Abstract
Despite the huge health and economic burden of migraine headache, few medications have been approved for its prophylactic treatment, most of which can potentially induce serious adverse effects. Coenzyme Q10 (CoQ10) is a supplement and has shown preliminary benefits in migraine prophylaxis. We aimed to assess this effect in an adult population. This is an open-label, parallel, add-on, match-controlled trial. Eighty patients diagnosed with migraine headache based on International Headache Society criteria were allocated to receiving only their current preventive drugs or their current preventive drugs plus 100 mg CoQ10 daily, matching for their baseline characteristics, and were assessed for frequency and severity of attacks, and ≥50 % reduction in attack frequency per month. Thirty-six and 37 patients were analyzed in CoQ10 and control groups, respectively. Number of attacks per month dropped significantly in the CoQ10 group (mean decrease: 1.6 vs. 0.5 among CoQ10 and control groups, respectively, p < 0.001). A significant reduction was also evident in the severity of headaches (mean decrease: 2.3 vs. 0.6 among CoQ10 and control groups, respectively, p < 0.001). For ≥50 % reduction in the frequency of attacks per month, the number needed to treat was calculated as 1.6. No side effects for CoQ10 were observed. This study suggests that CoQ10 might reduce the frequency of headaches, and may also make them shorter in duration, and less severe, with a favorable safety profile.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Migraine headache is the third most prevalent medical disorder and considered to be a major constituent of the global burden of disease, with worldwide estimated prevalence of 14.7 and 2.9 % share of all years lived with disability, which makes it the eighth most burdensome disease globally [1]. Despite this huge health and economic burden imposed by this episodic disorder to the populations, few medications have been approved for its prophylactic treatment, most of which can potentially induce serious adverse effects. In response to the demand for drugs with better safety profiles, the role of certain supplements and herbals has been studied widely in the recent years, including riboflavin, vitamin B12, and magnesium. Coenzyme Q10 (CoQ10) is among these agents [2–7].
Coenzyme Q10 is a mitochondrial electron transporter and antioxidant which transfers electron from complexes I and II (nicotinamide adenine dinucleotide dehydrogenase and succinate-Q-reductase, respectively) to the cytochrome C. CoQ10 has been used in the treatment of numerous disorders including mitochondrial disorders, fibromyalgia, diabetes, cardiovascular disease, and some neurological conditions including Parkinson’s disease, Huntington’s disease, Alzheimer’s disease, and Friedreich’s ataxia [8, 9].
Investigations using phosphorus magnetic resonance spectroscopy has shown abnormal brain metabolism in anaerobic conditions in the interictal period in migraineurs, resulting in diminished tolerance in high energy-demanding situations. Accumulation of lactate has been considered to trigger migraine attacks in patients who already have abnormal sensory processing [10]. Specific genetic abnormalities in mitochondrial DNA are an area of avid investigations. On the basis of the mitochondrial theory for migraine pathogenesis, CoQ10, as a supplement without any major side effects, has been tried by researchers in the prophylactic treatment of migraine [2–6].
However, to the best of our knowledge, there have been very few controlled clinical trials of adult population but without a significant-enough population size in this area, and therefore, in this study, we investigated the effectiveness of CoQ10 in migraine prophylaxis including an Iranian adult population following a controlled design.
