Abstract
The growing interest and major advances of the last decades in evolutionary developmental biology (EvoDevo) have led to the recognition of the incompleteness of the Modern Synthesis of evolutionary theory. Here we discuss how paleontology makes significant contributions to integrate evolution and development. First, extinct organisms often inform us about developmental processes by showing a combination of features unrecorded in living species. We illustrate this point using the vertebrate fossil record and studies relating bone ossification to life history traits. Second, we discuss exceptionally preserved fossils that document rare ontogenetic sequences and illustrate this case with the patterns of heterochrony observed in Cambrian crustacean larvae preserved three-dimensionally. Third, most fossils potentially document the evolutionary patterns of allometry and modularity, as well as some of the (paleo)ecological factors that had influenced them. The temporal persistence of adaptive patterns in rodent evolution serves to address the importance of ecological constraints in evolution. Fourth, we discuss how the macroevolutionary patterns observed in the tetrapod limb, in the mammal molar proportions, and in the molluscan shell provide independent tests of the validity of morphogenetic models proposed on living species. Reciprocally, these macroevolutionary patterns often act as a source of inspiration to investigate the underlying rules of development, because, at the end, they are the patterns that the neo-Darwinian theory was unable to account for.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most of evolution has happened in geological time, so if we wish to understand the origin of organismal diversity, it would be paramount to examine the contributions that fossils can make to this task (Raff 2007; Sánchez-Villagra 2012). The study of several morphological transformations in vertebrate evolution, such as those concerning the origin of the tetrapod limb (Shubin et al. 2009), the mammalian middle ear (Luo 2007; Luo et al. 2007) and the turtle shell (Scheyer et al. 2012) provide examples of the illuminating integration of embryological with paleontological data. Fossils serve to date the tree of life and provide estimates on when and under which paleoecological circumstances new developmental programs arose in evolution (Peterson et al. 2007; Schoch 2009). On the other hand, the more direct role that paleontology can play in studies of development is far from obvious. Fossils are incomplete and no experiments can be conducted with them. What, then, is the place of paleontology in the larger context of studies of morphology and its developmental origin? This is a large question, and to address it some fundamental historical developments about current evolutionary theory are here first summarized.
Gene-centric conceptualization characterized the Modern Synthesis (MS) that surged in the 1930s and 1940s. The paleontologist George Gaylord Simpson, considered one of the “architects” of the MS, assumed that microevolutionary data can be simply extrapolated to explain macroevolutionary patterns (Simpson 1944). The importance of processes above population-level was largely disregarded, and with time several authors raised the issue (e.g., Rensch 1959; Eldredge and Cracraft 1980; Gould 1980a; Vrba 1983; Erwin 2000; Leroi 2000; Grantham 2007). Population-genetic approaches (Charlesworth et al. 1982) face difficulties when addressing long-term patterns of evolution because they are unable to determine and account for the frequency and intensity of mass extinctions, and the large-scale modes and rates of evolution (e.g., Hunt 2007; Jablonski 2007, 2010). The growing interest and major developments of the last decades in evolutionary developmental biology (EvoDevo) have also led to the recognition of the incompleteness of the MS (Gould 1977; Alberch 1989). Some authors argued for an “extension” of the MS to incorporate development (Goodwin 1988; Gilbert et al. 1996; Pigliucci and Müller 2010). Central here has been the critique to an adaptationist program as it surged from the MS (Gould and Lewontin 1979), and the growing importance of the concept of constraints in its different forms (Alberch 1980, 1989; Smith et al. 1985; Oster et al. 1988; see Urdy and Chirat 2006 for a review). The approach of Konstruktionsmorphologie and the triad of factors involved in evolution advocated by Seilacher (1970) already contained the ideas of constraint that became so dominant in the English-speaking literature in later years.
During the time of the MS, researchers outside Anglo-American circles also developed approaches that took into account morphology and the developmental origin of organic form (Olsson et al. 2010). For example, the works of Alexei Nikolaevich Severtsov were influential to other authors around the time of the MS (Rensch 1959; Schmalhausen 1949). Most notably, Severtsov (1912) detailed reasons why he thought morphology was of key importance among the evolutionary sciences. These centered upon how evolutionary theory could not be independent of morphology (see Adams 1980 for review). One argument Severtsov put forth was that without knowing of the changes that have occurred in life’s history (without examining the fossil record), it is impossible to formulate a theory of how such changes might have happened. Essentially, paleontological data, in a unique manner, represent, “all we know directly about the actual course of life’s history” (Gould 1980b, p. 153), and thus define the patterns to be explained.
In the Anglo-American discourse of the late 1970s and early 80s embryology and morphology were reconnected to evolutionary questions (Gould 1977; Alberch 1980; Raff and Kaufman 1983; review by Gilbert 2003). Some authors repeatedly argued for the search of general rules of development advocating that at least some amount of biological order was caused by the dynamics of development (Gould and Lewontin 1979; Alberch 1980, 1989; Webster and Goodwin 1982; Alberch and Gale 1985; Goodwin 1988; Oster et al. 1988; Kauffman 1993). The debate concentrated mainly on the nature and clarification of the concept of constraints (Alberch 1980; Smith et al. 1985) and the specific role that one has to attribute to natural selection and development, respectively, to account for the origin of order (Kauffman 1993). But the debate on constraints also produced some ambiguity, given the different conceptions of constraint (Amundson 1994). One relevant point, raised by Salazar-Ciudad (2006), is that the selection/developmental constraints debate relies on two different assumptions about the relationship between genotype and phenotype (linear/non-linear) and what kind of morphological variation is produced by development (gradual/discrete, unbounded/limited).
Developmental Biases in Evolution
Historically, the concept of developmental constraints was cited to argue against the view that without selection, phenotypic variation would be random (e.g., Alberch 1980). Putting aside the complications in defining “random phenotypic variation” (Eble 1999), it appeared that in practice, the MS assumed that variation was gradual and “in every direction.” This view has been much debated since the late 1970s under the umbrella of “developmental constraints,” to bring about the notion that, as all morphologies are constrained by the rules of chemistry, physics, and geometry (Thompson 1917), development is a source of order structuring variation into discrete phenotypes. The dynamical interactions of various developmental factors then set out the possibilities for variation of morphologies during development and evolution. For instance, it has been argued that the relative level of conservation of body plans could be an expression of the limited possibilities of development rather than an expression of adaptation by natural selection (Hall 1996). Likewise, the broad distribution of homoplasies could also be due to developmental constraints (Wake 1991).
The term “constraints” has also been used in a somehow different meaning than discussed above, as in the mathematical constraints that correspond to the initial and boundary conditions of the system under study. In mathematical models, the behaviors of developmental systems are described, characterized, and predicted thanks to the rules of interaction between molecules, proteins, cells, and/or tissues under a particular set of constraints and initial boundary conditions. Without such constraints, the behavior of a system cannot be predicted. In this view, constraints are given a decisive and “creative” role (Urdy 2012).
When critics of the MS pointed out that not every kind of variation is developmentally possible (first sense), they were arguing that development was limiting the range of possible variation on one side, and that development was creative on the other side (second sense). In this way, the first and second meanings of constraints partially overlap, the first building extensively on the second. Probably because of this ambiguity, it has been advocated that this term would be best replaced by “developmental bias” (Arthur 2004).
Independent of the operational issues related to constraints and developmental biases, it is currently agreed that some phenotypes are possible and extremely probable whereas others are unlikely or even impossible. The morphological space occupied by a clade of organisms is then a reflection of the robustness of development on one side and a reflection of its developmental plasticity on the other side (Kaneko 2011). Looking at the space occupied by a clade considering only extant species can be misleading since fossils often record a vast number of phenotypes that do not exist anymore. As Wilson (2013) stated, “the vast record of geological time provides the richest account of what is possible to do.” Modern molecular EvoDevo methods will not inform us about the development of phenotypes that no longer exist, and a theory of evolution that does not account for those cannot be complete.
Unique Patterns of Life History Revealed by the Vertebrate Fossil Record
Adult phenotypes of extinct organisms can inform us about developmental processes by showing a combination of features or levels of integration unrecorded in living species. Fossils chronicle how phenotypic evolution progressed and the (paleo)ecological factors that had shaped them. These phenotypic character transformations serve to confirm the constraint hypotheses of developmental evolution based on extant forms, or expand the range of morphospace that a clade can occupy. Without inspecting this evidence, the subject matter of developmental studies remains incomplete (Wagner and Larsson 2003). The study of vertebrate fossilized ontogenies is mostly restricted to postnatal and late stages of growth, but nevertheless can deliver great insights into life history and evolutionary mechanisms affecting development in general. The following examples serve to illustrate this point.
Island Mammals’ Metabolism and Bone Growth
Paleohistology, the fastest growing area of research in developmental paleontology of vertebrates, can address many aspects of postnatal/post-hatching life history. It can serve to estimate the age of sexual maturity and of death, to understand activity cycles and reproductive cycles as well as to decipher growth patterns (e.g., Chinsamy-Turan 2005; Sander and Klein 2005; Cubo et al. 2011). Among the main features studied by paleohistologists are the “lines of arrested growth” (LAGs), which mark the cessation of appositional bone growth. Endogenous and exogenous conditions most likely trigger the formation of these growth marks, and their cyclical appearance, probably coupled with seasonal changes in the environment, is used to estimate the age of fossil individuals at the time of their death. It has been generally assumed that this feature is “erased” in animals with a high metabolic rate and an associated high degree of bone remodeling. Whereas LAGs are commonly recorded and studied in reptiles, mammals are not expected to have them or rather have them mostly obliterated during growth. However, a recent report of a dwarf bovid from the Pleistocene of Mallorca, Myotragus balearicus, indicated the presence of numerous LAGs in adult individuals. This finding, coupled with a low degree of bone resorption and remodeling, suggest that Myotragus had a crocodile-like mode of growth and physiology unrecorded among living species (Köhler and Moyá-Solá 2009). Current work on other island mammal dwarf forms, including the deer Candiacervus from the Pleistocene of Crete, shows that the bone specializations in Myotragus are not universal for other island mammals (Kolb et al. 2011).
Skeletal Formation in Placodont Reptiles
Placodontia is a group of armored marine reptiles restricted to the Triassic period and part of the sauropterygian radiation that also consists of plesiosaurs among other forms. All placodont species possess dermal armor plates, some building a single row dorsal to the spine, others superficially resembling turtles in forming an armor shell. In a study of bone microstructures in this group, Scheyer (2007) discovered the unique presence of cartilaginous tissue in some postcranial armor plates of placodonts. The developmental pathways leading to the “postcranial fibro-cartilaginous bone” tissue of placodont armor plates is unique among tetrapods, in which otherwise osteoderms develop intra-membraneously or through metaplastic ossification without cartilaginous preformation. Placodonts were aquatic, and as other groups living in this kind of environment, they possessed pachyostotic limb bones (e.g., de Ricqlès and de Buffrénil 2001). Scheyer (2007) interpreted the unique presence of compact “postcranial fibro-cartilaginous bone” as an osteosclerotic trend in the armor plates, which, as in the limbs, aided in buoyancy control, affecting manoeuvrability and swimming speed. The paleoecological context of placodont evolution in which the skeletogenetic innovation arose is well known (Scheyer et al. 2011).
