Abstract
Neuromyelitis optica spectrum disorder (NMOSD) has unknown risk factors. The aim of this study was to identify the environmental risk factors for NMOSD. A case–control study was conducted in Tehran from 2015 to 2016 among 100 patients with NMOSD. Sex-matched healthy controls (n = 400) were selected through random digit dialing (RDD). Logistic regression was used to estimate unadjusted and adjusted ORs (odds ratio) at 95% confidence intervals (CI) via SPSS. Compared with the control population, in NMOSD patients, the adjusted OR for low dairy consumption per week was (OR = 18.09; 95% CI 6.91, 47.37), following low sea food intake (OR = 13.91; 95% CI 6.13, 31.57) and low fruit and vegetable consumption (OR = 6.23; 95% CI 3.07, 12.62). The lower heavy physical activity (OR: 16.11, 95% CI 7.03, 36.91) among patients had risen the risk of NMOSD. A past history of head trauma was considered a risk for NMOSD (OR: 8.39, 95% CI 4.97, 14.16). The association between NMOSD and intentional abortion only among females (OR: 7.42, 95% CI 2.81, 19.55) was detected. This study indicates significant associations between dietary habits, life style, history of head trauma and intentional abortion in female and the later diagnosis of NMOSD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuromyelitis optica spectrum disorder (NMOSD) is an autoimmune inflammatory disorder of the central nervous system [1, 2]. Environmental factors have been consistently observed in multiple sclerosis (MS) operating between ages 13 and 18, while the effect of these factors on NMOSD risk has not identified [3]. The nutritional habits, smoking, exposure to infectious diseases, lack of sunlight, life style and hormonal factors are the most reported risk factors involved in the MS etiology [3, 4].
Unlike MS, NMOSD is more prevalent among Asians than Caucasians and expected to be a multifaceted illness with a significant non-genetic factor [5, 6].
Environmental factors may be important in determining NMOSD risk. Environmental exposures might occur long before the NMOSD becomes clinically evident, as suggested by the wide range in onset age.
The present study was set to discover the potential risk factors associated with dietary habit, smoking, environmental exposures, history of infection and autoimmune diseases, life style and hormonal factors among females in a large sample of people with NMOSD [3].
Methods
Study population and Case definition
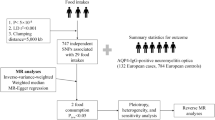
A case–control study was conducted in Tehran, Iran. This study was performed among patients with a definite diagnosis of NMOSD based on the international 2015 consensus criteria [7] at Sina Hospital, a tertiary care referral center to evaluate the possible risk factors for NMOSD incidence. The diagnosis of NMOSD was confirmed by neurologists. Clinical records and from all prevalent patients in this referral center were included from August 1, 2015 to September 21, 2016 through getting access to either paper or electronic based files [8].
Control definition
Controls were provided from all 15- to 50-year-old residents of 22 municipality zones of Tehran (total population approximately 5.11 million). The controls matched on sex and were from the same source of patient’s population. The controls had no history of any neurological disorder [4]. They were randomly selected through random digit dialing (RDD) using a standard RDD protocol [9].
In brief, from 4457 randomly created numbers, 2856 were inactive or non-residential numbers. Totally 1510 (94.3%) contactable households had an eligible person, and 91 (5.7%) had no eligible subject. To select a subject from all eligible people in one household, the Kish method was used [10].
Data collection protocol
The same questionnaire was applied for both patients and controls. Exposure to risk factors was determined prior to disease onset in NMOSD patients and in a parallel time period in controls.
Measurement
A structured questionnaire was designed in the MS research center of Tehran University of Medical Sciences to measure the main epidemiological variables, associated with the individual level. The NMOSD risk factors were considered according to the questionnaire designed for multinational case–control studies of environmental risk factors for multiple sclerosis (EnvIMS-Q) [11].
