Abstract
Spontaneous Intracranial Hypotension typically occurs from spontaneous CSF leak. CSF volume depletion rather than decrease in CSF pressure is thought to be the main causative feature for intracranial hypotension. More and more cases of intracranial hypotension are getting diagnosed with the advances in the imaging. The advances in the imaging have also led to the better understanding of the dynamic changes that occur with intracranial hypotension. The old theories of CSF overproduction or CSF underproduction have not been substantially associated with intracranial hypotension. It has also led to the fore different atypical clinical features and presentations. Although, it has been known for a long time, the diagnosis is still challenging and dilemma persists over one diagnostic modality over other and the subsequent management. Spontaneous CSF leaks occur at the spinal level and the skull base and other locations are rare. The anatomy of spontaneous intracranial hypotension is a very complex process with significant overlap in connective tissue disorders, previous dural weakness or meningeal diverticula. To localize the location of the CSF leak—CT myelography is the modality of choice. CSF cysternography may provide additional confirmation in uncertain cases and also MRI spine imaging may be of significant help in some cases. Spontaneous intracranial hypotension continues to be a diagnostic dilemma and our effort was to consolidate available information on the clinical features, diagnostics, and management for a practicing neurologist for a “15–20 min quick update of the topic”.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
“Doctor, my head hurts when I am standing. The pain gets better after I sit down or lay down flat!” This patient in the emergency room (ER) was a young female in her mid-thirties, who came that morning concerned that she might have had a “brain tumor”! ER physician saw the patient and promptly communicated her grief to me “I do not know, but doesn’t sound like a tumor” he exclaimed! Indeed, we are talking about a headache subtype related to low cerebral spinal fluid (CSF) pressure, that was first described in 1938 [32].
Intracranial hypotension is characterized by a low pressure headache secondary to CSF leak. In 1959, Dr. William Bell described 5 types of intracranial hypotension: (1) Spontaneous or primary, (2) postoperative (cranial), (3) head trauma, (4) post lumbar puncture or nerve sleeve tear, (5) secondary to other medical conditions, such as dehydration, or instances of decreased cerebral blood flow [3]. We will be focusing on the spontaneous intracranial hypotension in this review chapter.
Epidemiology
According to the current literature, the incidence of spontaneous intracranial hypotension is estimated to be around 2–5 per 100,000 in western countries [1–4, 6, 44]. It is one of the greatest masqueraders, and the current belief is that it is vastly underdiagnosed. Females are affected more than males in 2:1 ratio [1–6]. This condition gains more importance as the affected population is young with average age at presentation being 38 years [4–6], leading to considerable morbidity with headaches accounting more than 1.3 % YLD (years lost due to disability) [3].
Pathophysiology
As the name suggests, intracranial hypotension is caused by CSF leak resulting in low CSF pressure. The CSF leak usually arises from the spine, but rarely from the skull base. The headache is believed to be caused by a change in the hydrodynamics of CSF in the intracranial space by changing the volume, and hence the buoyancy effect on brain provided by CSF. Persistent CSF leak leads to sinking of the brain towards foramen magnum. If the leak continues, subdural hygromas, cranial nerve palsies, brainstem compression, and even coma may result [6, 17–22]. In addition, persistent intracranial hypotension can be complicated by cortical venous thrombosis secondary to reduced venous flow. This may lead to increased vessel shear and rupture, leading to subarachnoid hemorrhage.
Spontaneous intracranial hypotension is generally seen in patients with underlying structural weakness of the spinal dura. The triggers for the symptoms can be trivial or minor; however, no clear precipitant was identified in most cases. Schievink et al. studied a group of 80 consecutive patients with spontaneous intracranial hypotension. Precipitating events were recorded in only 35 % of their patients. Reported triggering events include straining, choking, exercising, sport activities, picking up small or large objects, positional changes, trivial falls, rollercoaster rides, or chiropractic neck manipulation.