The Marine Reptiles’ Case: Iguanas and Pachypleurosaurs
High bone compactness has evolved in several lineages of land vertebrates that have secondarily and independently adopted an aquatic lifestyle, related to the need for buoyancy control. Hugi et al. (2011) provided an example of how in some extinct marine reptiles bone compactness developed in a different way than in other, analogous marine reptiles. The groups in question are the pachypleurosaurs (extinct sauropterygians from the Triassic), and the marine iguana. In pachypleurosaurs from the Triassic of Monte San Giorgio in Switzerland, a medullary cavity never forms, and high bone compactness develops by adding layers of compact bone around a mineralized cartilaginous center (Hugi et al. 2011). In the iguana, in contrast, a medullary cavity is visible, and a higher bone compactness is achieved only by increased periosteal ossification (Hugi and Sánchez-Villagra 2012). The comparison of the living with the fossil provides an example of the flexibility of variation of developmental mechanisms.
Body Size and Life History
A seemingly simple feature that fossils document with large implications for life history evolution is body size. In the fossil record, the maximum body size appears to be much larger than one may have predicted from the living species, such as 73-ton sauropods, half-a-ton rodents, and 3-ton diprotodontian marsupials (Geiger et al. in press; Mazzetta et al. 2004; Wroe et al. 2004). This apparently simple variable is correlated with many life history variables. To attain those extreme sizes, growth could have occurred over a long period of time or have been accelerated. This matter has been examined in many groups of land vertebrates, with a main focus on dinosaurs. Scheyer et al. (2010) recently presented a summary of these studies (see also Erickson 2005). Living ectotherm reptilians like crocodylians and turtles display lower growth rates but prolonged time spans of continued growth between sexual and skeletal maturity, as well as extended life spans afterwards in which growth virtually ceases. With few exceptions (i.e., elephants), mammals reach sexual maturity shortly after reaching full adult size, and life spans are usually shorter. Birds reach full adult size extremely fast with extremely high growth rates, and determinate growth coupled to high metabolic rates is also characteristic of this growth pattern (Scheyer et al. 2010). Like other reptile groups (e.g., crocodylians or turtles), dinosaurs also exhibited continued growth until skeletal maturity, and extended life spans afterwards. By having high growth rates, non-avian dinosaurs could achieve giant sizes, as seen in large theropods or in the gigantic sauropods. Non-avian dinosaurs may thus have had a life history influenced by “increased physiological demands and/or predation exposure associated with reproduction” (Erickson et al. 2009, p. 1514).
Vertebral Numbers in Amniotes
The vertebral number in each region of the axial skeleton, easily recorded in fossils of adult individuals, provides indirect information about somitogenesis and Hox-gene expression boundaries (Thewissen et al. 2012). The number of somites has a one-to-one correspondence with that of segments in the axial skeleton, as two somite-halves are involved in the development of each vertebra (Head and Polly 2007). Another coupling is that of the boundaries among regions of the vertebral column—including cervical, thoracic, and lumbar anterior to the sacrum—and the expression domains of some Hox-genes, which then determine the morphological identity of the sections of the body (Wellik and Capecchi 2003). With this background, Müller et al. (2010) examined presacral vertebral counts across extant and extinct amniotes and mapped them onto phylogeny. They used the relationship between presacral and cervical numbers to infer the relative influence of homeotic effects and meristic changes, and found no correlation between somitogenesis and Hox-mediated regionalisation. Furthermore, they reconstructed ancestral states of major clades in amniote evolution using squared-change parsimony, thus tracing the evolution of segmentation and regionalization in that clade. The mammalian and the reptilian lineages show early in their evolutionary histories clear divergences in axial developmental plasticity, with basal stem mammals (the early synapsid lineage) sharing the conserved axial configuration of crown mammals, and basal reptiles exhibiting the plasticity of extant taxa. These results contradicted the hypothesis that a developmental constraint involving high metabolism is characteristic of mammals (see also Hautier et al. 2010), as the stem forms with a reptilian physiology already were conservative (Sánchez-Villagra 2010). Müller et al. (2010) also found that whereas conservatism in presacral numbers characterized early synapsid lineages, in some cases reptiles and synapsids exhibit the same developmental innovations in response to similar selective pressures. Conversely, increases in body mass are not coupled with meristic or homeotic changes, but mostly occur in concert with postembryonic somatic growth.
This examination of segmentation using the fossil record is preceded by a series of elegant works examining that in trilobites. In that group, a major discovery has been the increased canalization or lack of plasticity during trilobite evolution and how the diminution of regionalization led to more plasticity in some clades (Hughes et al. 2006), the latter a phenomenon also recorded in amniotes (Müller et al. 2010).
Exceptional Preservation of Mineralized Fossils and Soft Parts
Exceptional fossils of rarely preserved stages can resolve issues of species identification and document evolutionary changes in reproductive modes. In the case of vertebrates, a comprehensive survey of around 1,600 references on fossilized ontogenies (www.developmental-palaeontology.net) served to identify topics and taxa which are the subject of much investigation and others with much potential for research. There are extensive records of embryos or juveniles for “fish” (Cloutier 2010), amphibians (Fröbisch et al. 2010), reptiles (Delfino and Sánchez-Villagra 2010), synapsids (Sánchez-Villagra 2010), and hominins (Zollikofer and Ponce de Leόn 2010). In the latter case, further insights may be revealed through the study of dental development to reconstruct growth and the timing of developmental milestones over the course of hominin evolution (e.g., Humphrey et al. 2008; Dean 2010; Humphrey 2010). Advances in non-invasive imaging methods have started to allow the extraction of previously inaccessible data (Tafforeau et al. 2006). This is particularly true for very tiny specimens, such as fossilized embryos from the Neoproterozoic Doushantou Formation of China, which have been examined with the aid of synchrotron microtomography (e.g., Donoghue et al. 2006a, b). Such methods have also allowed the reconstruction of the movements of the oro-pharyngeal elements of conodonts operated during feeding, lending strong support to their interpretation as vertebrates (Goudemand et al. 2011). The origin of jaws can best be understood by comparing fossil and living jawless vertebrates, using insights from developmental studies and from the evolutionary record (Kuratani 2012).
Mineralized Skeletons and Biomineralization Modes
Ontogenetic information on fossil “invertebrates” is mostly restricted to animals with a hard or mineralized exoskeleton (e.g., Klug 2001; Nützel et al. 2007; Sumrall and Wray 2007; De Baets et al. 2012; Korn 2012). These exoskeletons usually preserve a record of ontogeny due to their accretionary growth.
Biomineralization has evolved many times independently in many clades of animals and plants, as also documented paleontologically (Murdock and Donoghue 2011). The study of the geochemical properties of fossils has revealed unsuspected capabilities to change a biomineralization system. However, developmental plasticity among groups varies. This must have affected past patterns of evolution and may potentially affect the future of shelled organisms in marine ecosystems, as exemplified by corals, which are among the most prolific biomineralizing organisms. Their case is of particular interest nowadays, as the increased atmospheric CO2 levels increase the acidity of sea water, leading to their decalcification (Anthony et al. 2008). What is the fate of “naked” corals in evolutionary terms? Can they resume calcification if atmospheric CO2 levels decrease? Looking at the geological past provides examples of several responses to environmental changes in biomineralizing organisms, including corals. The first corals to appear in the Middle Triassic—after the major extinction event at the end of the Permian—were scleractinian corals (Brayard et al. 2011). Their ancestors are supposed to have been “naked”, anemone-like corals that survived the Permian mass extinction. It had been assumed that scleractinian corals form purely aragonitic skeletons. But an exceptionally preserved fossil from the Upper Cretaceous possessed a purely calcitic skeleton (Stolarski et al. 2007). This implies that these corals could form skeletons of different carbonate polymorphs, as do some other but not all groups of marine, calcium carbonate-producing organisms (Stolarski et al. 2007).
Preservation of Soft Parts
Among arthropods the best fossils yielding ontogenetic data from their exoskeletons are trilobites (e.g., Barrande 1852; McNamara 1978; Tripp and Evitt 1986; Edgecombe et al. 1988, 1997; Chatterton and Speyer 1989; Chatterton et al. 1990, 1994; Lee and Chatterton 1997, 2003, 2005; Zhang and Pratt 1999; Clarkson and Ahlberg 2002; Lerosey-Aubril and Feist 2005a, b; Hughes et al. 2006, 2008) and bivalved arthropods, such as ostracods (e.g., Spjeldnaes 1951; Maness and Kaesler 1987; Hoare 1991; Tinn and Meidla 2003, 2004), bradoriids (in former times misidentified as ostracods; Zhang and Pratt 1993; Zhang 2007; Hou et al. 2010), or spinicaudate crustaceans (e.g., Olempska 2004; Tasch 1961; and references therein). Even for large arthropods with unmineralized exoskeletons such as sea scorpions (eurypterids), different developmental stages are preserved in the fossil record (Leutze 1958; Andrews et al. 1974; Cuggy 1994).
In some fossil sites there has been unusual preservation of soft parts, thus going beyond the usual exoskeleton or shells (Briggs et al. 2005). These sites include the Crato Formation in the Cretaceous of Brazil, Solnhofen in the Jurassic of Bavaria in Germany, Rhynie Chert in the Devonian of Scotland, and the “Orsten” Lagerstätten worldwide, best known from Sweden and expanding from the Lower Cambrian to the Lower Ordovician (Maas et al. 2006). As an example of how fossils from these exceptional sites can address the subjects treated in this article, we elaborate on the case of the “Orsten” fauna, which provides tiny fossils preserved in three dimensions, with minute details such as setae, setules, membraneous areas, and eyes.
The “Orsten” fauna is dominated by larval forms and some tiny adults of arthropods, mainly crustaceans of various groups. Based on the detailed material that is best studied with scanning electron microscopy (SEM), more or less complete ontogenetic series of fossil arthropods have been reconstructed (e.g., Müller and Walossek 1988; Walossek 1993) and have contributed to the knowledge of developmental patterns, as summarized in four major aspects:
-
(1)
Segment addition patterns absent in any extant taxon. Examples are (a) the extremely gradual mode of segment addition in the branchiopod Rehbachiella kinnekullenis Müller, 1983 that resembles the development in the ground pattern of Eucrustacea (the crown group of Crustacea; Walossek 1993), and (b) the trilobite-like development of the early crustacean Henningsmoenicaris scutula (Walossek and Müller, 1990) reflecting the development in the ground pattern of Crustacea sensu lato (Haug et al. 2010a).
-
(2)
Specialised larval stages. An example is the nauplius larva (one with three pairs of appendages), an autapomorphy of Eucrustacea. It is present in some taxa from the “Orsten,” rendering them representatives of the crown group (e.g., Müller and Walossek 1986, 1988; Walossek 1993), already in the lower Cambrian (Zhang et al. 2010), while other species possess a more plesiomorphic type of arthropod larva (e.g., Haug et al. 2009a, 2010a, b).
-
(3)
Specialized developmental rate. An example is the limb-bud delay, with first delayed and then accelerated development of trunk limbs in extant barnacles and their relatives (Thecostraca). This pattern is also found in Bredocaris admirabilis Müller, 1983 from the middle Cambrian, identifying this species as a representative of the thecostracan lineage (Müller and Walossek 1988).
-
(4)
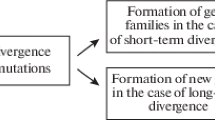
Specialized developmental timing of appearance of certain structures. An example is the proximal endite, an important feeding structure medio-proximally on the limbs, which appears in different stages of the larval sequence in several species of early Crustacea sensu lato (Fig. 1; Haug et al. 2009a, 2010a, b).