EnvIMS-Q contains six sections describing the most important environmental exposures including demographic data, subjective social status (rated via a visual ladder with 10 stairs (from poorest to richest), diet habits during 13–19 years of age, life style including duration of cigarette smoking (number of years), alcohol and drug abuse for a minimum of 6 months and no less than once per week, passive smoking (whether the person lived with a regularly smoking person and if yes, how many years), light and heavy physical activities (regularity; per week with average duration (min), sun exposure during 13–19 years of age (h/day), medical history of diseases diagnosed by a physician (a self-reported question) [12], and hormonal factors and delivery among females such as regular menstruation, use of oral contraceptive pill (OCP) and its duration, age at first delivery, abortion history and its type, and breast feeding to children for at least 2 months [4, 12]. The content validity and reliability of the translated questionnaire were approved [13].
Besides, several questions were added based on ethnicity, history of type-1 diabetes in first-degree relatives, history of head trauma (leading to loss of consciousness, vomiting, or bleeding), and having water-pipe smoking at least once per week for a minimum of 6 months. Detailed information on length (years) and amount (average frequency per week) was also obtained [4].
Protocol approval and patient consents
The main study objectives were fully explained to both study groups at the start of individual interview.
To excluding recall bias, the opportunity to remind and receiving information assist from relatives and physician for respond was given and informed consents were taken from all participants.
The study protocol was approved by the institutional review boards (IRB) at ethics committee of Tehran university of medical sciences (ref. no. IRTUMSREC13941195).
Statistical methods
Logistic regression was used to estimate unadjusted and adjusted odds ratio (OR) at 95% confidence intervals (CI) via SPSS 23. A large number of variables were examined to explore whether they are confounding or causally related to outcomes including age, education level, marital status, subjective social status, and mother/father’s ethnic group. The selection bias is remediated through appropriate analytical methods [14]. P values < 0.05 were considered significant based on two-tailed tests. Means, medians, and baseline characteristics of subjects were estimated. The Chi square test was used to analyze the association among variables. Equal percentiles on cases defined groups with respect to diet habit and life style with roughly the same number of observations done via the visual binning procedure.
Multiplicative interaction was measured by including a product term in the logistic model as an independent variable. Synergy Index (SI) was applied to discover any additive interaction, as authorized adjustment for confounders [15]. Finally based on multiple comparisons consideration, P values < 0.002 were considered significant.
Results
Study population characteristics
A total of 100 patients with NMOSD and 400 healthy controls participated in the survey. We checked presence of aquaporin-4 immunoglobulin G (AQP4-IgG) status of patients and results were positive for 44 (46.8%) and negative for 50 (53.2%) patients.
The response rates among patients and healthy control were 99.0 and 62%, respectively.
The characteristics of patients in comparison to healthy controls are shown in Table 1.
The average female-to-male ratio for NMOSD patients was 5.25:1.
A significant difference was seen with respect to age and father and mother’s ethnicity (P value < 0.05).
Weekly diet habits and monthly supplementary consumption
Table 2 shows the potential risk factors for NMOSD for the association between different types of dietary subgroup consumption during 13–19 years of age. Compared with the control population, in NMOSD patients, the adjusted OR for low dairy consumption per week was (OR = 18.09; 95% CI 6.91, 47.37), following low sea food intake (OR = 13.91; 95% CI 6.13, 31.57), low number of egg consumption (OR = 9.39; 95% CI 4.03, 21.86), low red meat consumption (OR = 3.07; 95% CI 1.68, 5.62), low chicken consumption (OR = 4.05; 95% CI 1.59, 10.35), low fat consumption (OR = 3.01; 95% CI 1.64, 5.49) and low fruit and vegetable consumption (OR = 6.23; 95% CI 3.07, 12.62). Lower consumption of multivitamins (OR = 5.49; 95% CI 1.90, 15.85), iron (OR = 7.64; 95% CI 2.98, 16.61) and Vitamin B12 and Vitamin C (P value < 0.03) was associated with an increased risk of NMOSD (Table 2).
Life style
Table 3 demonstrates increased NMOSD odds among passive smokers (OR = 1.79; 95% CI 1.12, 2.85) and who had water-pipe smoking (OR = 2.39; 95% CI 1.33, 4.29).
