Abstract
Piezodorus guildinii (Westwood) is a soybean pest that causes significant economic losses in the Americas. The variability of overwintering (diapause) traits was evaluated in populations of the Southwest (SW) (33°55′–34°17′S, 57°13′–57°46′W) during 2-year period (2011–2013) and of the Northwest (NW) (32°01′–33°02′S, 57°50′–57°24′W) during 1-year period (2014–2015) Regions of Uruguay. Samples were taken from different plant species (cultivated legumes, wild shrubs, and trees) and from overwintering sites (leaf litter and bark). Alfalfa, Medicago sativa L. was the main host, with a collection period of 10–11 months in the SW and 12 months in the NW. Cluster analysis for each sex was carried out to group the months according to the similarity in diapause traits of populations (body size, body lipid content, immature reproductive organs, and clear type of pronotum band and connexivum in females). Female diapause in the SW was longer (beginning of autumn to end of winter) than that in the NW (mid-autumn to mid-winter). Male diapause was longer (mid-autumn to mid-winter) in SW1 (1st year) than in SW2 (2nd year) and NW (late-autumn to mid-winter). In both regions, male diapause was shorter than female. Differences were associated with maximum temperature at daylight hours ≤ 12.1, being necessary maximum temperatures below 23.8 °C for females and 19.2 °C for males to initiate diapause.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Piezodorus guildinii (Westwood) (Hemiptera: Pentatomidae) (common names: Neotropical green stink bug and small green stink bug in South America, red-banded stink bug in the USA) is a Neotropical species found from Southern United States to South America (Panizzi & Slansky 1985). It is a significant pest of soybean in the Southern Cone of South America, particularly in Uruguay and Argentina, causing the largest economic loss in this crop (Zerbino et al2014). In the last decade, it has reached economic importance as a pest of soybean in Southern United States (Baur & Baldwin 2006; Kamminga et al2012).
Like most phytophagous stink bugs, P. guildinii spends about a third of its lifetime feeding usually on immature pods of soybean from summer to early-autumn (Panizzi 2000, Panizzi & Silva 2009). The rest of the year, adults inhabit alternate plants, shelters in overwintering sites in reproductive diapause (Panizzi 1997, Zerbino et al2013, 2014, 2015), which are essential links in the intricate net of the life history of phytophagous hemipterans (Panizzi 2000).
The majority of hemipteran species have facultative diapause and external token stimuli are necessary to induce diapause (Koštál 2006). In temperate populations, the induction of adult reproductive diapause is controlled by a long-day photoperiodic response (Ali & Ewiess 1977, Harris et al1984, Musolin & Numata 2003). Winter adult diapause is the most common diapause in Pentatomidae (Musolin & Saulich 2018). The main diapause-inductive factors are photoperiod, temperature, and food quality/shortage (Panizzi & Silva 2009, Musolin & Saulich 2018, Zerbino et al2014, 2015). The interaction between photoperiod and temperature factors creates a reliable ecological mechanism to regulate the timely onset of diapause (Musolin & Saulich 2018). The most reliable environmental cue is day length, because of its astronomic precision and the fact that no environmental factors can affect it. Studies evaluating the effect of photoperiod on reproductive diapause in different species of pentatomids showed that diapause is induced by a photophase of ≤ 12 h (Ali & Ewiess 1977, Albuquerque 1993, Hodek & Hodková 1993, Kobayashi & Numata 1995, Nakamura & Numata 1997, Musolin & Numata 2003, Mourão & Panizzi 2002, Chocorosqui & Panizzi 2003, Niva & Takeda 2003, Zerbino et al2014). Thermoperiod fluctuates among years, which makes it a less reliable seasonal indicator. However, temperature acts to modify or reinforce the effects of photoperiod (Leather et al1993; Zerbino et al2013, 2014). The number of annual generations largely depends on local thermal conditions, whereas day length acts as a cue and provides information for an optimal timing of the active development and dormancy in a given location (Musolin & Saulich 2018). In general, increase in temperature suppresses winter diapause, whereas drop in temperature facilitates it (Zerbino et al2014).
