Abstract
Parallels between cancer and ecological systems have been increasingly recognized and extensively reviewed. However, a more unified framework of understanding cancer as an evolving dynamical system that undergoes a sequence of interconnected changes over time, from a dormant microtumor to disseminated metastatic disease, still needs to be developed. Here, we focus on several examples of such mechanisms, namely, how in cancer niche construction a metabolic adaptation and consequent change to the tumor microenvironment (niche modification) becomes an important factor in evasion of the predator (immune system), facilitating disease progression; how tumor establishment and propagation is driven by the tumor’s own keystone species, the cancer stem cells; and how the succession of stages of metastatic dissemination can be informed by ergodic theory and forest ecology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cancer as an Evolving Ecosystem
It has become increasingly recognized that there exist a number of parallels between the disease of cancer and ecological systems (see, for instance, Kareva 2011; Gillies et al. 2012; Thomas et al. 2013; Korolev et al. 2014; Yang et al. 2014; Arnal et al. 2015). Cancer cells within a tumor are genetically and phenotypically heterogeneous; they compete for space and nutrients, interact with predators (the immune system), migrate (metastases), all within the local microenvironment of the tissue, and more globally, within the ecosystem of the human body (Kareva 2011). Moreover, tumors themselves are largely composed not of the malignant cancer cells but of the surrounding supporting cells, such as fibroblasts, adipocytes, pericytes, and so on (Kareva et al. 2015) Recognition of the importance of engaging and creating the tumor microenvironment has been emphasized in the seminal paper “Hallmarks of Cancer: The Next Generation” by Hannahan and Weinberg (2011). What makes many tumors so difficult to eradicate is the fact that they engage normal surrounding cells in their microenvironment, the niche that they occupy, and modify it in such a way that gives them a selective advantage over healthy cells.
Within this framework, each individual is a separate evolutionary experiment, where tumors act like parasites that are looking to survive and maximize their fitness within the ecosystem of their host’s body. In most cases, as has been shown by autopsy studies performed on people who died of non-cancer-related causes, tumors remain dormant. For instance, dormant breast microtumors were found in 39 % of 40- to 50-year-old women who died of trauma, and only 1 % were ever diagnosed in this age range. Similarly, dormant prostate tumors were found in 46 % percent of 60- to 70-year-old men who died of trauma, but only 1–1.5 % of men were diagnosed with prostate cancer in their lifetime. The most striking example, however, is that of thyroid cancer, which was found to be present in a dormant state in over 98 % of 50- to 70-year-old people who died of trauma, and only 0.1 % were diagnosed in their lifetime in this age range (Feldman et al. 1986; Montie et al. 1989). This indicates that cancer is in fact a successful parasite that for the most part does not cause the death of its host. However, when dormant tumors start growing (and the numerous reasons for this phenomenon are still being investigated), they begin the process that leads to the tragedy of the commons (Hardin 1968), a situation whereby overly adapted individuals consume shared resources, since it increases their fitness at each moment of time. If the population depends for survival solely on the shared resource, such adaptive behavior leads to evolutionary suicide, analogous to the situation whereby a disseminated cancer eventually kills its host. In closer examination, in a system such as cancer, the success of the parasitic tumor lies not only in the intrinsic properties of cancer cells, such as increased rate of proliferation or decreased intrinsic death rates, but also in the changes that are imposed on the tumor microenvironment, the niche that the tumor occupies and eventually modifies through its metabolic activity. Therefore, the key to understanding many cancers as parasites is to recognize them as being evolving ecosystems, where ecosystem mechanisms lead to evolutionary suicide.
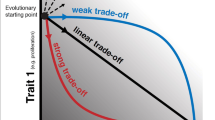
Dynamic Fitness Landscapes
In 1932 Sewall Wright introduced the notion of a fitness landscape, which can be represented as a map, on which the highest fitness (largest difference between birth and death rates) corresponds to the highest elevation. Within this construct the individuals are evolving in such a way as to “climb” the nearest peak, of which there can be just one (single peak, or simple landscape) or many (rugged landscape) (Page 2010). In an evolving complex system, such as cancer, one can think of microenvironmental changes that stem not only from external forces, such as competition for the resources, but also from internal forces, such as intraspecies competition, or cooperation, or other types of interactions, as making the fitness landscape on which the population evolves dynamic. This has been termed “dancing landscapes” (Page 2010) or seascapes (Mustonen and Lässig 2009) and refers to a situation where fitness peaks, whether single or multiple, can change over time. Because the environment in such a framework is dynamic, there exists a type of constant coadaptation between the individuals and their environment. This process underlies the ecological concept of niche construction, whereby organisms modify their microenvironments though their metabolism, activities, and choices (Odling-Smee et al. 1996). In cancer, growing tumors actively engage in metabolically driven modification of their microenvironment, which has important implications for tumor growth, progression, and disease dissemination.