The alcohol consumption at least once for 6 mounts had an OR of 2.11 (95% CI 1.08, 4.11) for whiskey intake and (OR = 2.32; 95% CI 1.21, 4.43) for wine intake.
Physical activity per week at 13–19 years of age was low for patients in comparison with the controls (P value < 0.001). Both lower light physical activity (OR: 6.32, 95% CI 3.08, 12.95) and lower heavy physical activity (OR 16.11, 95% CI 7.03, 36.91) was associated with an increased risk of NMOSD.
An association between low sunlight exposure at 13–19 years of age and an increased risk of NMOSD (OR: 17.97, 95% CI 7.11, 45.40, P < 0.001) was observed.
Medical history
History of ever had infectious mononucleosis (OR: 79.35, 95% CI 8.83, 712.75), depression (OR: 2.99, 95% CI 1.81, 4.94), cardiovascular diseases (OR: 28.06, 95% CI 2.87, 274.40), and kidney failure (OR: 5.48, 95% CI 7.03, 36.91) before NMOSD incidence, demonstrated significant differences between the two groups.
A past history of head trauma appeared as a risk of NMOSD (OR: 8.39, 95% CI 4.97, 14.16).
A strong family aggregation of type-1 diabetes in siblings and mother (OR: 12.63, 95% CI 2.91, 54.84) and (OR: 7.61, 95% CI 3.49, 16.59) was observed, respectively (Table 4).
Hormonal factors and delivery among females
The association between history of irregular menstruation and NMOSD (OR: 3.92, 95% CI 2.29, 6.71), oral contraceptive pill usage (OR: 2.11, 95% CI 1.21, 3.66), first delivery lower than 24 years of age (OR: 2.18, 95% CI 1.17, 4.07), and intentional abortion among females with positive history of abortion (OR: 7.42, 95% CI 2.81, 19.55) detected (Table 5).
Discussion
Regardless of quick increasing awareness in NMOSD studies, there is no information in risk factors on this topic in the literature [2]. This study contributed to the body of knowledge regarding the epidemiological risk factor assessment of NMOSD on a large sample size. The data indicated that the risk of NMOSD is significantly higher among younger age groups and females.
NMO-IgG is very specific test for NMOSD [16]. Our subjects seemed to have similar NMO-IgG status to Caucasians with lower frequency of serum positivity (46.80%). The seropositivity rate for NMO-IgG was higher in southeast Wales and northern Japan [17, 18].
Overall, 22 risk factors were studied with respect to their association with the diseases, including autoimmune and infection diseases, severe head traumatic events, and type-1 diabetes among first-degree relatives. Only seven risk factors including depression, migraine, infectious mononucleosis, cardiovascular disease, kidney failure, and positive history of type-1 diabetes among the siblings and mother were supported by evidence with potent epidemiological credibility regarding medical history of subjects.
The mean age of disease onset (35.83 years old) among the study patients was the same as the existing reports from southern Denmark [19] and it was lower when compared to southeast Wales’s report [17].
Age and mother/father’s ethnicity had significant association with NMOSD. A frequency difference was detected between various ethnic groups living in Tehran [20].
A detailed examination of low dairy and sea food consumption showed an 18- and 13.9-fold increased risk of NMOSD. A comprehensive study revealed that low milk consumption and dairy products can lead to MS development [21], especially, the reduction of milk consumption during adolescent growth [22].
Also, in other food groups, lower consumption of eggs per week, low fruit or vegetable intake), eating chicken less than 450 g, red meat consumption lower than 360 g, and fat consumption less than 150 g per week during adolescence may increase the risk of NMOSD.
Studies have indicated that low-fat dairy products, fish, and vegetable diet are inversely related to the risk of MS [22]. It was further indicated in the study that the use of supplements such as iron, multivitamin, vitamin C and B12 may be protective of later NMOSD. Interestingly, recent studies suggest that high serum vitamin D, as an immunomodulator, may decrease the risk of MS [23].