Diapause is a hormonally determined state, characterized by a complex of morphological, physiological, and behavioral traits known as the diapause syndrome (Tauber et al1986). An accumulation of energetic reserves, undeveloped reproductive structures, and changes in size, shape, and body coloration are among the most conspicuous alterations on pentatomids during the diapause period (e.g., McPherson 1974, Ali & Ewiess 1977, Ito 1985, Albuquerque 1989, Hodek & Hodková 1993, Kobayashi & Numata 1995, Nakamura & Numata 1997, Musolin & Numata 2003, Mourão & Panizzi 2002, Chocorosqui & Panizzi 2003, Niva & Takeda 2003, Zerbino et al2014, 2015). Increase of lipid reserves is one of the most consistent features of insects entering diapause (Ito 1985, Danks 1987, Chocorosqui & Panizzi 2003), which enhances survival and longevity under adverse conditions (Ito 1985, Panizzi & Hirose 1995). Diapausing adults change the structure external and internal systems, which often results in changes in color or size (Danks 1987). Variable body coloration during the season is typical of many true bugs usually associated with the physiological state of the individuals controlled by day length (Musolin & Numata 2003, Musolin et al2007, Saulich & Musolin 2007, 2012). In moderate winters, some pentatomid species continue feeding during the short photophase, while others reduce their feeding activity (Shearer & Jones 1996, Mourão & Panizzi 2002, Chocorosqui & Panizzi 2003, Zerbino et al2014, Musolin & Saulich 2018).
Diapause can also affect phenology, affecting voltinism (i.e., the number of generations per year) among and within species (Posledovich et al2015, Musolin & Saulich 2018, Saulich & Musolin 2018). The number of generations per year greatly influences the growth and dynamics of insect populations, important factors to manage pests (Schebeck et al2017). Despite abundant, few diapause studies are clearly understood, which requires a combination of physiological and ecological approaches (Musolin 2012). Therefore, the purpose of this work was to study the variability of the diapause traits and overwintering strategies of P. güildinii populations in the Southwest and Northwest Regions of Uruguay.
Materials and Methods
Sites and sampling methods
P. guildinii adults were weekly sampled during 2 years (May 2011 to April 2013) in the Southwest Region of Uruguay (Colonia) (SW, between 33°55′ and 34°17′S, and between 57°13′ and 57°46′W) and during 1 year (July 2013 to June 2014) in the Northwest Region (Paysandú and Río Negro) (NW, between 32°01′ and 33°02′S, and between 57°50′ and 57°24′W) (Fig 1). Samples were taken from different plant species, including cultivated legumes, and wild shrubs and trees, and at overwintering sites (leaf litter and bark) (Table 1). Adults caught in each sample were identified according to the sampling site, date, and plant species, and taken to the laboratory, where they were killed by freezing.
For sampling the bugs, on forage legumes, a 38-cm diameter sweep net was used (100 and 400 sweep/crop in SW and NW, respectively). During winter (June–September), when bugs were not caught by sweep net, they were sampled in a randomly selected area (1 m2) using an iron frame, and 10 sampling units were evaluated in each crop. In the case of tree and shrub species, samples were taken by shaking foliage plants over a 1 m2 white cloth. Fifteen sampling units were taken from each plant species. Eucalyptus spp. and soybean litter were sampled using the 1 m2 iron frame, in a similar way as described for the forage legumes in the SW during winter.
Seasonal morphological and physiological changes
According to Zerbino et al (2014), the criteria that indicate reproductive diapause are small body size, the accumulation of energy reserves, and undeveloped reproductive organs for both sexes, and a clear coloration of the pronotum band and the connexivum for females. For that reason, the variables considered in this study were body length and body lipid content for both sexes, the percentages of females with immature ovaries and a clear type of pronotum band and connexivum, and the the percentages of males with immature testes and collapsed ectodermic sac.
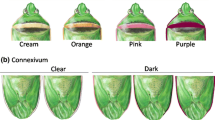
In each sampling date, collected adults were killed, sexed, and body length and color were evaluated. Individual digital pictures were taken, and the body length was evaluated using Image Pro-Express 5.1 software (MediaCybernetics, Silver Spring, MD). Females were characterized by the color of the pronotum band and of the connexivum (Zerbino et al2014). Females were typified as clear type when the color of the pronotum band was cream, yellow or orange, and dark type when they presented a pink, red, or purple band. The color of the connexivum varied from clear (whitish pink) to dark (blackish pink).
After the color assessment, females and males of each sampling date were divided into two groups. In the first one, lipid content was evaluated following Panizzi & Hirose (1995); insects were dried at 60 °C for 48 h, and dry mass (DW) was recorded to the nearest 0.01 mg (Scientech SA 210). For lipid extraction, a Twisselman extractor (IVA Co., Argentina) with six extraction tubes was used. Insects were individually identified with a number and placed in a cotton cloth bag (3.0 × 4.0 cm). Six cotton bags were conditioned in each extraction tube (7.5 × 3.0 cm); 250 mL of the extraction solvent (hexane) was added to a volumetric flask and placed in the Twisselman extractor. The equipment was heated to approximately 120 °C, after which the extraction process continued for 2 h. After that, each adult was placed back into the oven (60 °C) for 15 h and then weighed once more to obtain its weight without lipid (TW). The percentage stored lipid was calculated as Lipid content (%) = ((DW − TW) / DW) × 100.