Fig. 1 Simplified phylogeny of Crustacea sensu lato highlighting some heterochronic events within this lineage (cf. Haug et al. 2010b). Top: a–f 3D models of Cambrian “Orsten” crustacean larvae (head larva stages) and their left appendages two and three in anterior view. a, b Late planktotrophic head larva stage of Henningsmoenicaris scutula. Although already feeding, the legs lack the proximal endite, an important feeding structure that will not develop before stage seven, in which already several trunk segments are present. c, d Head larva stage of Goticaris longispinosa. A proximal endite is already developed on the third appendage (arrow), but not yet on the second appendage. e, f Martinssonia elongata, early head larva stage. Although lecitotrophic and still lacking a mouth opening, proximal endites are already developed (arrows). Bottom: Based on the phylogeny and the pattern of appearance of the proximal endites within the larval sequences, two heterochronic events can be identified, both pre-displacement (arrows). The proximal endite appears both times earlier in ontogeny than in the more plesiomorphic condition
Based on phylogenetic analyses including the developmental patterns discovered in fossils, the reconstruction of evolutionary scenarios and the detection of heterochronic events becomes feasible (Fig. 1; Haug et al. 2010a, b). Besides crustacean fossils, also other taxa are represented within the fauna with their early developmental stages, such as larvae or embryos of agnostines (trilobite-like arthropods, sister group to Crustacea sensu lato; Müller and Walossek 1987), chelicerates (Waloszek and Dunlop 2002), and different taxa of nemathelminths (Müller and Hinz-Schallreuter 1993; Maas et al. 2007, 2009; Haug et al. 2009b).
In addition to the “Orsten” in the strict sense, there are also different fossil deposits with “Orsten”-like fossils. Among these, several specimens of various taxa with preserved soft-parts yield developmental information. These taxa comprise ostracods (Weitschat 1983a, b; Smith 2000), decapod larvae (Maisey and de Carvalho 1995; Tanaka et al. 2009), insects (Duncan et al. 1998), nemathelminths (Dong et al. 2004; Dong 2007; Zhang et al. 2011), and forms of yet unknown affinities (Steiner et al. 2004).
Macroevolutionary Patterns of Allometry and Modularity
Allometry
First coined by Huxley and Teissier (1936a, b), the term allometry refers to the pattern of covariation among the size of two morphological traits. Stemming from the early works of Dubois (1897) and Lapique (1907) that examined relationships between brain weight and body weight in mammals, allometry studies have a long history in the literature, and have more recently begun to be examined from a mechanistic perspective, as EvoDevo has focused more sharply on identifying specific genes and developmental pathways that are responsible for the evolution of ontogenies (West and Brown 2005; Li et al. 2007; Sears et al. 2007).
The advent and application of geometric morphometrics—the statistical analysis of the variation in the Cartesian geometric coordinates of homologuous landmarks (Dryden and Mardia 1998; Bookstein 1991)—has reformed the ways in which morphological form can be described and has permitted intuitive visualizations of ontogenetic trajectories as vectors in multivariate morphospace (e.g., O’Higgins 2000; Ponce de Léon and Zollikofer 2001; Monnet et al. 2009). The use of ontogenetic data to create developmental morphospaces has great potential to yield a wealth of results comparable to those currently documented by the studies of adult form, which at present dominate the literature. Importantly, the study of morphological evolution in fossils and extant species is equally possible using these techniques.
Understanding the dynamics of allometric evolution can be greatly enriched by incorporating data from the fossil record. Such data provide the only opportunity to evaluate the evolutionary persistence of factors affecting morphospace occupation, on a geological time scale. Ontogenetic series are known for many fossils. Rodents, the most speciose mammalian order, represent an excellent case study. Their unparalleled taxonomic success among mammals, coupled with phenomenal levels of morphological diversity and a rich fossil record, provide a wealth of opportunities to explore phylogenetic, ecological, or functional hypotheses relating to morphospace occupation and structure. Among the Caviomorph rodents, a group endemic to South America, well-preserved ontogenetic material is known for several fossil Ctenomyid rodents. These include the Pliocene rodents Actenomys, Xenodontomys, and Praectenomys (e.g., Verzi 2008). Comparison of allometric patterns between extant and fossil ctenoymids has already revealed several insights into ontogenetic evolution above the species level, particularly in relation to the acquisition of digging adaptations (Vassallo and Mora 2007). In this regard, Actenomys is a key taxon, as it shows a plesiomorphic condition for several traits usually considered to be adaptations for digging in the Ctenomyidae. By comparing ontogenetic series of fossil and extant ctenomyids, Verzi et al. (2010) showed how changes in ontogenetic trajectories, for instance among traits for incisor and mandibular morphology, have led to diversification in cranial form within the group. These data can be further used to explore ontogenetic dynamics on a macroevolutionary scale, and to investigate the factors leading to the patterning of species in morphospace. Wilson and Sánchez-Villagra (2010) examined patterns of allometric trajectory evolution in two extant clades of rodents with differing levels of morphological diversity. Their study revealed that dietary habit played a crucial role in patterning allometric evolution in the cranium, regardless of phylogenetic relatedness. The temporal persistence of the adaptive evolutionary patterns revealed by Wilson and Sánchez-Villagra (2010) may be evaluated by incorporating growth series of fossil species (Fig. 2) that will allow a suite of questions to be addressed relating to the importance of ecological constraints in rodent evolution.
In a wider context, ontogenetic trajectories are commonly represented using the major axis of covariance, and consequently through the exploration of developmental morphospaces, the extent and patterning of modification to covariance structure can be revealed, reflecting the possibilities that remain for the functional or developmental differentiation of integrated phenotypes (Eble 2004). Genetic (G) and phenotypic (P) covariance matrices have been cited as the quantitative expression of constraints that shape evolution (Arnold 1992), and much attention has been devoted to the interaction between the two (e.g., Cheverud 1984; Marroig and Cheverud 2004; Revell 2007; Arnold et al. 2008) in an attempt to bridge the gap between microevolutionary processes and macroevolutionary patterns.
Integrating fossil data into estimates of phenotypic covariance structure—through allometric evolution, integration, or modularity studies—is of particular interest because one major issue of concern is the temporal stability of the P matrix (Arnold et al. 2008). Logic confers that over a long enough time period the P matrix will alter; otherwise all organisms would have morphologies of approximately the same form. What are the time frames for covariance structure change? How do these compare between clades and how are these differences related to evolutionary success and morphological diversity? These macroevolutionary questions center on the understanding of how morphologies are generated, and measurements from fossils provide the patterns to be explained by morphogenetic models.
Modularity
Modularity refers to the differential integration of sets of characters and is reflected in the relatively high degree of covariation of units within a module and the relative independence of these units from other modules (Berg 1960; Callebaut and Raskin-Gutman 2005; Klingenberg 2008). Since the seminal paper of Wagner and Altenberg (1996), modularity has become a central concept in evolutionary biology. Morphometric methods used to identify phenotypic modules (Goswami and Polly 2010) can be applied to extinct taxa, as has been done for some mammals (Goswami et al. 2011), and trilobites (Webster and Zelditch 2011). In the paleontological context, the goal is to identify integrated morphological traits acting as units of evolutionary change among species (Eble 2004; Schoch 2006; Young et al. 2010; Gerber and Hopkins 2011).
There has been much theoretical discussion regarding whether modularity constrains or facilitates morphological evolution (e.g., Wagner and Altenberg 1996; Kirschner and Gerhart 1998; Budd 2006; Marroig et al. 2009). Theoretically, both situations are equally likely (Fig. 3), and empirical data are needed to explicitly test these assumptions. Elucidating the dynamics of trait integrations and interactions is particularly important for the explanation of macroevolutionary trends.
A hypothetical phenotypic morphospace with species (circles) unequally distributed. Each extant or fossil species may be represented by a number of landmarks recorded on adult individuals or an ontogenetic series. Traits measured may have connective relationships with one another such that they form modules or sets of highly integrated traits that behave relatively autonomously. Modularity may constrain or facilitate morphological evolution, and thus the ability of a clade to explore morphospace, that is, to generate phenotypic variation. Under the constraint hypothesis (left: purple point cloud), strong correlations among traits within a module may limit the potential of an individual trait to vary and hence comparatively increased amounts of modularity may result in limited morphospace occupation. Fewer trait interactions and modules (right: green point cloud) may thus permit a greater dispersion in phenotypic space. Under the facilitation hypothesis, the reverse would be the case. Modularity evolves, as indicated by the different hypothetical patterns and magnitudes illustrated in the caption boxes, and therefore a clade-wide study of modularity essentially reflects a lineage-specific study of the evolution of evolvability, most basically the ability of an organism to evolve. Modules are expected to arise through developmental or functional interactions among traits. Urotrichus talpoides cranium illustrated after Wilson 2012
Concerning vertebrates, the complexity of mammalian skull growth and development provides an excellent opportunity to test hypotheses about the factors responsible for variability and evolutionary change, and for this reason many studies have taken advantage of the wealth of knowledge already available for this system. Among mammals, several macroevolutionary studies of primates and carnivorans have documented patterns of modularity in the adult cranium (e.g., Goswami 2006; Marroig et al. 2009; de Oliveira et al. 2009; Shirai and Marroig 2010). These studies have revealed differences among clades, indicating that modularity changes over time.
Macroevolutionary Patterns of Covariation, Convergences, and Morphogenetic Models
Segmentation
Although no genetic experiments can be performed on fossils, strong inferences on genetic mechanisms underlying development in extinct taxa can be made (Luo et al. 2007; Schmid and Sánchez-Villagra 2010; Schmid 2012). For example, the segmental morphology of extinct taxa can be studied by making comparison with patterns of morphological expression for regulatory genes in extant arthropods (Hughes et al. 2006; Hughes 2007) or vertebrates (Müller et al. 2010).
The consideration of developmental genetic changes in extant taxa can serve to understand morphological variation in extinct ones (Schmid 2012; Thewissen et al. 2012). In particular, extant mutants can serve as models to infer mechanisms that may have been responsible for morphological diversity in fossil lineages. For example, the molecular underpinning of the morphological diversification of the Triassic basal actinopterygian fish Saurichthys was inferred by Schmid and Sánchez-Villagra (2010) on these grounds. Originating from an ancestor covered entirely by uniform rhomboid scales with numerous, highly segmented fin rays, Saurichthys radiated into species diagnosed by different degrees of loss in rays, scales, and dermal bones (Romano et al. 2012). These changes are analogous to those reported in mutants of different extant species, such as zebrafish, sticklebacks, and medaka. Schmid and Sánchez-Villagra (2010) suggested one of two alternatives: (1) a mutation or a regulatory change of a signaling pathway, in which either the fibroblast growth factor pathway was affected, assuming that a gene duplication had occurred, for example in the closely related acipenserids or in teleosts; (2) the ectodysplasin pathway was involved, assuming that its pleiotropic effects led to viable morphological diversification.
The Tetrapod Limb
The vertebrate limb is one classic example illustrating the successful integration of developmental and evolutionary studies. Based on the consideration of living species alone, one could wrongly conclude that the last common ancestor of tetrapod vertebrates (land vertebrates) must have had five fingers and five toes and that evolutionary “innovations” have largely involved reductions in the number of digits. But stem tetrapods that lived between 385 and 360 million years ago possessed more than five fingers (Coates and Clack 1990). Polydactyly in the stem tetrapods would not have been predicted based on the study of the crown group alone (Shubin et al. 2009); fossil discoveries reshaped our knowledge of ancestral limb morphology. Furthermore, it has inspired the examination of phenotypes of extant species that are suggestive of polydactyly, such as that of the pseudo-thumb of some anurans (Tokita and Iwai 2010) and talpid moles (Mitgutsch et al. 2012).