The consequences of urbanized lifestyle including changes in dietary habits, smoking, alcohol intake, and lack of physical activity and sun exposure have been identified as potential risk factors for MS [24]. Multivariate regression models demonstrate that particular life style habits especially low physical activity and sun exposure during adolescence are associated with an increased risk of MS [25].
The present study for the first time examined the association between smoking and NMOSD. However, it was previously found that water-pipe smoking and passive smoking were associated with MS [4]. Strengthening these data, a strong association was observed between passive smoking and water-pipe smoking duration with an increase in NMOSD rate.
The results verified that those who had a history of passive smoking over 10 years had odds of over twofold higher risk than those who had history of passive smoking less than 10 years.
Water-pipe smoking is emerging globally as a health problem, mostly among the young population in the west Asian countries. There was an association between alcohol consumption and risk of developing NMOSD [26]. It seems that the amount of ethanol in some high alcoholic beverages like wine and whisky/vodka can increase the risk of NMOSD incidence at least two folds compared to low alcohol beverages such as bear.
Overall, unhealthy life style could play an important role in increasing chronic diseases and urbanization, modernization, and mechanical life could influence life style [27]. These factors cause lower physical activity and a decrease in sunlight exposure.
Low weekly sun exposure and physical activity during adolescence also received accuracy as risk factors for NMOSD. A similar effect has previously been seen in MS [28].
The present scale defining the weekly amount of light physical activity less than 195 min and no heavy physical activity can increase the risk of NMOSD diseases like MS. In summary, higher amounts of heavy physical activity during adolescence were found to be reversely associated with the risk of NMOSD; the same result was true for MS across different European countries [28].
Several studies show that a low degree of exposure to sunlight is associated with a higher risk of MS [29]. The UV radiation has immunomodulatory effects, enhancing the control of T-cell function [29]. The results indicated that in a tropical country, there is a strong association between low sunlight exposure and the risk to develop NMOSD.
Several marks of evidence associated with the risk of developing MS as a result of childhood infection such as measles and rubella and life time diseases such as migraine, thyroiditis, rheumatoid arthritis, and type-1 diabetes [21].
A history of infectious mononucleosis was a strong risk factor as for MS, cardiovascular diseases with 28-fold, and severe head trauma with 8.30-fold also had strong association with an increased risk of NMOSD. The female majority in autoimmune connective tissue disorders raises an instant question; whether the hormonal factors have any effect on increasing NMOSD risk.
A positive history of infection disease and comorbidity profile highlights the importance of environmental risk factors in MS pathogenesis [25, 30].
Great changes have been observed in women’s lifestyle over the last decades. Hormonal replacement therapy, later childbirth, using OCP, and abortion are potential lifestyle factors that may be related to the recent change of MNOSD epidemiology [25]. According to the results, un-regular menstruation can increase 3.92-fold risk of NMOSD following the increase of twofold risk among females who had history of OCP practice. Young age at the first delivery can increase the risk of NMOSD up to twofold in comparison to ages over 23. The intentional abortion history had strong association with the increasing risk of NMOSD. However, a current assessment of a Canadian population-based cohort did neither detect an association of maternal nor paternal age with risk for MS [31].
In confirmation of our results, several studies consistently report an increased risk of MS in association with emphasizing the role of environmental factors such as rapid changes in dietary habits, western lifestyle and past history of diseases [3, 4, 30] but unlike MS, smoking was not a risk factor for NMOSD [32].
We are the first to examine the environmental risk factors of NMOSD and collected large sample size for improving the statistical power of study. This study has a limitation as recall bias which is an inherent characteristic of case–control studies.
The importance of risk factors in NMOSD pathogenesis and role of lifestyle changes in the prevention of NMOSD were susceptibility confirmed. Additional future epidemiologic studies are recommended to improve exposure assessment and to define these risk factor for NMOSD more precisely.