A subset of patients with spontaneous intracranial hypotension has been shown to have stigmata of connective tissue disorders. These patients have been demonstrated to have dural abnormalities that would predispose them at risk for CSF leaks. Meningeal diverticula are known to occur in Marfan’s syndrome and neurofibromatosis. In a study of 83 patients with Marfan’s syndrome, 92 % of the patients were found to have dural ectasia on the MRI of the thoracic aorta and lumbosacral spine [34–36]. Therefore, it is conceivable that a trivial insult to patients with underlying connective tissue disorder can lead to spontaneous intracranial hypotension.
Clinical presentation
The hallmark of intracranial hypotension is postural or orthostatic headache [1–4]. This headache occurs upon assuming an upright posture and improves with recumbency. In an observational study by Schievink et al., 18/18 patients had orthostatic headache [17, 18]. The other symptoms that are mentioned by the patients include neck pain or stiffness (60 %), nausea and vomiting (50 %), hearing abnormalities (40 %), and visual abnormalities (28 %). [17–19]. Some of the patients have reported double vision and symptoms suggestive of parkinsonism and reversible coma [19–22], but these remain as reported case reports. Due to the common presentation of headache with neck stiffness, nausea and vomiting, clinicians often suspect meningitis and sometimes rightly so! However, in an appropriate clinical setting of orthostatic headache without fever and other meningeal signs, intracranial hypotension should be considered. It is important to recognize that in one of the case series from Cedar-Sinai, 28/80 patients had a preceding event within 15 min of symptoms onset, 13 had symptoms onset after 1 min of the precipitating factor, and 15 patients presented after 24 h of precipitating factor. The types of precipitating factors were most commonly (1) blunt non-penetrating trauma (57 %), (2) Valsalva maneuver (33 %), and (3) rapid positional change (10 %) [43]. In addition, persistent intracranial hypotension can be complicated by cortical venous thrombosis secondary to reduced venous flow. This may lead to increased vessel shear and rupture, leading to subarachnoid hemorrhage. Cardiac anomalies like bicuspid anomaly have been reported to be associated with intracranial hypotension [47]. We would also like to mention that although orthostatic hypotension is hallmark of intracranial hypotension, it may also be seen with headache associated with pneumocephalus [48].
Diagnosis
Clinical history alone is often highly suggestive of the diagnosis. Imaging study and lumbar puncture are used to confirm the diagnosis and localize the CSF leak. The proposed diagnostic criteria of headache due to spontaneous intracranial hypotension have undergone several revisions since 2004 to recognize the variability in the clinical presentation and the imaging findings. The criteria from the most recent publication (2011) are as follows [44]:
-
1.
Orthostatic headache;
-
2.
The presence of at least one of the following:
-
a.
Low opening pressure (≤60 mm H2O),
-
b.
Sustained improvement of symptoms after epidural blood patching,
-
c.
Demonstration of an active spinal cerebrospinal fluid leak,
-
d.
Cranial magnetic resonance imaging changes of intracranial hypotension (such as brain sagging or pachymeningeal enhancement);
-
a.
-
3.
No recent history of dural puncture; and
-
4.
Not attributable to another disorder.
Lumbar puncture
It is a simple bedside test. Opening pressure <60 mm H2O (6 cm) is typically found in patients with intracranial hypotension. It should be noted that a normal opening pressure does not rule out a CSF leak. CSF analysis can be normal. However, it is also not uncommon to see CSF pleocytosis (up to 200 cells/mm3) and elevated protein (up to 1000 mg/dl) reflecting an increased permeability of the dilated meningeal blood vessels and reduced CSF circulation [6, 8].
Imaging
Computed tomography (CT) imaging
The advantages of cranial CT imaging is the wide availability and in emergency setting evaluation. CT imaging can readily identify subdural fluid collection, obliteration of subarachnoid cistern and ventricular collapse [6].