Metabolic Adaptation and Evasion of the Predator (the Immune System)
Once a microtumor starts growing, it may eventually outgrow its blood and oxygen supply, leading to activation of oxygen-independent anaerobic metabolism, or glycolysis, as a normal physiological survival mechanism. Two important consequences can follow from this adaptation.
Firstly, a by-product of anaerobic metabolism is lactic acid, which can accumulate in the tumor microenvironment and lower the local pH to as low as 6.5 (Gatenby and Gillies 2004). Under normal physiological conditions, one can observe local acidosis either during exercise (hence the lactic acid buildup in the muscle tissue) or in the event of ruptured blood vessels, which interfere with normal blood flow and can create a hypoxic region and thus an acidic microenvironment downstream from the damaged vessel. A large amount of experimental evidence suggests that low pH can either interfere with activation of naive immune cells, or direct their activation away from the anti-tumor phenotype (Kareva and Hahnfeldt 2013). Under normal conditions, such a response could be warranted if the damage to the vessel were augmented by an overly activated immune system: its temporary downregulation, signaled through temporary local acidosis, would allow for reestablishment of the normal blood flow. However, since tumor cells continue producing lactic acid even in the areas of ample oxygen supply, a phenomenon known as the Warburg effect (Kim and Dang 2006), they continue mimicking a wound, and this downregulation of immune activation becomes more permanent.
Secondly, oxygen deprivation and turning to glycolysis as a primary mode of glucose metabolism results in upregulation of nutrient transporters, which in glycolytic cells can be upregulated as much as 30-fold (Ganapathy et al. 2009). This is a necessary adaptation, since glycolysis yields only two molecules of ATP, compared to up to 30 molecules of ATP produced during oxidative phosphorylation (Berg et al. 2002). However, cytotoxic cells of the immune system also use glycolysis as a primary mode of glucose metabolism, and also upregulate their nutrient transporters 20- to 40-fold (Greiner et al. 1994; Fox et al. 2005). Moreover, cytotoxic immune cells are incapable of killing tumor cells in a state of nutrient deprivation (MacIver et al. 2008). Therefore, in the local microenvironment of the tumor, one can expect to observe competition for a shared resource (glucose) between the predator (immune cells) and the prey (cancer cells), which both have the same adaptation—upregulated nutrient transporters. Should the prey be successful at outcompeting the predator for the shared resource, it will be able to escape and progress further to a malignant disease (Kareva and Berezovskaya 2015). The presence of theoretically predicted metabolic competition in the tumor microenvironment between cancer and immune cells has recently been confirmed experimentally by Chang et al. (2015).
This example demonstrates an important aspect of niche modification in the context of a tumor. Reversion to anaerobic glycolysis is an adaptation necessary for immediate survival, but its by-products (low pH and increased competitive ability of the cancer cells) become crucial in how the tumor can now interact with its natural predator. Notably, in cancer biology there exist notions of driver and passenger mutations (Stratton et al. 2009). A driver mutation gives direct selective advantage to a cell clone, increasing the carrier’s fitness in its microenvironment. A passenger mutation, also known as a hitchhiker mutation, has no direct effect on the fitness of a cell clone but is passed along because it occurs in the same genome with the driver mutation. Here, we observe “driver” and “passenger” processes, where consequences of the “passenger process”—anaerobic metabolism as a means for immediate survival but not as a direct means of increasing the cells’ fitness—lead to the “driver process,” evasion from the immune system, which allow for further tumor growth, taking the tumor one step closer to evolutionary suicide. It is likely that there exist a number of other mechanisms that start as a secondary adaptation and become driver processes, which would be able to provide further insights into dynamical processes that may underlie cancer progression and tumor dissemination. Within the framework of cancer as an ecosystem, these evolutionary adaptations advance successful tumors one step closer to evolutionary suicide.