Conclusion
Low and moderate dairy, sea food, egg, chicken, fruit/vegetable consumption, and low meat and fat consumption per week during adolescence and lack of monthly supplementary consumption such as multivitamin, iron, B12, and vitamin c during adolescence were identified as novel risk factors for NMOSD. The water-pipe smoking, history of lifetime passive smoking, and alcohol consumption are associated with NMOSD.
In summary, low physical activity and sun exposure per week during adolescence are strongly related with increasing NMOSD occurrences. Having positive history of depression, migraine, infectious mononucleosis, cardiovascular diseases, kidney failure, head trauma, and type-1 diabetes in first-degree relatives are considerably correlated with an increase in NMOSD.
In females, the history of irregular menstruation, use of OCP, and first delivery less than 24 years of age were among the risk factors for NMOSD.
References
Etemadifar M, Dashti M, Vosoughi R, Abtahi SH, Ramagopalan SV, Nasr Z (2014) An epidemiological study of neuromyelitis optica in Isfahan. Multiple sclerosis. 20(14):1920–1922
Etemadifar M, Nasr Z, Khalili B, Taherioun M, Vosoughi R (2015) Epidemiology of neuromyelitis optica in the world: a systematic review and meta-analysis. Multiple sclerosis international. 2015:174720
Hedstrom AK, Olsson T, Alfredsson L (2015) The role of environment and lifestyle in determining the risk of multiple sclerosis. Curr Top Behav Neurosci. 26:87–104
Abdollahpour I, Nedjat S, Sahraian MA, Mansournia MA, Otahal P, van der Mei I (2017) Waterpipe smoking associated with multiple sclerosis: a population-based incident case-control study. Mult Scler. 23(10):1328–1335
Eskandarieh S, Nedjat S, Abdollahpour I, Moghadasi AN, Azimi AR, Sahraian MA (2017) Comparing epidemiology and baseline characteristic of multiple sclerosis and neuromyelitis optica: a case-control study. Mult Scler Relat Disord. 12:39–43
Eskandarieh S, Heydarpour P, Minagar A, Pourmand S, Sahraian MA (2016) Multiple sclerosis epidemiology in East Asia, South East Asia and South Asia: a systematic review. Neuroepidemiology. 46(3):209–221
Tan CT, Mao Z, Qiu W, Hu X, Wingerchuk DM, Weinshenker BG (2016) International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 86(5):491–492
Eskandarieh S, Nedjat S, Azimi AR, Moghadasi AN, Sahraian MA (2017) Neuromyelitis optica spectrum disorders in Iran. Mult Scler Relat Disord. 18:209–212
Clagett B, Nathanson KL, Ciosek SL, McDermoth M, Vaughn DJ, Mitra N et al (2013) Comparison of address-based sampling and random-digit dialing methods for recruiting young men as controls in a case-control study of testicular cancer susceptibility. Am J Epidemiol 178(11):1638–1647
Ikeda N, Shibuya K, Hashimoto H (2011) Improving population health measurement in national household surveys: a simulation study of the sample design of the comprehensive survey of living conditions of the people on health and welfare in Japan. J Epidemiol. 21(5):385–390
Pugliatti M, Casetta I, Drulovic J, Granieri E, Holmoy T, Kampman MT et al (2012) A questionnaire for multinational case-control studies of environmental risk factors in multiple sclerosis (EnvIMS-Q). Acta Neurol Scand Suppl 195:43–50
Horton M, Rudick RA, Hara-Cleaver C, Marrie RA (2010) Validation of a self-report comorbidity questionnaire for multiple sclerosis. Neuroepidemiology. 35(2):83–90
Sahraian MA, Naghshineh H, Shati M, Jahromi SR, Rezaei N (2016) Persian adaptation of a questionnaire of environmental risk factors in multiple sclerosis (EnvIMS-Q). Mult Scler Relat Disord. 10:82–85