Magnetic resonance imaging (MRI)
Improvement in the MRI over the years has made it possible to understand many aspects of intracranial hypotension. Cranial MRI can be diagnostic of intracranial hypotension without resorting to invasive modalities, including lumbar puncture. Recognizing the characteristic imaging findings on cranial MRI have been responsible in the ever increasing number of intracranial hypotension being identified. It should be noted that about 20–30 % patients with intracranial hypotension have normal cranial MRI [25].
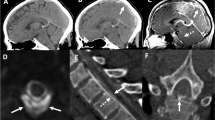
The mnemonic SEEPS can be helpful to remember the main findings on cranial MRI [37]:
- 1.
-
2.
Enhancement of pachymeninges (Fig. 3).
-
3.
Engorgement of venous structures.
-
4.
Pituitary hyperemia (Fig. 4).
-
5.
Sagging of the brain (Fig. 5).
Subdural fluid collections are the most common findings found on cranial MRI, accounting for as many as 50 % of patients in the published studies [24–26]. These subdual fluid collections are mostly bilateral, thin, on the cerebral convexities without any mass effect. However, subdural hematomas with varying degrees of mass effect are not uncommon in intracranial hypotension. Pachymeningeal enhancement is the most well-known imaging abnormality that is found with intracranial hypotension. The enhancement is non-nodular, diffuse, supra and infratentorial and sparing the leptomeninges. The venous engorgement becomes evident when, dural venous sinuses or large cerebral veins are involved. Of late, pituitary hyperemia is a well described imaging modality described in patient with intracranial hypotension and sometimes mimicking tumor or hyperplasia [27]. Sagging of the brain is a specific finding associated with this particular headache syndrome. It may be associated with slit like ventricles or frank ventricular collapse [24–26]. In addition, the sagging of brain is generally out of proportion to the subdural hygroma collection. All the imaging findings can be explained by the Monroe-Kellie hypothesis, which states that the sum total of CSF, intracranial blood and cerebral tissue remains constant [31]. Therefore, the intracranial pressure loss from the CSF volume is compensated by the vascular component causing venous engorgement and pituitary hyperemia [28]. The sagging of the brain occurs from the loss of buoyancy provided by the CSF volume. Improvement in the cranial MRI changes can be seen a few days (generally 6–7) to many weeks from the onset of the clinical symptoms. Therefore, it is not uncommon to observe clinical improvement before the radiologic improvement.
Spinal MRI is a new but important modality which has revealed many an important spinal manifestations like epidural and intradural veins, dural enhancement, meningeal diverticula, extra-thecal CSF collection and retrospinal C1–C2 fluid collection [24–26]. MRI with intrathecal gadolinium may also be a useful modality as the gadolinium stays in the cranial cavity for 24 h. This helps in detecting intermittent leaks. In addition, it is reported to be well tolerated with good contrast ratio, superior spatial, and temporal resolution [45].
Myelography
Myelography with iodinated contrast and thin slice computed tomography is the diagnostic modality of choice for defining the location and extent of the CSF leak [6, 10]. The extent of leak may range from small collection of the iodinated contrast limited to one single nerve root to extensive bilateral collection of the CSF in the paraspinal muscles. Majority of the leaks are at the cervico-thoracic level or thoracic levels [30]. In approximately 5 % of the patients, the symptoms are made worse by the procedure. The risk of cerebral herniation caused by the procedure is considered extremely low, and in fact has never been reported [6, 30].Retrospective studies confirm digital subtraction myelography as a useful test to localize spinal dural tears [46].
Radionuclide cysternography
Radionuclide cysternography involves injecting radioisotope by lumbar puncture into the spinal subarachnoid space. The radioisotope will then diffuse up the spinal column and into the brain. At predetermined intervals up to 24 or even 48 h, the progress of the radioisotope’s diffusion through the subarachnoid space will be recorded by a nuclear medicine camera. A radionuclide cysternography can be very helpful to confirm the presence of a CSF leak, especially in patients with normal myelography and atypical but suspected intracranial hypotension. Unfortunately, this technique is insensitive in localizing CSF leaks [40].