Methods
This is an open-label, parallel, add-on, match-controlled study. Subjects were recruited consecutively among patients who visited at the university clinic of Mashhad Medical University at Ghaem Hospital, during September 2014–September 2015 period. Mashhad is the capital of Khorasan-e-Razavi district and is Iran’s second largest city, located in northeast of the country, with an estimated population of about 3,000,000. Ghaem Hospital is the only referral center of Khorasan-e-Razavi district for neurological conditions. Prevalence of migraine has not yet been studied in Mashhad; however, a review of published studies of migraine prevalence in different cities of Iran showed that the prevalence of migraine is between 7.14 and 18.11 % in Iran [11]. Diagnosis of migraine was confirmed based on the International Headache Society criteria for inclusion of patients [12]. The applied inclusion criterion was having at least one attack a week, or 4 attacks per month, or if less frequent, having severe, debilitating, or long-lasting attacks. Our exclusion criteria were: (1) presenting serious comorbid conditions such as diabetes, hypertension, thyroid disease, and asthma, which would necessitate a change in preventive migraine medical therapy, (2) receiving CoQ10, for any reasons, 6 months prior to the start of the trial, (3) using known migraine deteriorating drugs including: oral contraceptives, nitrate-containing drugs, and vasodilators, etc. during the trial (4) patient’s choosing not to continue with CoQ10. Sample size was calculated using two-mean comparison equation, on the outcome of number of attacks per month, in compliance with previous studies [4]. Type 1 and 2 errors were assumed 5 and 20 % respectively.
Patients were allocated to either group 1 (Control), receiving only their current preventive drugs or group 2 (Intervention), taking their current preventive drugs plus 100 mg of CoQ10 daily (WN Pharmaceuticals Ltd., softgel formulation). The 100 mg dosage were used, because it is the only preparation available in the study setting, and we intended to avoid the gastrointestinal side effects, which thence increases adherence to the supplement. The groups were matched for their baseline characteristics, including the type of drugs they were using for migraine attacks and for prophylaxis (Table 1). At the baseline, 16 patients in the intervention group and 2 patients of the control group were receiving prophylactic treatment. However, considering the severity and frequency of headaches, prophylactic treatment started for all patients at the baseline; hence, all patients were receiving prophylactic treatment at least one month prior to the study in both control and intervention groups. The prophylactic medications used were a combination of a tricyclic antidepressant and the anticonvulsant, sodium valproate, for all patients.
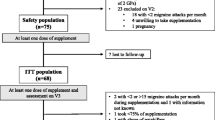
Originally, each group consisted of 40 subjects, but 2 patients from each group dropped out from the study as they withdrew their consent before institution of the trial treatment. Three (2 from intervention group and 1 from the control) left the study during the first post-intervention month because of personal issues unrelated to the treatment effects (3.9 % loss to follow-up) (Fig. 1).
The total duration of the study was 4 months, one month of pre-intervention and 3 months for recording the effects of the treatment. During the baseline period and the following 3 months of the study, all patients were allowed to continue their previous prophylactic drugs, in consideration of ethical issues regarding deprivation of previously established drugs. All patients were assessed for peak severity of attacks using the visual analogue scale (VAS), frequency of attacks per week and month, quality of attacks (including accompanying symptoms of nausea, vomiting, photophobia, and phonophobia), number of days with headache, and number of days of absence from work because of migraine, at the baseline and reassessed at the end of the first, second and third months after initiating treatment with CoQ10 during an interview session. Type of migraine was assessed for each patient based on their history of aura and classified as migraine with or without aura. Patients were also asked if they had any other comorbid diseases or conditions as well as about their family history of migraine and level of education. Our primary outcome measure was the proportion of patients with ≥50 % reduction in mean number of attacks per month comparing resultant evidence from first month with that of the third month. Secondary outcome measures were severity of attacks and change in symptoms accompanying headache.
Statistical analyses were made using SPSS version 20. Friedman’s test was used for comparing mean number of attacks per month and week, duration of attacks, number of days with headache and number of days of absence from work because of headache, among intervention and control groups. Dichotomous data on nausea, vomiting, photophobia and phonophobia were analyzed using Fisher’s exact test for each recoding separately and Cochrane Q test for comparing the three recordings between groups. Number needed to treat calculated as a primary outcome measure. We intended to evaluate if the CoQ10 plus standard treatment is superior to the standard treatment alone.
The study was approved by Research Ethics Committee of Mashhad University of Medical Sciences and Health Services. All patients signed an informed consent form before participation.