Cancer Stem Cells as Keystone Species
The concept of “keystone species” designates species that can exert an effect on ecosystem functionality that is disproportionate to the keystone species’ abundance or biomass (Paine 1969). The term was derived in analogy to a keystone in an arch, which is under the least pressure compared to other arch stones, but whose removal would result in the arch’s collapse. In the classic formulation, there exist several classes of keystone species, which perform their function through predation (e.g., wolves that caused a cascade of ecological changes in Yellowstone (Fortin et al. 2005)), through facilitating pollination (e.g., as southern cassowary, flightless birds that play a large role in seed dispersal (Gosper et al. 2005)), or through directly modifying their environment (e.g., beavers). In 2003, Davic (2003) proposed a further subdivision of the concept to distinguish keystone species from other strongly interacting species in the ecosystems. These include “keystone species” that regulate species diversity, “key species” that regulate energy and nutrient dynamics, “intraguild competitors and predators,” which structure niche partitioning, and “ecosystem engineers,” which modulate physical habitat. Determining which species are keystone in a particular population is important in conservation biology to help plan system management efforts. It is proposed here that the Davic concept of “keystone species” may be applicable to cancer stem cells, and could potentially be of equal importance in “reverse conservation” of tumor biology.
Cancer stem cells (CSCs), or tumor-initiating cells, are a subpopulation of cells within a tumor that have limitless replicative potential, capacity for self-renewal and the ability to seed new tumors (Al-Hajj et al. 2003; Yu et al. 2012). They are more resistant to most therapies and are believed to be the drivers of tumor progression (Magee et al. 2012; Yu et al. 2012). The proportion of cancer stem cells within a tumor appears to vary depending on the tissue of tumor origin (Enderling and Hahnfeldt 2011; Enderling 2015). A cancer stem cell can divide symmetrically or asymmetrically (Morrison and Kimble 2006; Yamashita et al. 2007), producing either stem cells or non-stem cells at various stages of differentiation, thus increasing the diversity of the tumor. This property of cancer stem cells, their importance for survival and propagation of the tumor, which is disproportionate to their relative abundance in the cell population, makes them the keystone species of the tumor (Bond 1994; Mouquet et al. 2013).
If cancer stem cells are the keystone species of the tumor population, then understanding the approaches that this specific subpopulation uses to maximize its fitness and evade predation are particularly important. (Notably, such approaches may be used by other cell types as well, but their success for CSCs would be particularly important.) One such approach may be connected with the niche-modification construct described above. As mentioned above, a cancer stem cell can divide symmetrically or asymmetrically (Morrison and Kimble 2006; Yamashita et al. 2007), producing either two stem cells or a stem cell and a non-stem cell. If a CSC divides symmetrically, then both the parent and the offspring are equally susceptible to predation by the immune cells. However, if it divides asymmetrically, the resulting offspring can populate the tumor, become oxygen deprived, revert to glycolysis as a primary mode of glucose metabolism, and thus incapacitate the immune predator in accordance with the scenario described above.
Therefore, in the presence of a local predator the CSC will maximize its fitness if in a fraction of times it will divide asymmetrically, creating a shield of offspring that will protect it from the predator. The implications of this hypothesis were explored in Kareva (2015) using an agent-based model, which has revealed that the proportion of cancer stem cells to non-stem cancer cells (or in other terms, of the keystone species of this community to the rest of the species) is determined by the strength of the predation. If the predation is very high, the CSCs cannot form a sufficiently large shield of differentiated offspring. If the predation is very low, the offspring outcompete the parents. This construct provides one potential explanation for differences in relative proportions of tumor-initiating cancer stem cells to non-stem cancer cells in different tissues and in different individuals, as well as within the same individual at different points of their life. It is also another adaptive mechanism whereby a successful tumor through adaptations that maximize the fitness of the tumor-propagating cells advances towards eventually destroying its environment and thus committing evolutionary suicide.