A recent mathematical model simulating the behavior of chondrogenic cells in the limb was capable of reproducing skeletal morphologies recorded in stem tetrapods (Zhu et al. 2010). This study is a significant achievement in that it brings together information on the regulatory network known for limb development in recent species, mathematical modeling, and information provided by fossils. The core mechanism for chondrogenesis consists of an activation subnetwork, an inhibition subnetwork, as well as adhesive and extracellular matrix molecules that promote pre-cartilage condensations. In conjunction with a gradient of a growth factor emanating from the apical ectodermal ridge situated at the distal part of the limb bud, the model accounts for the proximo-distal and antero-posterior layout of cartilaginous primordia in the chicken limb, as well as distal truncations and other patterns resembling mutational and experimental variants in various species. The model is able to reproduce the patterns of limbs observed in stem tetrapods like the transitional forms between fishes and amphibians or the secondarily marine ichthyosaurs. These morphologies result from variation in the kinetic parameters of topologically conserved networks acting in distinctly shaped limb buds. The model shows that the limb regulatory system is endowed with generic properties (Urdy 2012). Major features of normal, experimentally manipulated, genetically aberrant and evolutionary transitional limb forms emerge from the inherent self-organizing properties of this core mechanism. The model’s predictive power shows that the mechanism acting in the present, especially the network topology, can be used to explain the early evolution of the tetrapod limb. Reciprocally, information gleaned from the morphology of fossils provides an independent test for the relative success of morphogenetic models.
Molar Proportions
Much progress has been made in recent years in understanding the mechanisms of tooth development. For instance, a recent model, integrating gene networks and tissue mechanics, suggests that despite the complexity of development and teeth, there may be a simple basis for variation (Salazar-Ciudad and Jernvall 2010). These authors argued that changes in single parameters regulating signaling may underlie variation among individuals of ringed seals, whereas changes in the parameters regulating the growth of the epithelium may underlie tooth-to-tooth variation along the jaw. The model also generates 3D patterns of gene expression, changing over the course of development, as well as 3D morphologies that can be compared to real teeth using morphometric methods. Moreover, proportions of molars can be easily connected to experimentally developed models that predict a dynamic balance between inter-molar inhibition and mesenchymal activation, due to the sequential initiation of molar buds along the jaw (Kavanagh et al. 2007). It has been proposed that the second molar always makes up one-third of the total molar area. This simple coupling of proportions with developmental mechanisms makes the examination of fossils in a developmental perspective possible (Polly 2007; Renvoisé et al. 2009; Sánchez-Villagra 2010). Whereas this rule has been shown to hold among murine rodent species with various diets (Kavanagh et al. 2007), exceptions have been described in bears, horses (Polly 2007), voles (Renvoisé et al. 2009), and some extinct clades of South American “meridiungulates” (Wilson et al. 2012). Thus, exceptions to this simple rule point to slightly different mechanisms acting along the jaw, related to the increase or decrease of the inhibition of one molar relative to the next to form. Such comparisons have led to the discovery that in the Cenozoic radiation of now extinct ungulates from South America, novel molar proportions evolved that were outside those recorded among living species (Wilson et al. 2012). These novel proportions are related to abrasive diets and testify to the developmental plasticity under ecological conditions (Wilson et al. 2012). The abrasiveness of the foodstuff is related with the ashes produced by volcanic activity, a major geological feature in the South American Cenozoic (Williams and Kay 2001).
Evolutionary Recurrent Patterns in Mollusks
Thompson (1917) inspired a powerful approach to the study of biological development, which consists in unraveling the “laws of form” describing how developmental systems generate and constrain the variation of biological forms over short and long time scales. One of his favorite examples concerned the shell shape of mollusks, which generally conforms well to the logarithmic spiral. Mollusks are indeed well suited to address issues of developmental palaeontology, as they have an excellent fossil and living record and their accretionary mode of growth preserves shell ontogeny. The mantle, a soft, thin elastic tissue, secretes the shell. The shape of shell increments is equivalent to that of the mantle edge poking out of the aperture at the time of shell growth. However, the study of evolutionary changes occurring in fossil molluscan lineages relies nearly exclusively on the interpretation of shell morphologies, and the evolution of the molluscan shell is characterized by frequent convergences in form and ornamentation. Important taxonomic features of mollusks include the shape of the aperture, the degree of coiling of the shell tube, the ornamentation (ribs, tubercles, spines, keels) and growth features (growth halts, constrictions, varices).
The comparison of shell shape between and within different clades of mollusks can be informative with regards to the basic rules of accretionary growth. Common rules of growth could underlie the morphogenesis of the shell and its evolution in ammonoids and gastropods (Bucher 1997; Bucher and Guex 1990; Bucher et al. 1996; Checa and Jimenez-Jimenez 1997; Checa et al. 1998, 2002). Evidences come from the comparison of intraspecific and/or interspecific patterns of covariation among shell characters (Westermann 1966; Morita 1991a, b, 2003; Dagys and Weitschat 1993; Checa et al. 1996; Hammer and Bucher 2005a), from the description of changes occurring at maturity in different species or clades (Thompson 1917; Burnaby 1966; Bucher 1997; Chirat et al. 2008), and from the analysis of teratological shells in response to injuries (Thompson 1917; Guex 1967, 1968; Bayer 1970; Landman and Waage 1986; Bond and Saunders 1989; Hammer and Bucher 2005b) or to change in living conditions (Linsley 1977; Checa and Jimenez-Jimenez 1997; Checa et al. 2002).
It has been often observed that in variable ammonoid species, there is a tendency for the aperture shape to covary with the degree of whorl overlap and the robustness of ornamentation. The most ornamented specimens tend to exhibit a depressed aperture and small whorl overlap, whereas smooth specimens exhibit a laterally compressed aperture and a large whorl overlap (Fig. 4). These patterns of intraspecific variation, known as Buckman’s first law of covariation, have been observed in Triassic boreal ammonoids (Rieber 1972; Dagys and Weitschat 1993; Checa et al. 1996; Bucher 1997; Dagys et al. 1999; Hammer and Bucher 2005a; Monnet and Bucher 2005), and in Jurassic (Westermann 1966) and Cretaceous ammonites (Kennedy and Cobban 1976). As these patterns of covariation have been observed in phylogenetically distant ammonoids at several different time periods, they are evolutionary recurrent. Hammer and Bucher (2005a) proposed that the negative correlation between the compression of the aperture and the intensity of ornamentation can be satisfactorily accounted for by assuming that lateral rib heights increase isometrically with aperture width, whereas ventral rib heights increase isometrically with aperture height. Simple scaling relationships lead to produce proportionally stronger lateral ribs on depressed specimens than on compressed specimens, which only exhibit strong ribs on venter (Fig. 4). These observations indicate that molluscan shell shape variation is remarkably structured. Some studies highlighted the generic rules underlying the morphogenesis of the molluscan shell, using either geometrical (Thompson 1917; Raup 1961; Raup and Michelson 1965; Okamoto 1988a, b; Illert 1990; Savazzi 1990; Rice 1998; Ubukata 2003; Hammer and Bucher 2005b; Urdy et al. 2010a, b), mechanical (Morita 1991a, b; Morita 1993; Vermeij 2002; Hammer and Bucher 2005a), or chemical models (Hammer and Bucher 1999; Guex et al. 2003). Some other studies laid emphasis on the role of life orientation in the determination of growth direction (Linsley 1977, 1978; Checa and Jimenez-Jimenez 1997; Checa et al. 2002). Others suggested that the preceding whorl played a role in the regulation of coiling (Hutchinson 1989; Checa et al. 1998; Morita 2003).
Variation in an assemblage of juveniles of Amaltheus margaritatus from Jurassic, illustrating the so-called Buckman’s laws of covariation, which state that the coarseness of ribs tends to correlate negatively with the degree of aperture compression (ratio of whorl height against whorl width), whorl overlap (ratio of umbilical diameter against shell diameter) and spacing between septa. In this assemblage, the most robust variants (top) display relatively few strong ribs, a small whorl overlap and widely spaced septa, whereas compressed variants (bottom) exhibit numerous faint ribs, a high whorl overlap and closely spaced septa. During ontogenesis, specimens tend to display relatively narrower apertures, increased whorl overlap and fainted ornamentation, so that the most extreme variation is observed in the juveniles samples. Photographs by Noel Podevigne (Université Claude Bernard Lyon (UCBL), Lyon, France)
Indeed, teratological shells, including fossil ones, often provide a useful source of information about the way development generally proceeds. For instance, planispiral ammonites that were infested by epizoans during their lifetime exhibit alterations of their coiling geometry (Checa et al. 2002). These authors pointed out that, most commonly, the epizoans settled on the venter of ammonoids, and constituted an obstacle to the subsequent growth. This disturbance probably initiated changes in the hydrostatic conditions of the ammonite and caused a lateral shifting of the growth direction compared to the previous whorl in attempts to avoid the obstacle. Using a hydrostatic model, Checa et al. showed that the shell tube should periodically cross the venter, thus leading to zigzag coiling, if the ammonite tried to maintain the growth direction perpendicular to the substrate (sea bottom). If the epizoan was positioned on the midventer, the whorl could be detached from the previous whorl. Under constant growth direction relative to the substrate, a lateral placement of the epizoan would rather result in trochospiral coiling (Fig. 5), especially if the epizoan had a certain non-negligible weight, which could cause the tilting of the ammonite.
Example of teratological shells found in an assemblage of Aplococeras vogdesi from Triassic, Nevada. First row a regular planispiral specimen. Second and third rows two specimens exhibiting trochospiral coiling. No scars are visible and the trochospiral coiling appears in the early growth stages. The settlement of an epizoan on the left side of these specimens could have induced the tilting of the ammonite because of changes in the hydrostatic condition, especially if the weight of the epizoan was comparable to the weight of the ammonite. A trochospiral coiling is subsequently produced, if a constant growth direction with respect to the sea floor is maintained. Photographs by Rosi Roth (Paläontologisches Institut und Museum der Universität Zürich (PIMUZ), Zürich, Switzerland)
A similar role for life orientation in determining the growth direction has been experimentally tested in gastropods. In the benthic freshwater Planorbidae (Gastropoda), specimens experimentally altered by extra weights on one side of the shell revealed that the growth direction remained perpendicular to the substrate (Checa and Jimenez-Jimenez 1997). Similarly, the benthic prosobranch gastropods exhibiting a tangential aperture with regards to the coiling axis have been shown to live with the aperture parallel to the substrate (Linsley 1977). These gastropods have the ability to regulate the amount of torsion/detorsion of the foot to place the center of gravity of the shell and body over the midline of the cephalopodial mass, thus allowing the maintenance of a constant life orientation. A well-known example of the influence of change of mode of life on shell morphology is provided by the gastropod Distorsio, which, once settled on the substrate, displays distorted coiling. Well-known heteromorph ammonites like the late Cretaceous Nipponites were first viewed as a challenge to the simulation of their ontogeny and their evolution. However, Okamoto (1988b) derived a mathematical model of the peculiar meandering whorls of Nipponites. Assuming neutral buoyancy and a constant aperture angle relative to sea bottom, Okamoto‘s model showed that the meandering whorls morphology is controlled, under hydrostatic constraints, by the permitted range of variation in the growth direction relative to the sea bottom. The simulations suggest that the morphological transition from a simple helicoidal form to a meandering form occurs abruptly without any intermediary form as a result of a minor change in the upper and lower limits of variation in the growth direction.