Results
Patients in the CoQ10 and control groups did not differ significantly in terms of their number, severity and quality of attacks (before participation in the study) as well as their education and sex. All patients were receiving prophylactic medications at least one month prior to the start of the study. Thirty-seven patients in the control group and 36 in CoQ10 group completed the study and were included in the analyses. No adverse effects were reported by patients in the CoQ10 group. Descriptive statistics of post-intervention values and the level of significance of comparisons are shown in Table 2.
Mean number of migraine attacks per month dropped from 2.7 at the end of the first month to 1.0 at the end of the 3rd month in the CoQ10 group, and from 3.6 to 3.1 among the control subjects (p < 0.001, analyzed by Friedman’s test) (Fig. 2). Accordingly, identical analysis for number of attacks per week (reducing from 0.3 to 0.1 in CoQ10 compared to 0.8–0.5 in controls, p < 0.001), number of days with migraine per month (reducing from 4.5 to 1.4 in CoQ10 compared to 12.4–8.3 in controls, p < 0.001), and days of absence from work due to severe migraine (reducing from 0.71 to 0.65 in CoQ10 compared to 3.75 to 2.72 in controls, p < 0.001), yielded significant results. Severity of attacks, assessed using VAS, reduced from 3.6 to 1.3 in CoQ10 users significantly compared to the reduction from 6.4 to 5.7 in the control group (p < 0.001) (Fig. 3). Analysis for the symptoms accompanying headache, including nausea, photophobia, and phonophobia revealed significant reduction of these symptoms among CoQ10 compared to the control group (p < 0.001, using Cochran’s Q test) (Table 2). No significant change in vomiting symptom was observed.
The number of attacks per month has dropped to less than 50 % comparing to the first month in 29 (about 80 %) patients in the CoQ10 group, and 6 (16 %) patients in the control group. Considering ≥50 % reduction in the frequency of attacks per month, the absolute risk reduction (ARR) was 64.34 % (CI: 46.78–81.90 %), and NNT was calculated as 1.6 (CI: 1.2–2.1). Considering a worst-case analysis (2 and 1 lost to follow-up in CoQ10 and control groups, respectively), ARR for aforementioned outcome is 57.90 % (CI: 39.60–76.19 %) and NNT is 1.7 (CI: 1.3, 2.5).
Discussion
This study is one of a few controlled studies that evaluated the efficacy of CoQ10 in adult migraine prophylaxis. The findings indicated a significant prophylactic effect resulting from CoQ10 that not only seems to reduce the frequency of headaches in migraineurs, but also renders their attacks shorter in duration, less severe and more tolerable. It was also observed to reduce symptoms of nausea, photophobia and phonophobia in these patients. Although we did not find any significant reduction in vomiting comparing the first-month records to those of the third, when considering pre-intervention values, the number of patients with vomiting was actually 11 at the baseline which reduced to one in the third month in CoQ10 group, while a reduction from 13 to 7 occurred in the control group. We calculated an NNT of 1.6 (or 1.7 based on worst-case analysis) for ≥50 % reduction in number of attacks per month that provides a reasonable explanation for the use of CoQ10 in clinical settings of frequent and durable attacks as an add-on to the standard treatment. In view of these presumably significant findings in terms of relieving the accompanying symptoms of nausea, photophobia and phonophobia and also the severity of attacks, CoQ10 could probably be recommended as an effective drug for patients with less frequent, but more severe attacks.
Notably, we encountered no side effects for this drug during the study, which makes it a preferable choice for migraine prophylaxis, especially in patients with comorbid conditions using multiple drugs, in children, in those who discontinued other effective prophylactic drugs because of their side effects, in women of childbearing age, and possibly in pregnant women.