The second group of adults was dissected and adult reproductive organs development was evaluated. Alcohol (70%) was used to clean the abdominal cavity of the insects to allow the best view of the reproductive organs. Females were ranked according to Mourão & Panizzi (2002) in one of three categories: immature (ovaries with no differentiation or no development, and no vitellary constriction); intermediate (ovaries with differentiation, visible chambers, and vitellary constrictions with oocytes); and mature (ovaries totally developed with well-developed oocytes). For males, the condition of the ectodermal sac (collapsed or expanded) was evaluated (Esquivel 2009). In addition, testes were extracted from the abdominal cavity, a digital picture was taken, and their length was measured using Image Pro-Express 5.1 software; they were ranked as mature or immature when values were ≥ 1.55 mm and ≤ 1.54 mm, respectively, according to Zerbino et al (2014), who determined these ranges from a sample of 217 testes.
Statistical analysis
For the three decades of each month of each sampling year (SW1-Southwest 2011/12, SW2-Southwest 2012/2013, and NW-Northwest 2013/14), averages of body length and body lipid content, percentages of females with immature ovaries and clear type of pronotum band and connexivum, and percentages of males with immature testes and collapsed ectodermic sac were calculated. These decade averages and percentages were considered as replicates in statistical analysis. A cluster analysis for each sex was carried out (complete linkage method, Gower distance (Gower 1971), and standardization of the data), to group the months in which adults were collected according to their similarity. For females, the data set included body length (mm), body lipid content (%), and percentage of females with immature ovaries and a clear type of pronotum band and connexivum. For males, the data set included body length (mm), body lipid content (%), and percentage of males with immature testes and collapsed ectodermic sac. To check the right composition of the groups determined by the cluster analysis, a multivariate analysis of variance (MANOVA) was performed. A discriminant analysis was carried out to determine the cross-errors in the groups. Regression trees were performed (Breiman et al1984) with the percentage of females and males with immature reproductive organs as the response variables, and daily light hours and maximum temperature (monthly average) as predictor variables (Table 2). Infostat version 2017 was the software used for all the analysis.
Results
Adults P. guildinii were obtained from 24 plant species, 11 of which were common in both regions (SW and NW). Alfalfa, Medicago sativa L. was the plant in which the largest number of adults was collected in both regions, with a collection period of 10–11 months in SW, and 12 months in NW (Table 3). The wild plant Pittosporum undulatum Vent and Eucalyptus spp. litter stood out among the group of shrubs and hibernation sites, in where the highest number of adults was obtained over a prolonged period (Table 3).
Females
According to the female diapause traits, the dendrogram of the hierarchical cluster analysis classified the 3 years of sampling (SW1, SW2, and NW) into three groups (diapause, non-diapause, and intermediate) (Fig 2a, Table 4). The cophenetic correlation of this analysis was 0.915, indicating the adequacy of the correlation between the distances defined by the metric of the binary tree (Gower) and the original distances. The groups were statistically different from each other (Wilks statistic = 0.03, F10,58 = 29.59, p < 0.0001) for the Hotelling Vector Comparison test corrected by Bonferroni. The cross classification of the groups performed by discriminant analysis estimated a total error of 7.41%, with errors of 4.17, 2.78, and 20.83% in the differentiation of the diapause, non-diapause, and intermediate groups, respectively.
The diapause group of females was composed of those collected during May to August (mid-autumn to mid-winter) of the 3 years of sampling (SW1, SW2, NW), and those collected in April and September (early-autumn and late-winter) in the 2 years of sampling in SW (SW1 and SW2) (Fig 2a, Table 4). Females of this group averaged the smallest body length (10.2 mm), the highest lipid content (35.6%), and the highest percentages of females with immature ovaries (89.7%), clear pronotum band (81.2%), and clear connexivum (85.3 %) (Table 5).
The non-diapause group was composed of females collected between November and February (late-spring and mid-summer) of the 3 sampling years (SW1, SW2, and NW) (Fig 2a, Table 4). Females of this group averaged the largest body length (10.6 mm) and the lowest percentages with immature ovaries (3.0%), clear pronotum band (7.6%), and clear connexivum (8.0%). Body lipid content (11.7%) was significantly lower compared to those in the diapause group and similar to that of the intermediate group (Table 5).