Ecological Succession, Ergodic Theory, and Connections to Cancer Stages
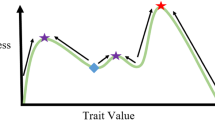
One can also think about the way that cancer colonizes both its primary and secondary (metastases) habitats from the point of view of ecological succession. Ecological succession (Clements 1916; Luken 1990; Prach and Walker 2011) refers to a process whereby an ecological community undergoes a series of more or less predictable steps following initial colonization of a habitat (establishment of a primary tumor or secondary metastatic tumor), or following a disturbance of the original habitat (tissue, as well as the whole body, after treatment, such as surgery, chemo- or radiation therapy). In forest succession, opportunistic species disseminate large quantities of seeds, very few of which manage to colonize distant areas. Once they become established, however, they may form a closed canopy, which is dense enough that the tree crowns preclude direct sunlight from reaching the ground, thereby putting the trees’ own seedlings at a competitive disadvantage. This provides an opportunity for shade-tolerant species to replace them. In the absence of disturbance, the ecosystem reaches an equilibrium state, known as “climax” (Clements 1916; Luken 1990).
A similar process occurs during tumor dissemination. It has been observed that in cancer metastases, only a small fraction of cells from the primary tumor are successful in establishing lesions (Luzzi et al. 1998; Cameron et al. 2000; Chaffer and Weinberg 2011). It is highly likely that the cells that are capable of successfully colonizing distant tissues are cancer stem cells (Chaffer and Weinberg 2011).
It is also possible that there exists a sequence of predictable processes, such as the one described above, that ensures establishment of the metastatic colony in the tissues, similarly to the process of ecological succession. The equilibrium “climax” stage, however, does not become established, since metastases are responsible for over 90 % of cancer-associated mortality. Nevertheless, perhaps understanding metastatic dissemination from the point of view of ecological succession can provide some insights.
Ergodic theory is mostly known through its applications in statistical physics; however, it has also been applied in forest ecology when studying ecological succession (Molchanov 1992; Karev 2006). According to ergodic theory, the climax state of a system that has undergone succession represents its spatially developed time history. And even more importantly, in an ergodic system the areas occupied by the population in each of the succession stages are approximately proportional to the expected development time of that stage (Karev 2006). That is, in terms of forest succession ecology, if one observes k different succession stages of a forest ecosystem in its climax state and sees that A i percent of the forest ecosystem territory is in the i-th particular stage, one can make the conclusion—given that conditions for ergodicity are satisfied—that the forest ecosystem will spend A i percent of time in this state. This provides a method for estimation of duration of the development time of each succession stage. This approach is particularly useful because the direct estimation may be impossible due to the duration of succession stages, which for forests may take tens or even hundreds of years.
Conversely, if the development time of each stage of a succession system is known, then one can estimate the percent of a whole territory that will be occupied by each stage in a stationary state of the system.
This can have important implications if the “ergodic hypothesis” holds for cancer (see Fig. 1). If, for instance, it is known how long a person spends in each cancer stage for his or her particular cancer type, one can then estimate how many people should be in this stage out of all cancer patients with this particular cancer type or stage. This hypothesis has the potential for very influential epidemiological applications and warrants further investigation.
If the ergodic hypothesis holds for a particular cancer type in a particular population subgroup, then one should expect points (Ti, xi), i = 1,…,4, to be on the same line. If an outlier, such as (T*, x*) is observed, it suggests that observations are incomplete and either there are likely to be undiagnosed patients in the population, or the duration of the stage has been overestimated, and the point should be moved leftward until it intersects the line. If an outlier such as (T**, x**) is observed, then either there have been false positive diagnoses, or the duration of the stage has been underestimated, and the point should be moved rightward until it intersects the line
Ecologically Motivated Cancer Treatment
The ideas discussed here provide a more unified understanding of cancer as an evolving ecological dynamical system. Different cell phenotypes or different clones have adaptations that may increase or decrease their fitness at different stages of tumor development and dissemination. In the framework of “dancing fitness landscapes” (Page 2010), or “seascapes” (Mustonen and Lässig 2009, 2010), this occurs due to the fact that fitness peaks “move” as a result of metabolically induced niche modifications. When the tumor becomes established, different clones might have different selective advantages at different stages of tumor development, as the environment and population composition change as a result of tumor-environment coevolution (Hanahan and Weinberg 2011; Kareva 2011). Furthermore, at the level of tumor dissemination and establishment of metastases, cell clones that are most successful during initial colonization of the tissue might not be the ones that have the highest fitness advantage later on, as the secondary tumor gets established and starts further modifying its environment. This understanding is important because it suggests that both different clones and different microenvironmental adaptations need to be targeted at different stages of tumor development and disease progression, supporting the growing need for personalized therapy.