In ammonoids, regenerated shells after damage are often found (Guex 1967, 1968; Bayer 1970; Landman and Waage 1986; Bond and Saunders 1989; Hammer and Bucher 2005b). Particularly, some changes in the ornamental features have been described in response to the location of injuries reaching the mantle (Guex 1967, 1968; Bayer 1970; Hammer and Bucher 2005b). For example, some shells with a ventral keel associated with ribs on the flanks can lose their keel in response to a wound located on the venter. Then, the ribs in the post-damaged shell cross the venter, whereas before they were interrupted by the keel. Some other shells bearing bifurcating ribs on the venter rather construct simple ribs after being damaged on one side. These examples are described in terms of “ornamental compensation” (Guex 1967, 1968). This phenomenon can be seen as a generic outcome of modes of shell growth, whether one interprets such results in terms of reaction–diffusion (Guex et al. 2003; Hammer and Bucher 2005b), or mechanical effects (Hammer and Bucher 2005a).
But probably the most famous work on fossil mollusks stems from Raup’s seminal (Raup 1961) paper, which triggered the emergence of theoretical morphology. Raup’s morphospace, particularly convenient to compare planispiral ammonites, has been extensively used to record changes in the patterns of morphological diversity of ammonites during the Mesozoic (Raup 1961, 1966; Raup and Michelson 1965; McGhee 1999). Since then, geometrical models progressively shifted from shape to growth description, by considering the timing of growth processes (Rice 1998; Urdy et al. 2010a, b). Such studies highlighted the role of growth rates and timing in the generation of allometries and phenotypic plasticity, providing a theoretical link between variation at the ontogenetic, population, and species levels (Urdy et al. 2010a, b). These approaches are expected to facilitate the comparison of theoretical and empirical data in the future, and to help interpreting mollusk fossil morphologies in a developmental, ecological, and evolutionary context.
Conclusions
Several examples show how paleontology can benefit from, but also contribute to the current conceptual and empirical advances to understand the evolution of development and constraints. Paleontological data can address directly mostly late aspects of ontogeny—a rich subject of study involving the origin of morphological diversity. Indirect information on developmental patterns can also be gathered from fossils. Even with a uniformitarian approach in which only mechanisms known from living organisms are used to make predictions about past phenomena, new discoveries about developmental evolution have been made. The fossil record provides numerous examples of extinct morphologies and developmental patterns. Given that 99.9 % of species are now extinct, neglecting the fossil species to construct morphospaces and infer developmental constraints may be highly misleading. Thus, the conceptual and empirical studies of development in a paleontological context constitute a significant contribution to the ongoing expansion of evolutionary theory, with the incorporation of EvoDevo themes not considered in the MS.
References
Adams MB (1980) Severtsov and Schmalhausen: Russian morphology and the evolutionary synthesis. In: Mayr E, Provine WB (eds) The evolutionary synthesis: perspectives on the unification of biology. Harvard University Press, Cambridge, MA, pp 193–225
Alberch P (1980) Ontogenesis and morphological diversification. Am Zool 20:653–667
Alberch P (1989) The logic of monsters: evidence for internal constraint in development and evolution. Geobios (Lyon) 22(Suppl 2):21–57
Alberch P, Gale EA (1985) A developmental analysis of an evolutionary trend: digital reduction in Amphibians. Evolution 39:8–23
Amundson R (1994) Two concepts of constraint: adaptationism and the challenge from developmental biology. Philos Sci 61:556–578
Andrews HE, Brower JC, Gould SJ, Reyment RA (1974) Growth and variation in Eurypterus remipes DeKay. Bull Geol Inst Univ Upsala N.S. 4:81–114
Anthony KRN, Kline DI, Diaz-Pulido G, Dove S, Hoegh-Guldberg O (2008) Ocean acidification causes bleaching and productivity loss in coral reef builder. Proc Natl Acad Sci USA 105:17442–17446
Arnold SJ (1992) Constraints on phenotypic evolution. Am Nat 140:85–107
Arnold SJ, Bürger R, Hohenlohe PA, Ajie BC, Jones AG (2008) Understanding the evolution and stability of the G-matrix. Evolution 62:2451–2461
Arthur W (2004) The effect of development on the direction of evolution: toward a twenty-first century consensus. Evol Dev 6:282–288
Barrande J (1852) Systême Silurien du centre de la Bohême. Iére partie. Recherches paléontologiques. Chez l’auteur et éditeur, Prague-Paris
Bayer U (1970) Anomalien bei Ammoniten des Aaleniums und Bajociums und ihre Beziehung zur Lebensweise. N Jahrb Geol Paläontol Abh 135:19–41
Berg RL (1960) The ecological significance of correlation Pleiades. Evolution 14:171–180
Bond PN, Saunders WB (1989) Sublethal injury and shell repair in upper Mississippian ammonoids. Paleobiologylogy 15:414–428
Bookstein FL (1991) Morphometric tools for landmark data: geometry and biology. Cambridge University Press, Cambridge
Brayard A, Vennin E, Olivier N, Bylund KG, Jenks J, Stephen DA, Bucher H, Hofmann R, Goudemand N, Escarguel G (2011) Transient metazoan reefs in the aftermath of the end-Permian mass extinction. Nat Geosci 4:693–697
Briggs DEG, Sutton MD, Siveter DaJ, Siveter DeJ (2005) Metamorphosis in a Silurian barnacle. Proc R Soc Lond B 272:2365–2369
Bucher H (1997) Caractères périodiques et mode de croissance des ammonites: comparaison avec les gastéropodes. Geobios Mém Spéc 20:85–99
Bucher H, Guex J (1990) Rythmes de croissance chez les ammonites triasiques. Bull Lab Géol Miner Géophys Mus Geol 308:191–209
Bucher H, Landman NH, Klofak SM, Guex J (1996) Mode and rate of growth in ammonoids. In: Landman NH, Tanabe K, Davis RA (eds) Ammonoid Paleobiologylogy. Plenum Press, New York, pp 407–461
Budd GE (2006) On the origin and evolution of major morphological characters. Biol Rev 81:601–628
Burnaby TP (1966) Allometric growth of ammonoid shells: a generalization of logarithmic spiral. Nature 209:904–906
Callebaut W, Raskin-Gutman D (eds) (2005) Modularity: understanding the development and evolution of natural complex systems. MIT Press, Cambridge, MA
Charlesworth B, Lande R, Slatkin M (1982) A neo-Darwinian commentary on macroevolution. Evolution 36:474–498
Chatterton BDE, Speyer SE (1989) Larval ecology, life history strategies and patterns of extinction and survivorship among Ordovician trilobites. Paleobiologylogy 15:118–132
Chatterton BDE, Siveter DJ, Edgecombe GD, Hunt AS (1990) Larvae and relationships of the Calymenina (Trilobita). J Paleontol 64:255–277
Chatterton BDE, Edgecombe GD, Speyer SE, Hunt AS, Fortey RA (1994) Ontogeny and relationships of Trinucleoidea (Trilobita). J Paleontol 68:523–540
Checa AG, Jimenez–Jimenez AP (1997) Regulation of spiral growth in planorbid gastropods. Lethaia 30:257–269
Checa A, Company M, Sandoval J, Weitschat W (1996) Covariation of morphological characters in the Triassic ammonoid Czekanowskites rieberi. Lethaia 29:225–235
Checa AG, Jimenez–Jimenez AP, Rivas P (1998) Regulation of spiral coiling in the terrestrial gastropod Sphincterochila: an experimental test of the road-holding model. J Morphol 235:249–257
Checa AG, Okamoto T, Keupp H (2002) Abnormalities as natural experiments: a morphogenetic model for coiling regulation in planispiral ammonites. Paleobiology 28:127–138
Cheverud JM (1984) Quantitative genetics and developmental constraints on evolution by selection. J Theor Biol 110:155–171
Chinsamy-Turan A (2005) The microstructure of dinosaur bone: deciphering biology with fine-scale techniques. Johns Hopkins University Press, Baltimore
Chirat R, Enay R, Hantzpergue P, Mangold C (2008) Developmental integration related to buoyancy control in Nautiloids: evidence from unusual septal approximation and ontogenetic allometries in a Jurassic species. Palaeontology 51:251–261
Clarkson ENK, Ahlberg P (2002) Ontogeny and structure of a new, miniaturised and spiny olenid trilobite from Southern Sweden. Palaeontology 45:1–22
Cloutier R (2010) The fossil record of fish ontogenies: insights into developmental patterns and processes. Semin Cell Dev Biol 21:400–413
Coates MI, Clack JA (1990) Polydactyly in the earliest known tetrapod limbs. Nature 347:66–69
Cubo J, Laurin M (2011) Perspectives on vertebrate evolution: topics and problems. C R Palevol 10:285–515
Cuggy MB (1994) Ontogenetic variation in Silurian eurypterids from Ontario and New York State. Can J Earth Sci 31:728–732
Dagys AS, Weitschat W (1993) Extensive intraspecific variation in a Triassic ammonoid from Siberia. Lethaia 26:113–121
Dagys A, Bucher H, Weitschat W (1999) Intraspecific variation of Parasibirites kolymensis Bychkov (Ammonoidea) from the Lower Triassic (Spathian) of Arctic Asia. Mitt Geol Paläontol Inst Univ Hambg 83:163–178
De Baets K, Klug C, Korn D, Landman N (2012) Evolutionary trends in ammonoid embryonal development. Evolution 66:1788–1806
de Oliveira FB, Porto A, Marroig G (2009) Covariance structure in the skull of Catarrhini: a case of pattern stasis and magnitude evolution. J Hum Evol 56:417–430
de Ricqlès A, de Buffrénil V (2001) Bone histology, heterochronies and the return of tetrapods to life in water: where are we? In: Mazin J-M, Buffrénil Vd (eds) Secondary adaptations of tetrapods to life in water. Friedrich Pfeil, München, pp 289–310
Dean MC (2010) Retrieving chronological age from dental remains of early fossil Hominins to reconstruct human growth in the past. Philos Trans R Soc B 365:3397–3410
Delfino M, Sánchez-Villagra MR (2010) A survey of the rock record of reptilian ontogeny. Semin Cell Dev Biol 21:432–440
Dong X (2007) Developmental sequence of Cambrian embryo Markuelia. Chin Sci Bull 52:929–935
Dong X, Donoghue PCJ, Cheng H, Liu J (2004) Fossil embryos from the middle and late Cambrian period of Hunan, South China. Nature 427:237–240
Donoghue PCJ, Bengston S, Dong XP, Gostling NJ, Huldtgren T, Cunningham JA, Yin C, Yue Z, Peng F, Stampanoni M (2006a) Synchroton X-ray tomographic microscopy of fossil embryos. Nature 442:680–683