The intermediate group was composed of females collected during October and March (early-spring and late-summer) of the 3 sampling years (SW1, SW2, and NW) and during April and September (early-autumn and late-winter) in NW (Fig 2a, Table 4). Females of this group averaged the same body length than those of the diapause group (10.2 mm). Other evaluated diapause traits (body lipid content = 17.5%, immature ovaries = 23.8%, clear pronotum band = 37.5%, clear connexivum = 39.3%) were significantly higher than those of the diapause and significantly lower than those of the non-diapause group.
Based on the cluster analysis, months in which females were in diapause and non-diapause were set for each year of sampling (SW1, SW2, and NW) (Fig 2a and Table 4). Females collected in April and September in SW1 and SW2 were part of the diapause group, while those collected in those months in NW belonged to the intermediate group. The average values of all diapause traits in those months were significantly different for both regions (Table 6).
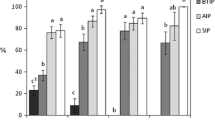
The regression tree allows identifying the threshold of the most critical variables to discriminate the effect in the percentage of females with immature reproductive organs (Fig 3a). Results indicate that the number of daylight hours was the main abiotic factor explaining percentage variability of females with immature ovaries: 83% with 12.1 or less hours of daylight, and 9% with more than 12.1 hours of daylight (Fig 3a). When the hours of daylight were ≤ 12.1, the average monthly maximum temperature explained the differences in the percentage of females with immature ovaries. At 23.8 °C or lower, the percentage of females with immature ovaries was 86%, while only 18% had immature ovaries at over 23.8 °C. At a maximum temperature of 19.2 °C or lower, the percentage of females with immature ovaries reached 93%.
Males
The dendrogram of the hierarchical cluster analysis shows months of the 3 years of sampling (SW1, SW2, and NW) classified into three groups, as it occurred with females (diapause, non-diapause, and intermediate group) (Fig 2b, Table 4). The cophenetic correlation of this analysis was 0.856, indicating the adequacy of the correlation between the distances defined by the metric of the binary tree (Gower) and the original distances. The groups were statistically different from each other (Wilks statistic = 0.08, F8,60 = 19.47, p < 0.0001) for the Hotelling Vector Comparison test corrected by Bonferroni. The cross classification of the groups performed by discriminant analysis estimated a total error of 7.41%, with errors of 5.56, 6.67, and 22.22% in the differentiation of the diapause, non-diapause, and intermediate groups, respectively.
The diapause group was composed of the males collected in May in SW1, and June to August (late-autumn to mid-winter) of the 3 years of sampling (SW1, SW2, and NW) (Fig 2b, Table 4). Males of this group averaged a smaller body length (9.9 mm) than those in the non-diapause group, and the highest percentages of body lipid content (38.3%), immature testes (79.9%), and collapsed ectodermal sac (97.8%) (Table 5).
The non-diapause group was composed of males collected from October to March (spring-summer) of the 3 years of sampling (SW1, SW2, and NW) (Fig 2b, Table 3). Males averaged the largest body length (10.1 mm), the lowest body lipid content (14.7%), and the lowest percentages of immature testes (13.9%) and collapsed ectodermal sac (24.8%) (Table 5).
The intermediate group was composed of males collected during April and September (early-fall and late-winter) of the 3 years of sampling (SW1, SW2, and NW), and those collected during May in SW2 and NW. Males averaged small body length (9.8 mm), similar to those of the diapause group. Mean values of the body lipid content (28.2%), and the percentage of males with immature testes (62.0%) and collapsed ectodermal sac (66.1%) were intermediate in relation to the diapause and non-diapause groups.
Based on the cluster analysis, males in diapause and non-diapause were set for each month/year and location of sampling (SW1, SW2, and NW) (Fig 2b and Table 4). Males collected in May in SW1 were part of the diapause group, while those collected in the same month in SW2 and NW belonged to the intermediate group. The percentages of males with immature testes and collapsed ectodermal sacs were significantly higher in SW1 as compared to SW2 and NW. Lipid content, however, was similar in all three cases (Table 7).
The regression tree (Fig 3b) allows to identify the threshold of the most critical variables to discriminate the effect in the percentage of males with immature testes. Results indicate that the number of daylight hours was the main abiotic factor in explaining the variability in the percentage of males with immature testes: 72% with 12.1 or less hours of daylight, and 14% with more than 12.1 h of daylight (Fig 3b). When daylight was ≤ 12.1 h, the mean monthly maximum temperature explained the differences in the percentage of males with immature testes. The percentages of males with immature testes were 80% at 19.2 °C or lower, and 59% at over 19.2 °C.