Coenzyme Q10 has been studied in pediatric and adolescent patients with variable results. In a placebo-controlled and crossover study involving 120 children and adolescents, researchers found no durable effect for CoQ10 on attack frequency or severity; however, it should be mentioned that in this study, only 50 cases were available to be analyzed at the endpoint, and researchers found significant effects on migraine frequency only in the first 4 weeks of the study in the CoQ10 group [6]. In another study, CoQ10 was evaluated in 1550 pediatric and adolescent patients, and CoQ10 supplement was given to 252 who had CoQ10 deficiency. Results showed a significant decrease in attack frequency and disability among these patients; however, this study was not a controlled one [2].
In a study involving an adult population, 31 patients were treated with 150 mg CoQ10 daily for 3 months, and although there was no control group in this study, in comparison with the relevant baseline records, 60 % of patients presented a 50 % reduction in number of days with headache. Researchers concluded that CoQ10 was effective in preventing migraine [3]. Higher doses of CoQ10 (100 mg three times a day) were administrated in another study [4], with smaller sample size (18 and 19 were eventually analyzed in CoQ10 and placebo groups, respectively), which resulted in a significant reduction in the number of attacks (NNT at 50 % reduction in frequency of headaches per month = 3). It should be noticed that the study groups were not similar at the baseline in terms of headache duration.
The theory of mitochondrial pathology in migraine headache has been supported by a multitude of studies [10, 13–21]. Biochemical evidence showed that energy failure on account of a failure in oxidative phosphorylation altered the vascular tone (due to lower levels of platelet superoxide dismutase enzyme and impaired calcium ion metabolism) and hindered the recycling of reactive oxygen species, which are probable mechanisms responsible for triggering headache in at least a subgroup of patients. Based on these findings, the role of supplements such as CoQ10, which have proved to generate mitochondrial enhancing effects, has been studied among migraineurs.
Considering the non-randomized and non-blind nature of this study, any reference to its results should be treated with caution. The placebo effect could potentially have interfered with the results of this trial. Therefore, it could not be ruled out that the observed therapeutic effects have been produced, at least partly, by the placebo effect of the supplement. Also, this was an add-on study and patients were allowed to continue using their previous prophylactic drugs, and hence, results could be confounded with other drugs that patients were already using, particularly as there was a difference in the proportion between the intervention and the control group using prophylactic medication longer than one month before starting the study. Considering the use of interview for recording the results, our results might be confounded with the recall bias; the same explanation could be pertinent for the report of no side effects for CoQ10 in this study. Further studies addressing these limitations are recommended.
Despite all, the present study was, indeed, singular in the sense that it was a match-controlled trial with acceptable sample size and minimal loss to follow-up which assessed all aspects of migraine attacks in adult population with noteworthy findings.
References
Vos T, Flaxman AD, Naghavi M et al (2012) Years lived with disability (YLD) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2163–2196
Hershey A, Powers S, Vockell A, LeCates S, Ellinor P, Segers A et al (2007) Coenzyme Q10 deficiency and response to supplementation in pediatric and adolescent migraine. Headache 47(1):73–80
Rozen T, Oshinsky M, Gebeline C, Bradley K, Young W, Shechter A et al (2002) Open label trial of coenzyme Q10 as a migraine preventive. Cephalalgia 22(2):137–141