Donoghue PCJ, Kouchinsky A, Waloszek D, Bengtson S, Dong X, Valkov AK, Cunningham JA, Repetski JE (2006b) Fossilized embryos are widespread but the record is temporally and taxonomically biased. Evol Dev 8:232–238
Dryden IL, Mardia KV (1998) Statistical shape analysis. Wiley, New York
Dubois E (1897) Sur le rapport de l’encéphale avec la grandeur du corps chez les Mammifères. Bull Mémo Soc d’Anthropol Paris, 4e série 8:337–376
Duncan IJ, Briggs DEG, Archer M (1998) Three-dimensionally mineralized insects and millipedes from the Tertiary of Riversleigh, Queensland, Australia. Palaeontology 41:835–851
Eble GJ (1999) On the dual nature of chance in evolutionary biology and Paleobiology. Paleobiology 25:75–87
Eble GJ (2004) The macroevolution of phenotypic integration. In: Pigliucci M, Preston KA (eds) Phenotypic integration. Oxford University Press, New York, pp 253–273
Edgecombe GD, Speyer SE, Chatterton BDE (1988) Protaspid larvae and phylogenetics of encrinurid trilobites. J Paleontol 62:779–799
Edgecombe GD, Chatterton BDE, Vaccari NE, Waisfeld BG (1997) Ontogeny of the proetoid trilobite Stenoblepharum, and relationships of a new species from the Upper Ordovician of Argentina. J Paleontol 71:419–433
Eldredge N, Cracraft J (1980) Phylogenetic patterns and the evolutionary process. Columbia University Press, New York
Erickson GM (2005) Assessing dinosaur growth patterns: a microscopic revolution. Trends Ecol Evol 20:677–684
Erickson GM, Makovicky PJ, Inouye BD, Zhou C-F, Gao K (2009) Life table for Psittacosaurus lujiatunensis: the first glimpse into ornithischian dinosaur population biology. Anat Rec 292:1514–1521
Erwin DH (2000) Macroevolution is more than repeated rounds of microevolution. Evol Dev 2:78–84
Fröbisch NB, Olori J, Schoch RR, Witzmann F (2010) Amphibian development in the fossil record. Semin Cell Dev Biol 21:424–431
Geiger M, Wilson LAB, Costeur L, Sánchez R, Sánchez-Villagra MR (in press) Diversity and growth in giant caviomorphs from the northern neotropics: a study of femoral variation in Phoberomys (Rodentia). J Vertebr Paleontol
Gerber S, Hopkins MJ (2011) Mosaic heterochrony and evolutionary modularity: the trilobite genus Zacanthopsis as a case study. Evolution 65:3241–3252
Gilbert SF (2003) The morphogenesis of evolutionary developmental biology. Int J Dev Biol 47:467–477
Gilbert SF, Opitz JM, Raff RA (1996) Resynthesizing evolutionary and developmental biology. Dev Biol 173:357–372
Goodwin BC (1988) Problems and prospects in morphogenesis. Experientia 44:633–637
Goswami A (2006) Cranial modularity shifts during mammalian evolution. Am Nat 168:270–280
Goswami A, Polly PD (2010) Methods for studying morphological integration and modularity. In: Alroy J, Hunt G (eds) Quantitative methods in Paleobiology. Paleontological Society Special Publications. Yale University Printing and Publishing, New Haven, pp 213–243
Goswami A, Milne N, Wroe S (2011) Biting through constraints: cranial morphology, disparity, and convergence across living and fossil carnivorous mammals. Proc R Soc B 1713:1831–1839
Goudemand N, Orchard M, Urdy S, Bucher H, Tafforeau P (2011) Synchrotron-aided reconstruction of the conodont feeding apparatus and implications for the mouth of the first vertebrates. Proc Natl Acad Sci USA 108:8720–8724
Gould SJ (1977) Ontogeny and phylogeny. Harvard University Press, Cambridge
Gould SJ (1980) Is a new evolutionary theory emerging? Paleobiology 6:119–130
Gould SJ, Lewontin RC (1979) The spandrels of San Marco and the Panglossian paradigm: a critique of the adaptationist programme. Proc R Soc B 205:581–598
Gould SJ, Simpson GG (1980) Paleontology, and the Modern Synthesis. In: Mayr E, Provine WB (eds) The evolutionary synthesis: perspectives on the unification of biology. Harvard University Press, Cambridge, MA, pp 193–225
Grantham T (2007) Is macroevolution more than successive rounds of microevolution? Palaeontology 50:75–85
Guex J (1967) Contribution à l’étude des blessures chez les ammonites. Bull Lab Géol Miner Géophys Mus Geol 165:1–23
Guex J (1968) Sur deux conséquences particulières des traumatismes du manteau des ammonites. Bull Societé Vaud Sci Nat 175:1–11
Guex J, Koch A, O’Dogherty L, Bucher H (2003) A morphogenetic explanation of Buckman’s law of covariation. Bull Soc Géol Fr 174:603–606
Hall BK (1996) Baupläne, phylotypic stages, and constraint: why there are so few types of animals. Evol Biol 29:215–261
Hammer O, Bucher H (1999) Reaction-diffusion processes: application to the morphogenesis of ammonoid ornamentation. Geobios 32:841–852
Hammer O, Bucher H (2005a) Buckman’s first law of covariation: a case of proportionality. Lethaia 38:67–72
Hammer O, Bucher H (2005b) Models for the morphogenesis of the molluscan shell. Lethaia 38:111–122
Haug JT, Maas A, Waloszek D (2009a) Ontogeny of two Cambrian stem crustaceans, †Goticaris longispinosa and †Cambropachycope clarksoni. Palaeontogr Abt A 289:1–43
Haug JT, Maas A, Waloszek D, Donoghue PCJ, Bengtson S (2009b) A new species of Markuelia from the Middle Cambrian of Australia. Mem Assoc Australas Palaeontol 37:303–313
Haug JT, Maas A, Waloszek D (2010a) †Henningsmoenicaris scutula, †Sandtorpia vestrogothiensis gen. et sp. nov. and heterochronic events in early crustacean evolution. Earth Environ Sci Trans R Soc Edinb 100:311–350
Haug JT, Waloszek D, Haug C, Maas A (2010b) High-level phylogenetic analysis using developmental sequences: the Cambrian †Martinssonia elongata, †Musacaris gerdgeyeri gen. et sp. nov. and their position in early crustacean evolution. Arthropod Struct Dev 39:154–173
Hautier L, Weisbecker V, Sánchez-Villagra MR, Goswami A, Asher RJ (2010) Skeletal development in sloths and the evolution of mammalian vertebral patterning. Proc Natl Acad Sci USA 107:18903–18908
Head JJ, David Polly P (2007) Dissociation of somatic growth from segmentation drives gigantism in snakes. Biol Lett 3:296–298
Hoare RD (1991) Ontogeny and variation in Glyptopleura costata (McCoy) (Ostracoda: Mississippian, Chesterian) from Ohio. J Paleontol 65:760–766
Hou X, Williams M, Siveter DaJ, Aldridge RJ, Sansom RS (2010) Soft-part anatomy of the early Cambrian bivalved arthropods Kunyangella and Kunmingella: significance for the phylogenetic relationships of Bradoriida. Proc R Soc B 277:1835–1841
Hughes NC (2007) The evolution of trilobite body patterning. Annu Rev Earth Planet Sci 35:401–434
Hughes NC, Minelli A, Fusco G (2006) The ontogeny of trilobite segmentation: a comparative approach. Paleobiology 32:602–627
Hughes NC, Haug JT, Waloszek D (2008) Basal euarthropod development: a fossil-based perspective. In: Minelli A, Fusco G (eds) Evolving pathways: key themes in evolutionary developmental biology. Cambridge University Press, Cambridge, pp 281–298
Hugi J, Sánchez-Villagra MR (2012) Life history and skeletal adaptations in the Galapagos marine iguana (Amblyrhynchus cristatus) as reconstructed with bone histological data: a comparative study of iguanines. J Herpetol 46:312–324
Hugi J, Scheyer TM, Sander PM, Klein N, Sánchez-Villagra MR (2011) Long bone microstructure gives new insights into the life of pachypleurosaurids from the Middle Triassic of Monte San Giorgio, Switzerland/Italy. C R Palevol 10:413–426
Humphrey LT (2010) Weaning behaviour in human evolution. Semin Cell Dev Biol 21:453–461
Humphrey LT, Dean MC, Jeffries TE, Penn M (2008) Unlocking evidence of early diet from tooth enamel. Proc Natl Acad Sci USA 105:6834–6839
Hunt G (2007) The relative importance of directional change, random walks, and stasis in the evolution of fossil lineages. Proc Natl Acad Sci USA 104:18404–18408
Hutchinson JMC (1989) Control of gastropod shell shape: the role of the preceding whorl. J Theor Biol 140:431–444
Huxley JS, Teissier G (1936a) Terminology of relative growth. Nature 137:780–781
Huxley JS, Teissier G (1936b) Terminologie et notation dans la description de la croissance relative. C R Seances Soc Biol Fil 121:934–937
Illert C (1990) Nipponites mirabilis: a challenge to seashell theory. Nuovo Cimento Soc Ital Fis D 12:1405–1421
Jablonski D (2007) Scale and hierarchy in macroevolution. Palaeontology 50:87–109
Jablonski D (2010) Origination patterns and multi-level processes in macroevolution. In: Pigliucci M, Müller GB (eds) Evolution, the extended synthesis. MIT Press, Cambridge, MA, pp 335–354
Kaneko K (2011) Characterization of stem cells and cancer cells on the basis of gene expression stability, plasticity, and robustness. BioEssays 33:403–413
Kauffman SA (1993) The origin of order: self-organization and selection in evolution. Oxford University Press, New York
Kavanagh KD, Evans AR, Jernvall J (2007) Predicting evolutionary patterns of mammalian teeth from development. Nature 449:427–432
Kennedy WJ, Cobban WA (1976) Aspects of ammonite biology, biogeography, and biostratigraphy. Spec Pap Palaeontol 17:1–94
Kirschner M, Gerhart J (1998) Evolvability. Proc Natl Acad Sci USA 95:8420–8427
Klingenberg CP (2008) Morphological integration and developmental modularity. Annu Rev Ecol Evol Syst 39:115–132
Klug C (2001) Life-cycles of Emsian and Eifelian ammonoids (Devonian). Lethaia 34:215–233
Köhler M, Moyà-Solà S (2009) Physiological and life history strategies of a fossil large mammal in a resource-limited environment. Proc Natl Acad Sci USA 106:20354–20358
Kolb C, De Vos J, Scheyer TM, Sánchez-Villagra MR (2011) The ontogeny of bone histology in the dwarfed island deer Candiacervus from the Late Pleistocene of Crete. In: Program and Abstracts of the 9th EAVP Annual Meeting, pp 31–32
Korn D (2012) Quantification of ontogenetic allometry in ammonoids. Evol Dev 14:501–514
Kuratani S (2012) Evolution of the vertebrate jaw from developmental perspectives. Evol Dev 14:76–92
Landman NH, Waage KM (1986) Shell abnormalities in scaphitid ammonites. Lethaia 19:211–224
Lapique L (1907) Tableau général des poids somatiques et encéphaliques dans les espèces animales. Bull Mémo Soc Anthropol Paris, 5e sér 8:248–269
Lee D, Chatterton BDE (1997) Three new proetide trilobite larvae from the Lower Ordovician Garden City Formation in southern Idaho. J Paleontol 71:434–441
Lee D, Chatterton BDE (2003) Protaspides of Leiostegium and their implications for membership of the order Corynexochida. Palaeontology 46:431–445
Lee D, Chatterton BDE (2005) Protaspides of Upper Cambrian Aphelaspis (Ptychopariida, Trilobita) and related species with their taxonomic implications. Palaeontology 48:1351–1375