Discussion
In the studied regions, adults showed the same preferences in colonizing plant species (alfalfa almost throughout the year, and P. undulatum during diapause), and in hibernation sites (Eucalyptus spp. litter during diapause). However, there were differences between regions when adults initiated colonization, and in the period during which they remained in the different plant species and overwintering sites. Colonization of P. undulatum and hibernation in SW started a month earlier than in NW. Adults remained in alfalfa for a longer period in NW (12 months) than in SW (10–11 months); this indicates that alfalfa may play a key role in the life history of P. guildinii. One of the aspects to consider in the development of integrated pest management strategies is the management of plant biodiversity. In the particular case of the stink bugs, the management of host plants is fundamental (Panizzi & Silva 2009). According to the results obtained in the field and in the laboratory, Zerbino et al (2015, 2016) established that implementing management tactics during spring on alfalfa may be successful in reducing bug populations prior to their colonization of soybean.
Adults of P. guildinii collected in SW and NW in different months showed differences in physiological (development of reproductive organs and body lipid content) and morphological (body length and body color in females) traits. Zerbino et al (2015) reported that this insect used diapause as a strategy to face unfavorable conditions in SW. Our result indicates that in NW, the insect also faces unfavorable environmental conditions as an adult in reproductive diapause, which was expected due to the small differences in the daylight conditions between both regions throughout the year.
Piezodorus guildinii, like many pentatomids, responds in different ways to day length; such physiological responses are called photoperiodic responses (PhPR) (Musolin & Saulich 2018, Zerbino et al2013, 2014, 2015). Studies with different species of stink bugs indicate that diapause is induced when photophase is ≤ 12 h (Ali & Ewiess 1977, Albuquerque 1989, 1993, Hodek & Hodková 1993, Kobayashi & Numata 1995, Nakamura & Numata 1997, Mourão & Panizzi 2002, Chocorosqui & Panizzi 2003, Musolin & Numata 2003, Niva & Takeda 2003, Zerbino et al2013, 2014). In our study, the threshold of daylight hours for both sexes was 12.1. The percentage of adults with immature reproductive organs increased significantly below this value, which coincides with the results obtained in the laboratory (Zerbino et al2013, 2014). A critical photoperiod determines the timing to switch from a reproducing population to a diapausing one, thus acting as a cue for the cessation of active development and the transition to seasonal dormancy (Musolin & Saulich 2018).
According to the annual variation of the evaluated diapause traits, three distinct groups were characterized: diapause, non-diapause, and intermediate. Additionally, for each year, it was possible to establish the months that defined these groups. Adults in the diapause group had a small body size, with high body lipid content, immature reproductive organs, and females, in particular, had clear coloration pronotum band and connexivum. The opposite was observed to the non-diapause group. Adults in the intermediate group had a body size similar to the adults in diapause, but the values of lipid content, the percentages of adults with immature reproductive organs and females with a clear type of pronotum band and connexivum were intermediate between those recorded in the diapause and non-diapause groups.
Geographical variation in diapause response allows for the efficient exploitation of development and reproduction resources in different environments, especially in species with wide distribution (Schebeck et al2017, Posledovich et al2015). In this study, we found differences between regions (SW and NW) at the beginning and the end of the diapause.
Each group of females in SW (diapause, non-diapause, and intermediate) in the 2 sampling years (SW1 and SW2) comprised the same months. April (early-autumn) was the first month in which a high percentage of females was in diapause (SW1 = 78% and SW2 = 87%). The average maximum temperatures recorded in that month were 21.5 °C in SW1 and 23.5 °C in SW2. Females collected in SW in April (early-autumn) and September (late-winter) exhibited a significantly higher frequency of immature ovaries and clear pronotum band and connexivum than those collected in NW. This defined that the females collected in April and September in SW were part of the diapause group, while those collected in NW belonged to the intermediate group. Thus, the diapause period of females in SW is longer than in NW; the beginning and the end of the period are 1 month earlier and 1 month later, respectively. When the length of diapause increases at higher latitudes, there is a change in voltinism (e.g., from two to one generations per year) along the latitudinal gradient (Posledovich et al2015).
The differences found are explained by the maximum average temperature recorded in both regions in April and September. The maximum temperatures in April in SW during the 2 years (SW1 = 21.5 °C, SW2 = 23.5 °C) were lower than those in NW (24.2 °C). The same pattern of temperature occurred in September (SW1 = 20 °C, SW2 = 19 °C, and NW = 21 °C). The maximum temperatures registered during the winter months (June–August) in NW were higher than those registered in SW. With an earlier resumption of seasonal development in spring, a faster development during summer, and a later termination of reproductive activity in autumn, the number of generations per year may increase (Musolin & Saulich 2018).