Treatment
There are several treatment options that we will discuss in the following paragraphs. However, due to lack of randomized controlled trials, the treatment of choice remains solely on the physician’s preference and expertise.
Conservative management
Traditionally accepted view is that many improve spontaneously without any intervention within a period of 1–2 weeks from the symptom onset. Initial management often involves bed rest, oral hydration, oral caffeine, abdominal binders, or simple analgesia. However, some patients may have persistent symptoms after the initial treatment or may desire more timely resolution of symptoms. For these patients, intravenous caffeine, theophylline infusions or corticosteroids can be considered [41, 42]. Most of the patients improve with the above treatment but some progress to continue having debilitating headaches.
Epidural patching
For patients with continued symptoms despite the above conservative measures, the mainstay first-line treatment is injection of autologous blood in the epidural space, also known as “epidural blood patch” [29]. Initially about 10–20 ml of blood should be used. This modality not only can provide instant relief but also may serve as a diagnostic modality. The mechanism of action is thought to be dural tamponade or reducing CSF absorption by restricting the CSF flow in the spinal epidural space. If this does not work in the first setting, it may be repeated for a second time with higher amount of blood (20–100 ml) [29]. It is recommended that generally 5 days should be given in between the two procedures in view of relatively large amount of blood used in the second setting. If the blood patch does not work, directed epidural blood patch or placement of fibrin sealant is recommended [29]. For the placement of the fibrin sealant, it is required that the exact site and extent of the leak is known; hence, providing with the best chance of symptomatic relief [6, 10, 29].
Surgical management
Surgical management is reserved only for patients who do not respond to the above management options or the symptoms are too severe, necessitating immediate surgical intervention. Surgical repair has been reported to be safe [6, 9, 29], and helpful in patients with focal or identified position of the leak. Leaking meningeal diverticula is usually ligated with a suture or metal aneurysm clip [6, 9, 10, 29]. Dural holes or tear may be repaired with a small tissue of muscle and gel foam and fibrin sealant. Intrathecal artificial CSF or saline infusion is sometimes used in severe cases to replenish the lost volume until a permanent solution is found or in patients with rapidly declining symptoms such as altered mental status [9, 10, 20].
Outcomes
Overall, outcomes are good for the majority of patients with spontaneous intracranial hypotension. However, recurrence of headache was estimated to be around 10 % [29]. Outcome studies indicate that patients with abnormal cranial MRI imaging and focal CSF leak do better than the rest [4, 6]. The exact mechanism for this is unclear at this point.
Following successful treatment, some patients may experience a different type of headaches. These headaches are typically opposite of their headaches from intracranial hypotension, waking them from sleep and improving with upright positioning. In addition, these headaches can be associated with nausea, vomiting, pulsatile tinnitus, or double vision. These findings are suggestive of elevated intracranial pressure, termed rebound intracranial hypertension [38, 39]. The proposed mechanism is a temporary increase in CSF production after the onset of the symptoms. After successful treatment of the CSF leak, it may take days, weeks or months for the CSF production to return to normal. Patients with mild symptoms may not require treatment. However, typical treatments for idiopathic intracranial hypertension (e.g., acetazolamide or CSF shunting) can be considered for patients with more severe or debilitating symptoms.
