventually gets exhausted.
Conclusion
In this study, biological solubilization of non-flocculated sludge and flocculated sludge was performed. The bioflocs that were formed in the sludge were most likely due to the extracellular polymeric substances secreted by the bacterial consortium present in the sludge. The formation of flocs leads to reduced bacterial enzymatic activity. However, after the removal of EPS by EDTA, the sludge became non-flocculated and this change was accompanied by enhanced bacterial activity leading to an increase in COD solubilization and SS reduction. Usually waste activated sludge is normally dominated by aerobic, gram negative bacteria. Such bacteria can effectively degrade the recalcitrant compound EDTA after the removal of EPS. EDTA is environmentally non-toxic, causing no harm to aquatic organisms. The SS reduction in flocculated sludge was about 12 % and about 23 % in non-flocculated sludge. The COD solubilization was about 18 % in flocculated sludge and 25 % in non-flocculated sludge. From the present investigations, the removal of EPS in sludge resulted in an increased release of entrapped organic matter from the sludge.
References
Abzac PD, Francois B, Ericl VH, Piet NLL, Gilles G (2009) Extraction of extracellular polymeric substances from anaerobic granular sludges: comparison of chemical and physical extraction protocols. Appl Microbiol Biotechnol 85:1589–1599. doi:10.1007/s00253-009-2288-x
American Public Health Association (APHA) (2005) Standard methods for the examination of water and wastewater, 21st edn. APHA, Washington, DC
Bomio M, Sonnleitner B, Fiechter A (1989) Growth and biocatalytic activities of aerobic thermophilic populations in sewage sludge. Appl Microbiol Biotechnol 32(3):356–362. doi:10.1007/BF00184989
Burgess JE, Pletschke BI (2008) Hydrolytic enzymes in sewage sludge treatment: a mini-review. Water SA (Online) 34(3):343–349. http://eprints.ru.ac.za/1371/. http://eprints.ru.ac.za/1371/1/Pletschke_hydrolic_enzymes.pdf
Chenel JP, Tyagi RD, Surampalli RY (2008) Production of thermostable protease enzyme in wastewater sludge using thermophilic bacterial strains isolated from sludge. Water Sci Technol 57(5):639–645. doi:10.2166/wst.2008.004
Chul-Hwi P, Yoon-Sun B, Gee-Bong H (2010) Implementation of an excess sludge reduction step in an activated sludge process. J Environ Sci Health A Tox Hazard Subst Environ Eng 45(6):709–718. doi:10.1080/10934521003648925
Comte S, Guibaud G, Baudu M (2006) Relations between extraction protocols for activated sludge extracellular polymeric substances (EPS) and EPS complexation properties part 1. Comparison of the efficiency of eight EPS extraction methods. Enzyme Microb Technol 38(1–2):237–245. doi:10.1016/j.enzmictec.2005.06.016
Daniel HZ, Carlan CJ, Richard ES (2008) Metal stimulation and municipal digester thermophilic/mesophilic activity. J Env Eng 134(1):42–47. doi:10.1061/(ASCE)0733-9372(2008)134:1(42)
Greg J (2010) Universal bacterial identification by PCR and DNA sequencing of 16S rRNA gene. PCR Clin Microbiol 3:209–214. doi:10.1007/978-90-481-9039-3_28
Guo-Ping S, Han-Qing Y, Zhou Y (2004) Extraction of extracellular polymeric substances from the photosynthetic bacterium Rhodopseudomonas acidophila. Appl Microbiol Biotechnol 67:125–130. doi:10.1007/s00253-004-1704-5
Henneken L, Nortemann B, Hempel D (1995) Influence of physiological conditions on EDTA degradation. Appl Microbiol Biotechnol 44(1–2):190–197. doi:10.1007/BF00164501
Hirst CN, Cyr H, Jordan IA (2003) Distribution of exopolymeric substances in the littoral sediments of an oligotrophic lake. Microb Ecol 46(1):22–32. doi:10.1007/s00248-002-1064-6
Jeongsik K, Chulhwanpark P, Tak-Hyun K, Myunggu L, Sangyong K, Seung-Wook K, Jinwon L (2003) Effects of various pretreatments for enhanced anaerobic digestion with waste activated sludge. J Biosci Bioeng 95(3):271–275. doi:10.1263/jbb.95.271
Jorand F, Boue-Bigne F, Block JC, Urbain V (1998) Hydrophobic/hydrophilic properties of activated sludge exopolymeric substances. Water Sci Technol 37(4–5):307–315. doi:10.1016/S0273-1223(98)00123-1
Kim YH, Bae JH, Oh BK, Lee WH, Choi JW (2002) Enhancement of proteolytic enzyme activity from Bacillus stearothermophilus for a thermophilic aerobic digestion process. Bioresour Technol 83(2):157–164. doi:10.1016/S0960-8524(01)00177-8
Krishna SBN, Kodidhela LD (2005) Optimization of thermostable alkaline protease production from species of Bacillus using rice bran. Afr J Biotechnol 4(7):724–726
Leroy C, Delbarre C, Gillebaert F, Compere C, Combes D (2008) Effect of commercial enzymes on the adhesion of a marine biofilm forming bacterium. Biofouling 24(1):11–22. doi:10.1080/08927010701784912
Li XI, Yang SF (2007) Influence of loosely bound extracellular polymeric substances (EPS) on flocculation, sedimentation and dewaterability of activated sludge. Water Res 41(5):1022–1030. doi:10.1016/j.watres.2006.06.037