There were also differences in the composition of the diapause and intermediate groups for males. In May (mid-autumn), the percentage with immature reproductive organs was significantly higher in SW1 than in SW2 and NW; this determined that SW1 belonged to the diapause group, while SW2 and NW belonged to the intermediate group. This difference may likely be associated with the maximum average temperature recorded in that month; in SW1 (18.3 °C), it was lower than in SW2 (20.6 °C) and NW (19.5 °C). It is noteworthy that, throughout the year, the minimum air temperature in NW is lower than in SW.
The majority of insect species in the temperate zone is characterized by the potentially multi-voltine seasonal cycle with a facultative winter adult diapause regulated by the PhPR of diapause induction of the long-day type. This occurs in the regions with moderate humidity and temperatures at middle and low latitudes, given the optimal trophic conditions and the choice of host plants (Saulich & Musolin 2018).
The effect of temperature as a modifier of the PhPR during diapause induction seems to be important and widespread (Numata et al1993, Hodek & Hodková 1993, Kobayashi & Numata 1995, Niva & Takeda 2003, Saulich & Musolin 2012, Musolin & Saulich 2018). The main factors limiting the number of generations are food and temperature (Musolin & Saulich 2018). High temperatures can strongly suppress the effect of day length and even completely prevent the induction of winter diapause in some species (Saulich & Musolin 2018). The data from many insect species shows that within a range of 20 to 27 °C, a temperature shift of 5 °C can cause a shift in critical photoperiod of approximately 1 h (Danilevsky 1961). On the other hand, low temperatures enhance the tendency towards winter diapause and all individuals enter diapause irrespective of the preceding day length (Musolin & Saulich 2018). The results obtained in our study allow to establish that when the number of daylight hours is ≤ 12.1, for females and males to be in diapause, it is necessary to record maximum temperatures below 23.8 °C and 19.2 °C, respectively.
In laboratory studies, Zerbino et al (2013, 2014) demonstrated that the photoperiodic response of P. guildinii was influenced by the decrease of temperature as in other insect species (Danks 1987, Cho et al2008; Saulich & Musolin 2012). The accumulation of lipids was more pronounced in adults raised at a 10-h photophase and 20 °C than in those reared at 25 °C; under short photophase, high temperatures delay the accumulation of lipids. Females significantly increased the tendency to enter into a reproductive diapause when reared in short photophase (10 h) and at 20 °C. The percentages of females (15 to 30 days old) with immature ovaries were significantly higher under a short photophase at 20 °C than at 25 °C. In our study, the effects of temperature under short photoperiod conditions on the development of reproductive organs were less pronounced for males than for females. The lowest temperature evaluated (20 °C) was above the threshold temperature determined (19.2 °C).
The different temperature requirements of each sex determine that the diapause duration is longer in females. Zerbino et al (2015) established that P. guildinii male diapause is shorter and/or less intense than female diapause. Therefore, males are physiologically ready to start reproduction earlier in the season than females. The proportion of females with immature ovaries between early-autumn and late-winter (April–September) was higher than 80%, while the proportion of males with immature testes from mid-autumn to late-winter (May–September) was higher than 70%.
In conclusion, results obtained elucidate which are the main host plants and overwintering niches, and the occurrence of geographical variation in the diapause response of P. guildinii in Uruguay. Alfalfa, P. undulatum and Eucalyptus spp. litter are the preferred habitats. In alfalfa, adults are found throughout the year; therefore, population management in this crop during the spring can reduce the impact of P. guildinii on the ensuing crops, for example, soybean. The two studied regions do not have important differences in the number of daylight hours. The differences recorded at the time of the beginning and the end of diapause are associated with the average maximum air temperature. When the number of daylight hours is equal or less than 12.1, it is necessary to record average maximum temperatures below 23.8 °C and 19.2 °C, for most females and males to begin the diapause stage. As a consequence of the thermal requirements of adults and the maximum temperatures recorded, the diapause period in SW may be longer than that in NW. These results suggest that the time to apply management practices in forage legumes with the purpose of reducing P. guildinii populations may differ depending of the region.