Sándor PS, Di Clemente L, Coppola G, Saenger U, Fumal A, Magis D et al (2005) Efficacy of coenzyme Q10 in migraine prophylaxis: a randomized controlled trial. Neurology 64(4):713–715
Gaul C, Diener H-C, Danesch U (2015) Improvement of migraine symptoms with a proprietary supplement containing riboflavin, magnesium and Q10: a randomized, placebo-controlled, double-blind, multicenter trial. J Headache Pain 16(1):32
Slater SK, Nelson TD, Kabbouche MA, LeCates SL, Horn P, Segers A et al (2011) A randomized, double-blinded, placebo-controlled, crossover, add-on study of CoEnzyme Q10 in the prevention of pediatric and adolescent migraine. Cephalalgia 31:897–905
Sasannejad P, Saeedi M, Shoeibi A, Gorji A, Abbasi M, Foroughipour M (2012) Lavender essential oil in the treatment of migraine headache: a placebo-controlled clinical trial. Eur Neurol 67(5):288–291
Pagano G, Talamanca A, Castello G, Cordero M, d’ Ischia M, Gadaleta M et al (2014) Current experience in testing mitochondrial nutrients in disorders featuring oxidative stress and mitochondrial dysfunction: rational design of chemoprevention trials. Int J Mol Sci 15(11):20169–20208. doi:10.3390/ijms151120169
Garrido-Maraver J, Cordero MD, Oropesa-Ávila M, Vega A, Mata M, Pavón A et al (2014) Coenzyme Q10 therapy. Mol Syndromol 5(3–4):187–197
Sparaco M, Feleppa M, Lipton R, Rapoport A, Bigal M (2006) Mitochondrial dysfunction and migraine: evidence and hypotheses. Cephalalgia 26(4):361–372
Sadeghi O, Nasiri M, Saiedi S (2015) The prevalence of migraine in different parts of Iran: review of the current evidence. Jundishapur J Chronic Dis Care 4(3):e27678
Headache Classification Committee of the International Headache Society (IHS) (2013) The international classification of headache disorders, 3rd edn (beta version). Cephalalgia 33(9):629–808
Yorns WR, Hardison HH (2013) Mitochondrial dysfunction in migraine. Semin Pediatr Neurol 20:188–193
Roos-Araujo D, Stuart S, Lea RA, Haupt LM (2014) Epigenetics and migraine; complex mitochondrial interactions contributing to disease susceptibility. Gene 543(1):1–7
Fried NT, Moffat C, Seifert EL, Oshinsky ML (2014) Functional mitochondrial analysis in acute brain sections from adult rats reveals mitochondrial dysfunction in a rat model of migraine. Am J Physiol Cell Physiol 307:C1017–C1030
Okada H, Araga S, Takeshima T, Nakashima K (1998) Plasma lactic acid and pyruvic acid levels in migraine and tension-type headache. Headache 38:39–42
Zaki EA, Freilinger T, Klopstock T, Baldwin EE, Heisner KR, Adams K et al (2009) Two common mitochondrial DNA polymorphisms are highly associated with migraine headache and cyclic vomiting syndrome. Cephalalgia 29:719–728
Di Lorenzo C, Pierelli F, Coppola G, Grieco GS, Rengo C, Ciccolella M et al (2009) Mitochondrial DNA haplogroups influence the therapeutic response to riboflavin in migraineurs. Neurology 72:1588–1594
Stuart S, Griffiths LR (2012) A possible role for mitochondrial dysfunction in migraine. Mol Genet Genomics 287:837–844
Welch K, Levine SR, D’andrea G, Schultz LR, Helpern JA (1989) Preliminary observations on brain energy metabolism in migraine studied by in vivo phosphorus 31 NMR spectroscopy. Neurology 39:538
Sparaco M, Feleppa M, Lipton RB, Rapoport AM, Bigal ME (2006) Mitochondrial dysfunction and migraine: evidence and hypotheses. Cephalalgia 26:361–372
Acknowledgments
We are thankful to the patients who participated in this study; we acknowledge Research Vice Chancellor of Mashhad University of Medical Sciences for funding and supporting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
Our study has been approved by Research Ethics Committee of Mashhad University of Medical Sciences.
Informed consent
Written informed consent was obtained from all participants before institution of trial.
Rights and permissions
About this article
Cite this article
Shoeibi, A., Olfati, N., Soltani Sabi, M. et al. Effectiveness of coenzyme Q10 in prophylactic treatment of migraine headache: an open-label, add-on, controlled trial. Acta Neurol Belg 117, 103–109 (2017). https://doi.org/10.1007/s13760-016-0697-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-016-0697-z