Leroi AM (2000) The scale independence of evolution. Evol Dev 2:67–77
Lerosey-Aubril R, Feist R (2005a) First Carboniferous protaspid larvae (Trilobita). J Paleontol 79:702–718
Lerosey-Aubril R, Feist R (2005b) Ontogeny of a new cyrtosymboline trilobite from the Famennian of Morocco. Acta Palaeontol Pol 50:449–464
Leutze WP (1958) Eurypterids from the Silurian Tymochtee Dolomite of Ohio. J Paleontol 32:937–942
Li H, Huang Z, Gai J, Wu S, Zeng Y, Li Q, Wu R (2007) A conceptual framework for mapping quantitative trait loci regulating ontogenetic allometry. PLoS ONE 2:e1245
Linsley RM (1977) Some “laws” of gastropod shell form. Paleobiology 3:196–206
Linsley RM (1978) Shell form and the evolution of gastropods. Am Sci 66:432–441
Luo Z-X (2007) Transformation and diversification in the early mammalian evolution. Nature 450:1011–1019
Luo Z-X, Chen P-J, Li G, Chen M (2007) A new eutriconodont mammal and evolutionary development of early mammals. Nature 446:288–293
Maas A, Braun A, Dong X, Donoghue PCJ, Müller KJ, Olempska E, Repetski JE, Siveter DJ, Stein M, Waloszek D (2006) The ‘Orsten’: more than a Cambrian Konservat-Lagerstatte yielding exceptional preservation. Palaeoworld 15:266–282
Maas A, Waloszek D, Haug JT, Müller KJ (2007) A possible larval roundworm from the Cambrian ‘Orsten’ and its bearing on the phylogeny of Cycloneuralia. Mem Assoc Australas Palaeontol 34:499–519
Maas A, Waloszek D, Haug JT, Müller KJ (2009) Loricate larvae (Scalidophora) from the Middle Cambrian of Australia. Mem Assoc Australas Palaeontol 37:281–302
Maisey JG, de Carvalho MdGP (1995) First records of fossil sergestid decapods and fossil brachyuran crab larvae (Arthropoda, Crustacea), with remarks on some supposed palaemonid fossils, from the Santana Formation (Aptian-Albian, NE Brazil). Am Mus Novit 3132:1–17
Maness TR, Kaesler RL (1987) Ontogenetic changes in the carapace of Tyrrhenocythere amnicola (Sars) a hemicytherid ostracode. Univ Kans Paleontol Contrib 118:1–15
Marroig G, Cheverud JM (2004) Cranial evolution in sakis (Pithecia, Platyrrhini) I: interspecific differentiation and allometric patterns. Am J Phys Anthropol 125:266–278
Marroig G, Shirai LT, Porto A, de Oliveira FB, De Conto V (2009) The evolution of modularity in the mammalian skull II: evolutionary consequences. Evol Biol 36:136–148
Maynard Smith J, Burian R, Kauffman S, Alberch P, Campbell J, Goodwin B, Lande R, Raup D, Wolpert L (1985) Developmental constraints and evolution: a perspective from the Mountain Lake conference on development and evolution. Q Rev Biol 60:265–287
Mazzetta GV, Christiansen P, Fariña RA (2004) Giants and bizarres: body size of some southern South American Cretaceous dinosaurs. Hist Biol 65:1–13
McGhee GR (1999) Theoretical morphology. Columbia University Press, New York
McNamara KJ (1978) Paedomorphosis in Scottish olenellid trilobites (Early Cambrian). Palaeontology 21:635–655
Mitgutsch C, Richardson MK, Jiménez R, Martín JE, Kondrashov P, de Bakker MAG, Sánchez-Villagra MR (2012) Circumventing the pentadactyly ‘constraint’: autopodial recruitment of pre-axial structures in true moles. Biol Lett 8:74–77
Monnet C, Bucher H (2005) New middle and late Anisian (Middle Triassic) ammonoid faunas from northwestern Nevada (USA): taxonomy and biochronology. Foss Strat 52:1–60
Monnet C, Zollikofer CP, Bucher H, Goudemand N (2009) Three-dimensional morphometric ontogeny of mollusc shells by micro-computed tomography and geometric analysis. Palaeontol Electron 12:12A
Morita R (1991a) Finite element analysis of a double membrane tube (DMS-tube) and its implication for gastropod shell morphology. J Morphol 207:81–92
Morita R (1991b) Mechanical constraints on aperture form in gastropods. J Morphol 207:93–102
Morita R (1993) Development mechanics of retractor muscles and the “Dead Spiral Model” in gastropod shell morphogenesis. N Jahrb Geol Paläontol Abh 190:191–217
Morita R (2003) Why do univalve shells of gastropods coil so tightly? A head-foot guidance model of shell growth and its implication on developmental constraints. In: Sekimura T, Noji S, Ueno N, Maini PK (eds) Morphogenesis and pattern formation in biological systems: experiments and models. Springer, Tokyo, pp 345–354
Müller KJ, Hinz-Schallreuter I (1993) Palaeoscolecid worms from the Middle Cambrian of Australia. Palaeontology 36:549–592
Müller KJ, Walossek D (1986) Arthropod larvae from the Upper Cambrian of Sweden. Trans R Soc Edinb Earth Sci 77:157–179
Müller KJ, Walossek D (1987) Morphology, ontogeny, and life habit of Agnostus pisiformis from the Upper Cambrian of Sweden. Foss Strat 19:1–124
Müller KJ, Walossek D (1988) External morphology and larval development of the Upper Cambrian maxillopod Bredocaris admirabilis. Foss Strat 23:1–70
Müller J, Scheyer TM, Barrett P, Werneburg I, Head JJ, Ericson PGP, Pol D, Sánchez-Villagra MR (2010) Homeotic effects, somitogenesis, and the evolution of vertebral numbers in recent and fossil amniotes. Proc Natl Acad Sci USA 107:2118–2123
Murdock DJE, Donoghue PCJ (2011) Evolutionary origins of animal skeletal biomineralization. Cells Tissues Organs 194:98–102
Nützel A, Frýda J, Yancey TE, Anderson JR (2007) Larval shells of Late Palaeozoic naticopsid gastropods (Neritopsoidea: Neritimorpha) with a discussion of the early neritimorph evolution. Paläontol Z 81:213–228
O’Higgins P (2000) Quantitative approaches to the study of craniofacial growth and evolution: advances in morphometric techniques: development, growth and evolution. Academic Press, San Diego
Okamoto T (1988a) Analysis of heteromorph ammonoids by differential geometry. Palaeontology 31:35–52
Okamoto T (1988b) Developmental regulation and morphological saltation in the heteromorph ammonite Nipponite. Paleobiology 14:272–286
Olempska E (2004) Late Triassic spinicaudatan crustaceans from southwestern Poland. Acta Palaeontol Pol 49:429–442
Olsson L, Levit GS, Hoßfeld U (2010) Evolutionary developmental biology: its concepts and history with a focus on Russian and German contributions. Naturwissenschaften 97:951–969
Oster GF, Shubin N, Murray JD, Alberch P (1988) Evolution and morphogenetic rules: the shape of the vertebrate limb in ontogeny and phylogeny. Evolution 42:862–884
Peterson KJ, Summons RE, Donoghue PCJ (2007) Molecular palaeobiology. Palaeontology 50:775–809
Pigliucci M, Müller GB (2010) Evolution. The extended synthesis. MIT Press, Cambridge, MA
Polly PD (2007) Development with a bite. Nature 449:413–415
Ponce de Léon MS, Zollikofer CP (2001) Neanderthal cranial ontogeny and its implications for late hominid diversity. Nature 412:534–538
Raff RA (2007) Written in stone: fossils, genes, and evo–devo. Nat Rev Genet 8:911–920
Raff RA, Kaufman TC (1983) Embryos, genes, and evolution: the developmental-genetic basis of evolutionary change. Macmillan, New York
Raup DM (1961) The geometry of coiling in gastropods. Proc Natl Acad Sci USA 47:602–609
Raup DM (1966) Geometric analysis of shell coiling: some general problems. J Paleontol 40:1178–1190
Raup DM, Michelson A (1965) Theoretical morphology of the coiled shell. Science 147:1294–1295
Rensch B (1959) Evolution above the species level. Wiley, New York
Renvoisé E, Evans AR, Jebrane A, Labruère C, Laffont R, Montuire S (2009) Evolution of mammal tooth patterns: new insights from a developmental prediction model. Evolution 63:1327–1340
Revell LJ (2007) The G matrix under fluctuating correlational mutation and selection. Evolution 61:1857–1872
Rice SH (1998) The bio-geometry of mollusc shells. Paleobiology 24:133–149
Rieber H (1972) Die Triasfauna der Tessiner Kalkalpen. XXII. Cephalopoden aus der Grenzbitumenzone (Mittelere Trias) des Monte San Giorgio (Kanton Tessin, Schweiz). Schweiz Paläontol Abh 93:1–95
Romano C, Kogan I, Jenks J, Jerjen I, Brinkmann W (2012) Saurichthys and other fossils from the late Smithian (Early Triassic) of Bear Lake County (Idaho, USA) with a discussion of saurichthyid palaeogeography and evolution. Bull Geosci 87:543–570
Salazar-Ciudad I (2006) Developmental constraints vs. variational properties: how pattern formation can help to understand evolution and development? J Exp Zool B 306B:107–125
Salazar-Ciudad I, Jernvall J (2010) A computational model of teeth and the developmental origins of morphological variation. Nature 464:583–586
Sánchez-Villagra MR (2010) Developmental palaeontology in synapsids: the rock record of ontogeny in mammals and their closest relatives. Proc R Soc B 277:1139–1147
Sánchez-Villagra MR (2012) Embryos in deep time. University of California Press, San Francisco
Sander PM, Klein N (2005) Developmental plasticity in the life history of a prosauropod dinosaur. Science 310:1800–1802
Savazzi E (1990) Biological aspects of theoretical shell morphology. Lethaia 23:195–212
Scheyer TM (2007) Skeletal histology of the dermal armor of the Placodontia: the occurrence of ‘postcranial fibro-cartilaginous bone’ and its developmental implications. J Anat 211:737–753
Scheyer TM, Klein N, Sander PM (2010) Developmental palaeontology of Reptilia as revealed by histological studies. Semin Cell Dev Biol 21:462–470
Scheyer TM, Neenan JM, Renesto S, Saller F, Hagdorn H, Furrer H, Rieppel O, Tintori A (2011) Revised paleoecology of placodonts—with a comment on ‘The shallow marine placodont Cyamodus of the central European Germanic Basin: its evolution, Paleobiologygeography and paleoecology’ by CG Diedrich. Hist Biol 24:257–267
Scheyer TM, Werneburg I, Mitgutsch C, Delfino M, Sánchez-Villagra MR (2012) Three ways to tackle the turtle: integrating fossils, comparative embryology and microanatomy. In: Brinkman DB, Holroyd PA, Gardner JD (eds) Morphology and evolution of turtles: origin and early diversification: vertebrate Paleobiologylogy and paleoanthropology. Springer, Dordrecht, pp 63–70
Schmalhausen II (1949) Factors of evolution: the theory of stabilizing selection. Blakiston, Philadelphia
Schmid L (2012) Reconstructing the molecular underpinnings of morphological diversification. A case study of the Triassic fish Saurichthys. In: Asher RJ, Müller J (eds) From clone to bone: the synergy of morphological and molecular tools in Paleobiologylogy. Cambridge University Press, Cambridge, pp 135–165
Schmid L, Sánchez-Villagra MR (2010) The potential genetic bases of morphological evolution in the Triassic fish Saurichthys. J Exp Zool B 314B:519–526