References
Albuquerque GS (1989) Ecologia de populações, biologia e estrategias da história de vida de Oebalus poecilus (Dallas, 1851) (Hemiptera: Pentatomidae) [Tesis de Mestría]. UFRGS, Porto Alegre, 309 p
Albuquerque GS (1993) Planting time as a tactic to manage the small rice stink bug, Oebalus poecilus (Hemiptera, Pentatomidae), in Rio Grande do Sul, Brazil. Crop Prot 12:627–630
Ali M, Ewiess MA (1977) Photoperiodic and temperature effects on rate of development and diapause in the green stink bug, Nezara viridula L. (Heteroptera: Pentatomidae). Z Ang Entomol 84:256–264
Baur ME, Baldwin J (2006) Red banded stink bug trouble in Louisiana. Louisiana Agric 49:9–10
Breiman L, Friedman J, Stone CJ, Olshen RA (1984) Classification and regression trees. CRC Press, Boca Raton, p 368
Cho JR, Lee M, Kim HS, Boo KS (2008) Effect of photoperiod and temperature on reproductive diapause of Scotinophara lurida (Burmeister) (Heteroptera: Pentatomidae). J Asia Pac Entomol 11:53–57
Chocorosqui VR, Panizzi AR (2003) Photoperiodic influence on the biology and phenological characteristics of Dichelops melacanthus (Dallas, 1851) (Heteroptera: Pentatomidae). Braz J Biol 63:655–664
Danilevsky AS (1961) Photoperiodism and seasonal development of insects. Leningrad University Press, Leningrad, the USSR. (in Russian). (Translated into English by J. Johnson assisted by N. Waloff, Oliver & Boyd, Edinburgh, UK, 1965)
Danks HV (1987) Insect dormancy: an ecological perspective. Biological Survey of Canada Monographs series No 1
Esquivel JF (2009) Stages of gonadal development of the southern green stink bug (Hemiptera: Pentatomidae): improved visualization. Ann Entomol Soc Am 102:303–309
Gower JC (1971) A General Coefficient of Similarity and Some of Its Properties. Biometrics 27:857–871
Harris VE, Todd JW, Mullinix BG (1984) Color change as an indicator of adult diapause in the southern green stink bug, Nezara viridula. J Agric Entomol 1:82–91
Hodek I, Hodková M (1993) Role of temperature and photoperiod in diapause regulation in Czech populations of Dolycoris baccarum (Heteroptera: Pentatomidae). Eur J Entomol 90:95–98
Ito K (1985) Seasonal changes of lipid content in adult Cletus punctiger. Appl Entomol Zool 20:350–351
Kamminga KL, Davis JA, Stock SP, Richter AR (2012) First report of a mermithid nematode infecting Piezodorus guildinii and Acrosternum hilare (Hemiptera: Pentatomidae) in the United States. Florida Entomol 95:214–217
Kobayashi S, Numata H (1995) Effects of temperature and photoperiod on the induction of diapauses and the determination of body coloration in the bean bug, Riptortus clavatus. Zool Sci 12:343–348
Koštál V (2006) Eco-physiological phases of insect diapause. J Insect Physiol 52:113–127
Leather SR, Walker KFA, Bale JS (1993) The ecology of insect overwintering. Cambridge University Press, Cambridge, p 268
McPherson JE (1974) Photoperiodic effects in a southern Illinois population of the Euschistus tristigmus complex (Hemiptera: Pentatomidae). Ann Entomol Soc Am 67:943–952
Mourão APM, Panizzi AR (2002) Photophase influence on the reproductive diapauses, seasonal morphs, and feeding activity of Euchistus heros (Fabr., 1978) (Hemiptera Pentatomidae). Braz J Biol 62:231–238
Musolin DL (2012) Surviving winter: diapause syndrome in the southern green stink bug Nezara viridula in the laboratory, in the field, and under climate change conditions. Physiol Entomol 37:309–322
Musolin DL, Numata H (2003) Photoperiodic and temperature control of diapause induction and colour change in the southern green stink bug Nezara viridula. Physiol Entomol 28:65–74
Musolin DL, Saulich AK (2018) Diapause in Pentatomoidea. In: McPherson JE (ed) Invasive stink bugs and related species (Pentatomoidea): biology, higher systematics, semiochemistry, and management, 1st edn. CRC Press, Boca Raton, pp 497–564
Musolin DL, Fujisaki K, Numata H (2007) Photoperiodic control of diapause termination, color change and postdiapause reproduction in the southern green stink bug, Nezara viridula. Physiol Entomol 32:64–72
Nakamura K, Numata H (1997) Seasonal life cycle of Aelia fieberi (Hemiptera: Pentatomidae) in relation to the phenology of its host plants. Ann Entomol Soc Am 90:625–630
Niva CC, Takeda M (2003) Effects of photoperiod, temperature and melatonin on nymphal development, polyphenism and reproduction in Halyomorpha halys (Heteroptera: Pentatomidae). Zool Sci 20:963–970