A number of modifications to treatment options have been proposed to account for the ecological properties of the cancer disease. The primary change in treatment approaches has been to move away from attempts at eradication, which mostly lead to selection for resistant clones, and towards making cancer a chronic disease, much like has been done with AIDS. Gatenby (2009) has made a comparison of cancer treatment to the use of pesticides, which are most effective when used judiciously, keeping the pest population at a manageable level. Experimental mouse models suggest that this can indeed be a more successful treatment strategy (Gatenby et al. 2009). Using pH buffer therapy has also been suggested to slow down development of aggressive tumors (Robertson-Tessi et al. 2015).
Targeting the environment on which cancer cells depend for survival, through changing the timing and dosage of therapy administration, known as metronomic chemotherapy (Kareva et al. 2015), has also shown promise. The success of this treatment approach is connected to recognizing that cancer is a complex ecological system and not a conglomeration of individual malignant cells that are disengaged from their environment. Only through incorporating our knowledge and understanding of managing other ecosystems might we be able to successfully manage cancer.
References
Al-Hajj M, Wicha MS, Benito-Hernandez A et al (2003) Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 100:3983–3988
Arnal A, Ujvari B, Crespi B et al (2015) Evolutionary perspective of cancer: myth, metaphors, and reality. Evol Appl 8:541–544
Berg JM, Tymoczko JL, Stryer L (2002) The regulation of cellular respiration is governed primarily by the need for ATP. In: Biochemistry, 5th edn. WH Freeman, New York
Bond WJ (1994) Keystone species. In: Shultze E-D, Mooney HA (eds) Biodiversity and ecosystem function. Springer, Berlin, pp 237–253
Cameron MD, Schmidt EE, Kerkvliet N et al (2000) Temporal progression of metastasis in lung: cell survival, dormancy, and location dependence of metastatic inefficiency. Cancer Res 60(9):2541–2546
Chaffer CL, Weinberg RA (2011) A perspective on cancer cell metastasis. Science 331:1559–1564
Chang CH, Qiu J, O'Sullivan D et al (2015) Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 162(6):1229–1241
Clements FE (1916) Plant succession: an analysis of the development of vegetation (No. 242). Carnegie Institution of Washington, Washington, DC
Davic RD (2003) Linking keystone species and functional groups: a new operational definition of the keystone species concept. Conserv Ecol 7(1):r11
Enderling H (2015) Cancer stem cells: small subpopulation or evolving fraction? Integr Biol 7(1):14–23
Enderling H, Hahnfeldt P (2011) Cancer stem cells in solid tumors: is ‘evading apoptosis’ a hallmark of cancer? Prog Biophys Mol Biol 106(2):391–399
Feldman AR, Kessler L, Myers MH, Naughton MD (1986) The prevalence of cancer. N Engl J Med 315(22):1394–1397
Fortin D, Beyer HL, Boyce MS et al (2005) Wolves influence elk movements: behavior shapes a trophic cascade in Yellowstone National Park. Ecology 86(5):1320–1330
Fox CJ, Hammerman PS, Thompson CB (2005) Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol 5(11):844–852
Ganapathy V, Thangaraju M, Prasad PD (2009) Nutrient transporters in cancer: relevance to Warburg hypothesis and beyond. Pharmacol Ther 121(1):29–40
Gatenby RA (2009) A change of strategy in the war on cancer. Nature 459:508–509
Gatenby RA, Gillies RJ (2004) Why do cancers have high aerobic glycolysis? Nat Rev Cancer 4(11):891–899
Gatenby RA, Silva AS, Gillies RJ, Frieden BR (2009) Adaptive therapy. Cancer Res 69(11):4894–4903
Gillies RJ, Verduzco D, Gatenby RA (2012) Evolutionary dynamics unifies carcinogenesis and cancer therapy. Nat Rev Cancer 12(7):487–493. doi:10.1038/nrc3298
Gosper CR, Stansbury CD, Vivian-Smith G (2005) Seed dispersal of fleshy-fruited invasive plants by birds: contributing factors and management options. Divers Distrib 11(6):549–558
Greiner EF, Guppy M, Brand K (1994) Glucose is essential for proliferation and the glycolytic enzyme induction that provokes a transition to glycolytic energy production. J Biol Chem 269(50):31484–31490