Liu H, Fang HHP (2002) Extraction of extracellular polymeric substances (EPS) of Sludges. J Biotechnol 95(3):249–256. doi:10.1016/S0168-1656(02)00025-1
Morgan JW, Forster CF, Evison L (1990) A comparative study of the nature of biopolymers extracted from anaerobic and activated sludges. Water Res 24(6):743–750. doi:10.1016/0043-1354(90)90030-A
Nybroe O, Jorgensen PE, Henze M (1992) Enzyme activities in waste water and activated sludge. Water Res 26(5):579–584. doi:10.1016/0043-1354(92)90230-2
Olajuyigbe FM, Ajene OJ (2005) Production dynamics of extracellular protease from bacillus species. Afr J Biotechnol 4(8):776–779
Orgaz B, Kives J, Pedregosa AM, Monistrol IF, Laborda F, Sanjose C (2006) Bacterial biofilms removal using fungal enzymes. Enzyme Microb Technol 40:51–56. doi:10.1016/j.enzmictec.2005.10.037
Ramesh A, Duu-Jong L, Hong SG (2006) Soluble microbial products (SMP) and soluble extracellular polymeric substances (EPS) from wastewater sludge. J Env Biotechnol 73:219–225. doi:10.1007/s00253-006-0446-y
Rosenberger S, Kraume M (2003) Filterability of activated sludge in membrane bioreactor. Desalination 151(2):195–200. doi:10.1016/S0011-9164(02)00998-0
Kashid SG, Ghosh JS (2010) Production, isolation and characterization of exotoxin produced by Bacillus cereus NCIM-2156 and Bacillus licheniformis NCIM-5343. Br J Pharmacol Toxicol 1(1):50–55
Sponza DT (2004) Properties of four biological flocs as related to settling. J Environ Eng 130(11):1289–1300. doi:10.1061/(ASCE)0733-9372(2004)130:11(1289)
Subramanian SB, Yan S, Tyagi RD, Surampalli RY (2008) Isolation and molecular identification of extracellular polymeric substances (EPS) producing bacterial strains for sludge dewatering. J Environ Sci Health A Tox Hazard Subst Environ Eng 43(13):1495–1503. doi:10.1080/10934520802293602
Sun Y, Clinkenbeard KD, Clarke C, Cudd L, Hagh-Lander SK, Dubo SM (1999) Pasteurella haemolytica leukotoxin induced apoptosis of bovine lymphocytes involves DNA fragmentation. Vet Microbiol 65(2):153–166. doi:10.1016/S0378-1135(98)00286-7
Takahashi E, Ledauphin J, Goux D, Orvain F (2009) Optimising extraction of extracellular polymeric substances (EPS) from benthic diatoms: Comparison of efficiency of six EPS extraction methods. Mar Freshw Res 60(12):1201–1210
Tapia JM, Munoz JA, Gonzalez F, Blazquez LM, Malki M (2009) Extraction of extracellular polymeric substances from the acidophilic bacterium Acidiphilium 3.2Sup(5). Water Sci Technol 59(10):1959–1967. doi:10.2166/wst.2009.192
Victoria EBL, Deokjin J (2004) Isolation of protease producing aerobic thermophilic bacteria for digestion of excess sludge. Korean J Env Eng 28(4):604–610
William WB, David LS (2000) Effects of EDTA on pollutant metal removal by municipal wastewater treatment plants. In: International symposium on specialty chemicals in the environment, San Francisco, vol 40(1), pp 150–152
Wingender J, Neu TR, Flemming HC (1999) What are bacterial extracellular polymeric substances? In: Wingender J, Neu TR, Flemming HC (eds) Microbial extracellular substances: characterization, structures and function, chapter 3. Springer, Berlin, pp 1–19
Xiangliang P, Jing L, Daoyong Z, Xi C, Lanhai L, Weljuan S, Jainying Y (2010) A comparison of five extraction methods for extra polymeric substances (EPS) from biofilm by using three-dimensional excitation-emission matrix (3DEEM) fluorescence spectroscopy. Water SA (Online) 36(1):111–116
Xuesong L, Hongzhi M, Qunhui W, Shoichiro M, Toshinari M, Ogawa HI (2009) Isolation, identification of sludge-lysing strain and its utilization in thermophilic aerobic digestion for waste activated sludge. Bioresour Technol 100(9):2475–2481. doi:10.1016/j.biortech.2008.12.019
Yang Q, Luo K, Li X, Wang D, Zheng W, Zeng G, Liu J (2010) Enhanced efficiency of biological excess sludge hydrolysis under anaerobic digestion by additional enzymes. Bioresour Technol 101:2924–2930. doi:10.1016/j.biortech.2009.11.012
Zhang J, Wang R, Ziang P, Liu Z (2002) Production of an exopolysaccharides bioflocculant by Sorangium cellulosum. Lett Appl Microbiol 34(3):178–181. doi:10.1046/j.1472-765x.2002.01068.x
Zhang X, Bishop PL, Kinkle BK (1999) Comparison of extraction methods for quantifying extracellular polymers in biofilms. Water Sci Technol 39(7):211–218. doi:10.1016/S0273-1223(99)00170-5
Acknowledgments
The authors would like to thank the Department of Biotechnology, India for financial assistant for this project (BT/PR13124/GBD/27/192/2009) under their Rapid Grant for Young Investigator (RGYI) scheme.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Merrylin, J., Kaliappan, S., Adish Kumar, S. et al. Effect of extracellular polymeric substances on sludge reduction potential of Bacillus licheniformis . Int. J. Environ. Sci. Technol. 10, 85–92 (2013). https://doi.org/10.1007/s13762-012-0141-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-012-0141-8