Numata H, Saulich AH, Volkovich TA (1993) Photoperiodic responses of the linden bug, Pyrrhocoris apterus, under conditions of constant temperature and under thermoperiodic conditions. Zool Sci 10:521–527
Panizzi AR (1997) Wild hosts of pentatomids: ecological significance and role in their pest status on crops. Annu Rev Entomol 42:99–122
Panizzi AR (2000) Suboptimal nutrition and feeding behavior of hemipterans on less preferred plant food sources. An Soc Entomol Brasil 29:1–12
Panizzi AR, Hirose E (1995) Seasonal body weight, lipid content, and impact of starvation and water stress on adult survivorship and longevity of Nezara viridula and Euchistus heros. Entomol Exp Appl 76:247–253
Panizzi AR, Silva FAC (2009) Insetos sugadores de sementes (Heteroptera). In: Panizzi AR, Parra JRP (eds) Bioecologia e nutrição de insetos; base para o manejo de pragas. Embrapa Informacão Tecnológica, Brazil, pp 465–522
Panizzi AR, Slansky F Jr (1985) Review of phytophagous pentatomids (Hemiptera: Pentatomidae) associated with soybean in the Americas. Fla Entomol 68:184–214
Posledovich D, Toftegaard T, Wiklund C, Ehrlén J, Gotthard K (2015) Latitudinal variation in diapause duration and post-winter development in two pierid butterflies in relation to phonological specialization. Oecol 177:181–190
Saulich AKh, Musolin DL (2007) Four seasons: diversity of seasonal adaptations and ecological mechanisms controlling seasonal development in true bugs (Heteroptera) in the temperate climate. In: Stekolnikov AA (ed.) Adaptive strategies of terrestrial arthropods to unfavourable environmental conditions: a collection of papers in memory of Professor Viktor Petrovich Tyshchenko, (Proceedings of the Biological Institute of St. Petersburg State University, 53:25–106 (in Russian, with expanded 4-page English summary)
Saulich AK, Musolin DL (2012) Diapause in the seasonal cycle of stink bugs (Heteroptera, Pentatomidae) from the temperate zone. Entomol Rev 92:1–26
Saulich AK, Musolin DL (2018) Seasonal Cycles of Pentatomoidea. In: McPherson JE (ed) Invasive stink bugs and related species (Pentatomoidea): biology, higher systematics, semiochemistry, and management, 1st edn. CRC Press, Boca Raton, pp 567–607
Schebeck M, Hansen EM, Schopf A, Ragland GJ, Stauffer C, Bentz BJ (2017) Diapause and overwintering of two spruce bark beetle species. Physiol Entomol 42:200–210
Shearer PW, Jones VP (1996) Diel feeding pattern of adult female southern green stink bug (Hemiptera: Pentatomidae). Environ Entomol 25:599–602
Tauber MJ, Tauber CA, Masaki S (1986) Seasonal adaptations of insects. Oxford University Press, New York, p 411
USNO (The United States Naval Observatory). Queries (8/27/2013, 4/23/2017) http://aa.usno.navy.mil/data/docs/RS_OneYear.php)
Zerbino MS, Altier N, Panizzi AR (2013) Effect of photoperiod and temperature on nymphal development and adult reproduction of Piezodorus guildinii (Heteroptera: Pentatomidae). Fla Entomol 96:572–582
Zerbino MS, Altier N, Panizzi AR (2014) Phenological and physiological changes in adult Piezodorus guildinii (Hemiptera: Pentatomidae) due to variation in photoperiod and temperature. Fla Entomol 97:734–743
Zerbino MS, Altier N, Panizzi AR (2015) Seasonal occurrence of Piezodorus guildinii on different plants including morphological and physiological changes. J Pest Sci 88:495–505
Zerbino MS, Altier N, Panizzi AR (2016) Performance of nymph and adult of Piezodorus guildinii (Westwood) (Hemiptera: Pentatomidae) feeding on cultivated legume. Neotrop Entomol 45:114–122
Acknowledgments
We thank Mabel Pessio and Eduardo García for their support working at the laboratory. We gratefully acknowledge Public Translator Gonzalo Peralta for the contributions made to improve this manuscript. This research is part of a project sponsored by the National Research Council of Brazil (CNPq) process number 490315/2008-9 in collaboration with INIA of Uruguay.
Author Contribution Statement
MSZ, LM, NAA, and ARP conceived and designed research. MSZ and LM conducted experiments. MSZ and LM analyzed data. MSZ, LM, NAA, and ARP wrote the manuscript. All authors read and approved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by R M Pitta – Embrapa
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zerbino, M.S., Miguel, L., Altier, N.A. et al. Overwintering of Piezodorus guildinii (Heteroptera, Pentatomidae) Populations. Neotrop Entomol 49, 179–190 (2020). https://doi.org/10.1007/s13744-019-00743-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-019-00743-z