References
Lindquist T, Moberg E (1949) Spontaneous hypoliquorrea. Acta Med Scand 132:556–561
Bell WE, Joynt RJ, Sahs AL (1960) Low spinal fluid pressure syndromes. Neurology 10:512–521
Schievink WI, Maya MM, Louy C (2005) Cranial MRI predicts outcome of spontaneous intracranial hypotension. Neurology 64:1282–1284
Lasater GM (1970) Primary intracranial hypotension: the low spinal fluid pressure syndrome. Headace 10:63–66
Schievink WI, Meyer FB, Atkinson JLD et al (1996) Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. J Neurosurg 84:598–605
Mokri B, Piepgras DG, Miller GM (1997) Syndrome of orthostatic headaches and diffuse pachymeningeal gadolinium enhancement. Mayo Clin Proc 72:400–413
Miyazawa K, Shiga Y, Hasegawa T et al (2003) CSF hypovolemia vs intracranial hypotension in “spontaneous intracranial hypotension syndrome”. Neurology 60:941–947
Schievink WI, Morreale VM, Atkinson JLD et al (1998) Surgical treatment of spontaneous spinal cerebrospinal fluid leaks. J Neurosurg 88:243–246
Schievink WI (2000) Spontaneous spinal cerebrospinal fluid leaks. Neurosurg Focus 9:1–9
Mokri B, Maher CO, Sencakova D (2002) Spontaneous CSF leaks: underlying disorder of connective tissue. Neurology 58:814–816
Schievink WI, Gordon OK, Tourje J (2004) Connective tissue disorders with spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension: a prospective study. Neurosurgery 54(65):70
Davenport RJ, Chataway SJ, Warlow CP (1995) Spontaneous intracranial hypotension from a CSF leak in a patient with Marfan’s syndrome. J Neurol Neurosurg Psychiatry 59:516–519
Mokri B (1999) Spontaneous cerebrospinal fluid leaks: from intracranial hypotension to cerebrospinal fluid hypovolemia—evolution of a concept. Mayo Clin Proc 74:1113–1123
Levine DN, Rapalino O (2001) The pathophysiology of lumbar puncture headache. J Neurol Sci 192:18
Headache Classification Subcommittee of the International Headache Society (2004) The international classification of headache disorders, 2nd ed. Cephalalgia 24(suppl 1):1–160
Ferrante E, Savino A, Brioschi A et al (1998) Transient oculomotor cranial nerves palsy in spontaneous intracranial hypotension. J Neurosurg Sci 42:177–179
Warner GT (2002) Spontaneous intracranial hypotension causing partial third cranial nerve palsy: a novel observation. Cephalalgia 22:822–823
Brady-McCreery KM, Speidel S, Hussein MA et al (2002) Spontaneous intracranial hypotension with unique strabismus due to third and fourth cranial neuropathies. Binocul Vis Strabismus Q 17:43–48
Binder DK, Dillon WP, Fishman RA (2002) Intrathecal saline infusion in the treatment of obtundation associated with spontaneous intracranial hypotension:technical case report. Neurosurgery 51:830–836
Whiteley W, Al-Shahi R, Myles L et al (2003) Spontaneous intracranial hypotension causing confusion and coma: a headache for the neurologist and the neurosurgeon. Br J Neurosurg 17:456–458
Kashmere JL, Jacka MJ, Emery D et al (2004) Reversible coma: a rare presentation of spontaneous intracranial hypotension. Can J Neurol Sci 31:565–568
Pakiam AS, Lee C, Lang AE (1999) Intracranial hypotension with parkinsonism, ataxia, and bulbar weakness. Arch Neurol 56:869–872
Mokri B, Krueger BR, Miller GM et al (1991) Meningeal gadolinium enhancement in low pressure headaches [abstract]. Ann Neurol 30:294–295
Schievink WI, Tourje J (2000) Intracranial hypotension without meningeal enhancement on magnetic resonance imaging: case report. J Neurosurg 92:475–477
Schoffer KL, Benstead TJ, Grant I (2002) Spontaneous intracranial hypotension in the absence of magnetic resonance imaging abnormalities. Can J Neurol Sci 29:253–257
Mokri B, Atkinson JLD (2000) False pituitary tumor in CSF leaks. Neurology 55:573–575