Schoch RR (2006) Skull ontogeny: developmental patterns of fishes conserved aross major developmental stages. Evol Dev 8:524–536
Schoch RR (2009) Developmental evolution as a response to diverse lake habitats in Paleozoic amphibians. Evolution 63:2738–2749
Sears KE, Goswami A, Flynn JJ, Niswander LA (2007) The correlated evolution of Runx2 tandem repeats, transcriptional activity and facial length in Carnivora. Evol Dev 9:555–565
Seilacher A (1970) Arbeitskonzept zur Konstruktions-Morphologie. Lethaia 3:393–396
Severtsov AN (1912) Etiudy po teorii evoliutsii: individual’noe razvitie i evoliutsiia. [Studies on the theory of evolution: individual development and evolution]. Gosizdat, Kiev
Shirai LT, Marroig G (2010) Skull modularity in neotropical marsupials and monkeys: size variation and evolutionary constraint and flexibility. J Exp Zool B 314B:663–683
Shubin N, Tabin C, Carroll S (2009) Deep homology and the origins of evolutionary novelty. Nature 457:818–823
Simpson GG (1944) Tempo and mode in evolution. Columbia University Press, New York
Smith RJ (2000) Morphology and ontogeny of Cretaceous ostracods with preserved appendages from Brazil. Palaeontology 43:63–98
Spjeldnaes N (1951) Ontogeny of Beyrichia jonesi Boll. J Paleontol 26:745–755
Steiner M, Zhu M, Li G, Qian Y, Erdtmann B (2004) New Early Cambrian bilaterian embryos and larvae from China. Geology 32:833–836
Stolarski J, Meibom A, Przeniosto R, Mazur M (2007) A Cretaceous Scleractinian coral with a calcitic skeleton. Science 318:92–94
Sumrall CD, Wray GA (2007) Ontogeny in the fossil record: diversification of body plans and the evolution of “aberrant” symmetry in Paleozoic echinoderms. Paleobiology 33:149–163
Tafforeau P, Boistel R, Boller E, Bravin A, Brunet M, Chaimanee Y, Cloetens P, Feist M, Hoszowska J, Jaeger J–J, Kay RF, Lazzari V, Marivaux L, Nel A, Nemoz C, Thibault X, Vignaud P, Zabler S (2006) Applications of X-ray synchrotron microtomography for non-destructive 3D studies of paleontological specimens. Appl Phys A 83:195–202
Tanaka G, Smith RJ, Siveter DJ, Parker AR (2009) Three-dimensionally preserved decapod larval compound eyes from the Cretaceous Santana Formation of Brazil. Zool Sci 26:846–850
Tasch P (1961) Pemphilimnadiopseidae, a new family of fossil conchostracans. J Paleontol 35:1117–1120
Thewissen J, Cooper L, Behringer R (2012) Developmental biology enriches paleontology. J Vertebr Paleontol 32:1223–1234
Thompson D’A W (1917) On growth and form, 1st edn. Cambridge University Press, Cambridge
Tinn O, Meidla T (2003) Ontogeny and thanatocoenoses of early Middle Ordovician palaeocope ostracode species Brezelina palmata (Krause, 1889) and Ogmoopsis bocki (Opik, 1935). J Paleontol 77:64–72
Tinn O, Meidla T (2004) Phylogenetic relationships of Early-Middle Ordovician ostracods of Baltoscandia. Palaeontol 47:199–221
Tokita M, Iwai N (2010) Development of the pseudothumb in frogs. Biol Lett 6:517–520
Tripp RP, Evitt WR (1986) Silicified trilobites of the family Asaphidae from the Middle Ordovician of Virginia. Palaeontology 29:705–724
Ubukata T (2003) Pattern of growth rate around aperture and shell form in Bivalvia: a theoretical morphological study. Paleobiology 29:480–491
Urdy S (2012) On the evolution of morphogenetic models: mechano-chemical interactions and an integrated view of cell differentiation, growth, pattern formation and morphogenesis. Biol Rev 87:786–803
Urdy S, Chirat R (2006) Snail shell coiling (re-)evolution and the evo–devo revolution. J Zool Syst Evol Res 44:1–7
Urdy S, Goudemand N, Bucher H, Chirat R (2010a) Allometries and the morphogenesis of the molluscan shell: a quantitative and theoretical model. J Exp Zool B 314B:280–302
Urdy S, Goudemand N, Bucher H, Chirat R (2010b) Growth dependent phenotypic variation of molluscan shell shape: implications for allometric data interpretation. J Exp Zool B 314B:303–326
Vassallo AI, Mora MS (2007) Interspecific scaling and ontogenetic growth patterns of the skull in living and fossil ctenomyid and octodontid rodents (Caviomorpha: Octodontoidea). In: Kelt DA, Lessa E, Salazar-Bravo JA, Patton JL (eds) The quintessential naturalist: honouring the life and legacy of Oliver P. Pearson. University of California Publications in Zoology, Berkeley, pp 945–968
Vermeij GJ (2002) Characters in context: molluscan shells and the forces that mold them. Paleobiology 28:41–54
Verzi DH (2008) Phylogeny and adaptive diversity of rodents of the family Ctenomyidae (Caviomorpha): delimiting lineages and genera in the fossil record. J Zool 274:386–394
Verzi DH, Álvarez A, Olivares I, Morgan CC, Vassallo AI (2010) Ontogenetic trajectories of key morphofunctional cranial traits in South American subterranean ctenomyid rodents. J Mammal 91:1508–1516
Vrba ES (1983) Macroevolutionary trends: new perspective on the roles of adaptation and incidental effect. Science 221:387–389
Wagner GP, Altenberg L (1996) Perspective: complex adaptations and the evolution of evolvability. Evolution 50:967–976
Wagner GP, Larsson HCE (2003) What is the promise of developmental evolution? III. The crucible of developmental evolution. J Exp Zool B 300B:1–4
Wake DB (1991) Homoplasy: the result of natural selection, or evidence of design limitations? Am Nat 138:543–567
Walossek D (1993) The Upper Cambrian Rehbachiella and the phylogeny of Branchiopoda and Crustacea. Foss Strat 32:1–202
Waloszek D, Dunlop JA (2002) A larval sea spider (Arthropoda: Pycnogonida) from the Upper Cambrian ‘Orsten’ of Sweden, and the phylogenetic position of pycnogonids. Palaeontology 45:421–446
Webster G, Goodwin BC (1982) The origin of species: a structuralist approach. J Soc Biol Struct 5:15–47
Webster M, Zelditch ML (2011) Modularity of a Cambrian ptychoparioid trilobite cranidium. Evol Dev 13:96–109
Weitschat W (1983a) Ostracoden (O. Myodocopida) mit Weichkörper-Erhaltung aus der Unter-Trias von Spitzbergen. Paläontol Z 57:309–323
Weitschat W (1983b) On Triadocypris spitzbergensis Weitschat. Stereo Atlas Ostracod Shells 10:127–138
Wellik DM, Capecchi MR (2003) Hox10 and Hox11 genes are required to globally pattern the mammalian skeleton. Science 301:363–367
West GB, Brown JH (2005) The origin of allometric scaling laws in biology from genomes to ecosystems: towards a quantitative unifying theory of biological structure and organization. J Exp Biol 208:1575–1592
Westermann GEG (1966) Covariation and taxonomy of the Jurassic ammonite Sonninia adicra (Waagen). N Jahrb Geol Paläontol Abh 124:289–312
Williams SH, Kay RF (2001) A comparative test of adaptive explanations for hypsodonty in ungulates and rodents. J Mammal Evol 8:207–229
Wilson LAB (2012) Geographic variation in the greater Japanese shrew-mole Urotrichus talpoides: combining morphological and chromosomal patterns. Mammal Biol. doi:10.1016/j.mambio.2012.09.003
Wilson LAB (2013) The contribution of developmental palaeontology to extensions of evolutionary theory. Acta Zool (Stockholm) 94. In press. doi:10.1111/j.1463-6395.2011.00539.x
Wilson LAB, Sánchez-Villagra MR (2010) Diversity trends and their ontogenetic basis: an exploration of allometric disparity in rodents. Proc R Soc B 277:1227–1234
Wilson LAB, Madden RH, Kay RF, Sánchez-Villagra MR (2012) Testing a developmental model in the fossil record: molar proportions in South American ungulates. Paleobiology 38:308–321
Wroe S, Crowther M, Dortch J, Chong J (2004) The size of the largest marsupial and why it matters. Proc R Soc B 271:34–36
Young NM, Wagner GP, Hallgrímsson B (2010) Development and the evolvability of human limbs. Proc Natl Acad Sci USA 107:3400–3405
Zhang X (2007) Phosphatized bradoriids (Arthropoda) from the Cambrian of China. Palaeontogr Abt A 281:93–173
Zhang X, Pratt BR (1993) Early Cambrian ostracode larvae with a univalved carapace. Science 262:93–94
Zhang X, Pratt BR (1999) Early Cambrian trilobite larvae and ontogeny of Ichangia ichangensis Chang, 1957 (Protolenidae) from Henan, China. J Paleontol 73:117–128
Zhang X, Maas A, Haug JT, Siveter DJ, Waloszek D (2010) A eucrustacean metanauplius from the Lower Cambrian. Curr Biol 20:1075–1079
Zhang X, Pratt BR, Shen C (2011) Embryonic development of a middle Cambrian (500 myr old) scalidophoran worm. J Paleontol 85:898–903
Zhu J, Zhang YT, Alber MS, Newman SA (2010) Bare bones pattern formation: a core regulatory network in varying geometries reproduces major features of vertebrate limb development and evolution. PLoS ONE 5:e10892
Zollikofer CP, Ponce de Leόn MSP (2010) The evolution of hominin ontogenies. Semin Cell Dev Biol 21:441–452
Acknowledgments
We beg to be excused for the somewhat haphazard nature of the reference list of this article, as some of the topics treated here are very encompassing and have had a long history of investigation and thoughtful contributions. The article has been coordinated by MRSV, and all authors revised and commented on the different sections. MRSV thanks Jeff Schwartz and all those attending the Workshop, “The Evolution of Form,” held at the Konrad Lorenz Institute for Evolution and Cognition, Altenberg, September 2010, for fruitful discussions. SU thanks Hugo Bucher (PIMUZ) for having sampled the specimens illustrated in Figs. 4 (photographs by Noel Podevigne, UCBL) and 5 (photographs by Rosi Roth, PIMUZ), and allowing them to be published here. LABW thanks Andrea Elissamburu, Alejandro Donda, Fredy Carlini, and Diego Verzi for much help in Argentina and access to collections of Actenomys. We thank Z.-X. Luo (University of Chicago) for thoughtful suggestions. JTH was kindly supported by Yale University and by the Alexander von Humboldt Foundation with a Feodor Lynen Research Fellowship for postdoctoral researchers. LABW was supported by a Japanese Society for the Promotion of Science postdoctoral fellowship (PE 10075). SU was supported by the Swiss National Foundation (200021_124784/1 and PA00P3-136478) and the University of Zurich. This work is supported by the Swiss National Science Foundation to MRSV (31003A-133032/1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Urdy, S., Wilson, L.A.B., Haug, J.T. et al. On the Unique Perspective of Paleontology in the Study of Developmental Evolution and Biases. Biol Theory 8, 293–311 (2013). https://doi.org/10.1007/s13752-013-0115-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13752-013-0115-1