Morabia A (2014) Invited commentary: do-it-yourself modern epidemiology–at last! Am J Epidemiol 180(7):669–672
Andersen PK, Skrondal A (2015) A competing risks approach to “biologic” interaction. Lifetime Data Anal 21(2):300–314
Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG (2007) The spectrum of neuromyelitis optica. Lancet Neurol. 6(9):805–815
Cossburn M, Tackley G, Baker K, Ingram G, Burtonwood M, Malik G et al (2012) The prevalence of neuromyelitis optica in South East Wales. Eur J Neurol 19(4):655–659
Houzen H, Niino M, Hirotani M, Fukazawa T, Kikuchi S, Tanaka K et al (2012) Increased prevalence, incidence, and female predominance of multiple sclerosis in northern Japan. J Neurol Sci 323(1–2):117–122
Asgari N, Lillevang ST, Skejoe HP, Falah M, Stenager E, Kyvik KO (2011) A population-based study of neuromyelitis optica in Caucasians. Neurology. 76(18):1589–1595
Kashipazha D, Mohammadianinejad SE, Majdinasab N, Azizi M, Jafari M (2015) A descriptive study of prevalence, clinical features and other findings of neuromyelitis optica and neuromyelitis optica spectrum disorder in Khuzestan Province. Iran. Iranian journal of neurology. 14(4):204–210
Shaygannejad V, Rezaie N, Paknahad Z, Ashtari F, Maghzi H (2016) The environmental risk factors in multiple sclerosis susceptibility: a case-control study. Adv Biomed Res. 5:98
Jahromi SR, Toghae M, Jahromi MJ, Aloosh M (2012) Dietary pattern and risk of multiple sclerosis. Iran J Neurol. 11(2):47–53
Bagur MJ, Murcia MA, Jimenez-Monreal AM, Tur JA, Bibiloni MM, Alonso GL et al (2017) Influence of diet in multiple sclerosis: a systematic review. Adv Nutr. 8(3):463–472
Koch-Henriksen N, Sorensen PS (2010) The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. 9(5):520–532
Belbasis L, Bellou V, Evangelou E, Ioannidis JP, Tzoulaki I (2015) Environmental risk factors and multiple sclerosis: an umbrella review of systematic reviews and meta-analyses. Lancet Neurol. 14(3):263–273
Etemadi A, Khademi H, Kamangar F, Freedman ND, Abnet CC, Brennan P et al (2017) Hazards of cigarettes, smokeless tobacco and waterpipe in a Middle Eastern Population: a Cohort Study of 50,000 individuals from Iran. Tob Control. 26(6):674–682
Patil RR (2014) Urbanization as a determinant of health: a socioepidemiological perspective. Soc Work Public Health. 29(4):335–341
Wesnes K, Myhr KM, Riise T, Cortese M, Pugliatti M, Bostrom I et al (2017) Physical activity is associated with a decreased multiple sclerosis risk: the EnvIMS study. Mult Scler. https://doi.org/10.1177/1352458517694088
Hart PH, Gorman S, Finlay-Jones JJ (2011) Modulation of the immune system by UV radiation: more than just the effects of vitamin D? Nat Rev Immunol 11(9):584–596
Abbasi M, Nabavi SM, Fereshtehnejad SM, Jou NZ, Ansari I, Shayegannejad V et al (2017) Multiple sclerosis and environmental risk factors: a case-control study in Iran. Neurol Sci. 38(11):1941–1951
Ramagopalan SV, Dyment DA, Guimond C, Yee IM, Ebers GC, Sadovnick AD (2010) No effect of parental age on risk of multiple sclerosis: a population-based study. Neuroepidemiology. 34(2):106–109
Degelman ML, Herman KM (2017) Smoking and multiple sclerosis: a systematic review and meta-analysis using the Bradford Hill criteria for causation. Mult Scler Relat Disord. 17:207–216
Funding
Tehran University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Eskandarieh, S., Nedjat, S., Abdollahpour, I. et al. Environmental risk factors in neuromyelitis optica spectrum disorder: a case–control study. Acta Neurol Belg 118, 277–287 (2018). https://doi.org/10.1007/s13760-018-0900-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-018-0900-5