Alvarez-Linera J, Escribano J, Benito-Leon J et al (2000) Pituitary enlargement in patients with intracranial hypotension syndrome. Neurology 55:1895–1897
Schievink WI, Maya MM, Moser FM (2004) Treatment of spontaneous intracranial hypotension with Percutaneous placement of a fibrin sealant: report of four cases. J Neurosurg 100:1098–1100
Liong WC, Constantinescu CS, Jaspan T (2006) Intrathecal gadoliniumenhanced magnetic resonance myelography in the detection of CSF leak. Neurology 67:1522
Mokri B The Monro–Kellie hypothesis: applications in CSF volume depletion. Neurology 56 (12): 1746–8
Schievink WI (2003) Misdiagnosis of spontaneous intracranial hypotension. Arch Neurol 60(12):1713–1718
Schievink WI, Louy C (2007) Precipitating factors of spontaneous spinal CSF leaks and intracranial hypotension. Neurology 69:700–702
Davenport RJ, Chataway SJ, Warlow CP (1995) Spontaneous intracranial hypotension from a CSF leak in a patient with Marfan’s syndrome. J Neurol Neurosurg Psychiatry 59:516–519
Erkulvrawatr S, El Gammal T, Hawkins J, Green JB, Srinivasan G (1979) Intrathoracic meningoceles and neurofibromatosis. Arch Neurol 36:557–559
Fattori R, Nienaber CA, Descovich B et al (1999) Importance of dural ectasia in phenotypic assessment of Marfan’s syndrome. Lancet 354:910–913
Schievink WI, Deline CR (2014) Headache secondary to intracranial hypotension. Curr Pain Headache Rep 18(11):457
Mokri B (2002) Intracranial hypertension after treatment of spontaneous cerebrospinal fluid leaks. Mayo Clin Proc 77:1241–1246
Kranz PG, Amrhein TJ, Gray L (2014) Rebound intracranial hypertension: a complication of epidural blood patching for intracranial hypotension. AJNR Am J Neuroradiol 35:1237–1240
Mokri B (2014) Radioisotope cisternography in spontaneous CSF leaks: interpretations and misinterpretations. Headache 54(8):1358–1368
Scott S, Davenport R (2014) Low pressure headaches caused by spontaneous intracranial hypotension. BMJ 349:g6219
Graff-Radford SB, Schievink WI (2014) High pressure headaches, low pressure syndromes and CSF leaks: diagnosis and management. Headache 54(2):394–401
Schievink WI, Louy C (2007) Precipitating factors of spontaneous spinal CSF leaks and intracranial hypotension. Neurology 69(7):700–702
Schievink WI, Dodick DW, Mokri B, Siberstein S, Bousser MG, Goadsby PJ (2011) Diagnostic criteria for headache due to spontaneous intracranial hypotension: a perspective. Headache 51:1442–1444
Ducros A, Biousse V (2015) Headache arising from idiopathic changes in CSF pressure. Lancet Neurol 14(6):655–668
Vanopdenbosch LJ, Dedeken P, Casselman JW, Vlaminck SA (2011) MRI with intrathecal gadolinium to detect a CSF leak: a prospective open-label cohort study. J Neurol Neurosurg Psychiatry 82(4):456–458
Hoxworth JM, Trentman TL, Kotsenas AL, Thielen KR, Nelson KD, Dodick DW (2012) The role of digital subtraction myelography in the diagnosis and localization of spontaneous spinal CSF leaks. AJR Am J Roentgenol 199(3):649–653
Schievink WI, Raissi SS (2012) Spontaneous intracranial hypotension in patients with bicuspid aortic valve. J Heart Valve Dis 21(6):714–717
Limaye K, Mahuwala Z, Lee RW (2015) Not everything that worsens on standing is intracranial hypotension! Acta Neurol Belg 115(3):481–483
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Source of Funding
None.
Ethical and Informed Consent
No identifiable patient information was used in the making of this review article.
Rights and permissions
About this article
Cite this article
Limaye, K., Samant, R. & Lee, R.W. Spontaneous intracranial hypotension: diagnosis to management. Acta Neurol Belg 116, 119–125 (2016). https://doi.org/10.1007/s13760-015-0577-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-015-0577-y