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674
Hardin G (1968) The tragedy of the commons. Science 162:1243–1248
Karev GP (2006) Ergodic properties of steady state forest communitites. In: Burk AR (ed) New developments in ecology research. Nova Publishers, Hauppauge, pp 41–42
Kareva I (2011) What can ecology teach us about cancer? Trans Oncol 4(5):266–270
Kareva I (2015) Immune evasion through competitive inhibition: the shielding effect of cancer non-stem cells. J Theor Biol 364:40–48
Kareva I, Berezovskaya F (2015) Cancer immunoediting: a process driven by metabolic competition as a predator–prey–shared resource type model. J Theor Biol 380:463–472
Kareva I, Hahnfeldt P (2013) The emerging “hallmarks” of metabolic reprogramming and immune evasion: distinct or linked? Cancer Res 73(9):2737–2742
Kareva I, Waxman DJ, Klement GL (2015) Metronomic chemotherapy: an attractive alternative to maximum tolerated dose therapy that can activate anti-tumor immunity and minimize therapeutic resistance. Cancer Lett 358(2):100–106
Kim JW, Dang CV (2006) Cancer’s molecular sweet tooth and the Warburg effect. Cancer Res 66(18):8927–8930
Korolev KS, Xavier JB, Gore J (2014) Turning ecology and evolution against cancer. Nat Rev Cancer 14(5):371–380
Luken JO (1990) Directing ecological succession. Springer, Dordrecht
Luzzi KJ, MacDonald IC, Schmidt EE et al (1998) Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am J Pathol 153(3):865–873
MacIver NJ, Jacobs SR, Wieman HL et al (2008) Glucose metabolism in lymphocytes is a regulated process with significant effects on immune cell function and survival. J Leukoc Biol 84(4):949–957
Magee JA, Piskounova E, Morrison SJ (2012) Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer Cell 21(3):283–296
Molchanov AM (1992) Nonlinear biology. Puschino, pp 62–63 (in Russian)
Montie JE, Wood DP, Pontes JE et al (1989) Adenocarcinoma of the prostate in cystoprostatectomy specimens removed for bladder cancer. Cancer 63(2):381–385
Morrison SJ, Kimble J (2006) Asymmetric and symmetric stem-cell divisions in development and cancer. Nature 441:1068–1074
Mouquet N, Gravel D, Massol F, Calcagno V (2013) Extending the concept of keystone species to communities and ecosystems. Ecol Lett 16(1):1–8
Mustonen V, Lässig M (2009) From fitness landscapes to seascapes: non-equilibrium dynamics of selection and adaptation. Trends Genet 25(3):111–119
Mustonen V, Lässig M (2010) Fitness flux and ubiquity of adaptive evolution. Proc Natl Acad Sci USA 107:4248–4253
Odling-Smee FJ, Laland KN, Feldman MW (1996) Niche construction. Am Nat 147(4):641–648
Page SE (2010) Diversity and complexity. Princeton University Press, Princeton
Paine RT (1969) A note on trophic complexity and community stability. Am Nat 103(929):91–93
Prach K, Walker LR (2011) Four opportunities for studies of ecological succession. Trends Ecol Evol 26(3):119–123
Robertson-Tessi M, Gillies RJ, Gatenby RA, Anderson AR (2015) Impact of metabolic heterogeneity on tumor growth, invasion, and treatment outcomes. Cancer Res 75(8):1567–1579
Stratton MR, Campbell PJ, Futreal PA (2009) The cancer genome. Nature 458:719–724
Thomas F, Fisher D, Fort P et al (2013) Applying ecological and evolutionary theory to cancer: a long and winding road. Evol Appl 6(1):1–10
Yamashita YM, Mahowald AP, Perlin JR, Fuller MT (2007) Asymmetric inheritance of mother versus daughter centrosome in stem cell division. Science 315:518–521
Yang KR, Mooney SM, Zarif JC et al (2014) Niche inheritance: a cooperative pathway to enhance cancer cell fitness through ecosystem engineering. J Cell Biochem 115(9):1478–1485
Yu Z, Pestell TG, Lisanti MP, Pestell RG (2012) Cancer stem cells. Int J Biochem Cell Biol 44(12):2144–2151
Acknowledgments
This research was partially supported by NIH/NIGMS R01 GM093050-01A1 (to Giannoula Lakka Klement).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kareva, I. Cancer Ecology: Niche Construction, Keystone Species, Ecological Succession, and Ergodic Theory. Biol Theory 10, 283–288 (2015). https://doi.org/10.1007/s13752-015-0226-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13752-015-0226-y