Abstract
In the last decade, several studies demonstrated the effectiveness of ecological network analysis to a better understanding of the structure bee–plant interaction networks; however, such approaches involving urban areas are still scarce. Here, we analyzed two assemblages of corbiculate bees (Apoidea, Apidae) in two geographically distinct urban areas in Brazil. In both study areas, apid bees visiting flowers were captured with an insect net. Surveys were performed biweekly and alternately in each area, over a 1-year period. Both urban areas were very similar for most indices. The two social bee–plant networks were significantly nested, a pattern usually described for bee–plant networks and somehow expected in our study, considering the recognized behavior of social apid bees in exploring a wide range of plant species. The modularity measures were low and very similar for the networks of both urban areas, a finding that could be due at least in part to the low phylogenetic distance between corbiculate bees and the broad dietary habits of the social apid bees. Network-level indices showed that both bee assemblages had a relatively low niche overlap, indicating that the set of social apid species studied exploited differently the arrays of plants available. Species level index (resource range) showed that in both urban areas, Trigona spinipes (Fabr.) and Apis mellifera L. showed the higher number of interactions, a result that demonstrates the importance of these species in social bee–plant interaction networks in urban areas. Similarly to other ecosystems, these two apid species behaved as super-generalists in the two urban areas surveyed herein.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the Neotropical region, eusocial Apidae bees (stingless bees, bumble bees, and honeybees) are among the most frequent flower visitors in different ecosystems (Roubik 1989, Pedro & Camargo 1991, Zanette et al 2005, Aguiar & de Mendonça Santos 2007, de Mendonça Santos et al 2013). This high frequency of eusocial apid bees as flower visitors can be explained, at least in part, by the social behavior of these bees, which usually raise perennial colonies with thousands of individuals and exhibit efficient communication systems among members of the colony (Michener 2000, Nieh 2004). Furthermore, most eusocial apid bees use a wide range of plant species for nectar and pollen collection (Biesmeijer & Slaa 2006), exhibiting, thus, a broad diet breath (Cortopassi-Laurino & Ramalho 1988, de Mendonça Santos et al 2010, 2012, Kleinert & Giannini 2012).
Apparently, studies performed in tropical urban areas also show the eusocial apid as the commonest bees on flowers from parks and gardens (Laroca et al 1982, Liow et al 2001). Particularly, in Brazil, the studies carried out in urban biotopes have shown this trend (Laroca et al 1982, Knoll et al 1993, Bortoli & Laroca 1997, Taura & Laroca 2001, Zanette et al 2005, Taura et al 2007).
The high abundance of eusocial apid bees in different ecosystems and the high number of interactions made by this group of bees with several plant species suggest the importance of social apid, both in the structuring of the Apoidea community as well as in providing pollination services in these ecosystems. As a consequence, some studies have searched for patterns or trends involving tropical eusocial bees and their food sources and vice versa based on plant–bee network approaches (Biesmeijer et al 2005, Biesmeijer & Slaa 2006, de Mendonça Santos et al 2010). In fact, ecological networks, which involve the interactions between species in a community, have contributed to a higher understanding of the structural properties of different ecological systems (Stouffer et al 2012), consolidating itself as an important method to be used in ecological studies.
Studies involving plant–bee networks, which represent a type of facultative mutualism, have shown frequently a nested pattern (Bezerra et al 2009, Pigozzo & Viana 2010, de Mendonça Santos et al 2010, 2012, Mello et al 2013), with a core set of generalist species having key roles in network structure (Potts et al 2010).
In Brazil, among the most frequent eusocial apid species visiting flowers in different ecosystems, including urban areas, are the exotic bee Apis mellifera Linnaeus and the stingless bee Trigona spinipes (Fabricius) (Pedro & Camargo 1991, Taura & Laroca 2001, Aguiar & de Mendonça Santos 2007). The high abundance of these two species in urban areas has been recognized as a high level of adaptability of both species to urban biotopes (Cortopassi-Laurino & Ramalho 1988).
A recent study revealed that Apis mellifera occupied a central position in all six bee–plant networks studied, playing the role of a “super-generalist” species and thus being able to induce significant changes in the structure of these networks (de Mendonça Santos et al 2012). Thus, the analysis of bee–plant interactions under the perspective of networks can provide complementary information and a more complete understanding of the structure of plant–visitor community. Moreover, Biesmeijer et al (2005) observed that the level of generalization, an important measure involving ecological networks, in a plant–flower visitor community can be influenced by habitat even within a group of generalist flower visitors.
In urban environments, where the changes in the array of plants is usually frequent due to the strong anthropogenic interference (Gong et al 2013), approaches involving plant–bee interaction networks could provide useful information for further effective conservation measures in such ecosystems. The native vegetation is usually scarce in these areas, while parks and residential areas show a high number of alien species (Frankie et al 2005, 2009, Schwartz et al 2006). As a consequence, some studies have suggested that urban areas may be undergoing a process of homogenization (McKinney 2006, Schwartz et al 2006, Gong et al 2013), with potentially important implications for bee communities in these environments.
In recent years, urban areas have received more attention regarding the conservation of bees and other pollinators, since these areas have now been recognized as potential “refuges” for the native fauna, which search in urban biotopes for resources for their survival (Frankie et al 2009, Ernstson et al 2010).
Ernstson et al (2010) advocated that green areas such as squares, parks, urban forests, and even cemeteries, provide important ecosystem services, such as CO2 absorption, maintenance of pollination, seed dispersal, and other important ecological processes. Despite such importance, these authors state that, unlike other urban services, there has been a profound neglect in research and theorizing about the importance of ecosystems in urban landscapes. In this context, Wojcik & McBride (2012) affirmed that a successful management of pollination resources present in urban ecosystem, depends on understanding the factors that shape urban communities of bees, which further increases the need for such studies.
Thus, the aim of this study was to investigate and compare the social apid–bees plant interaction networks in two urban areas in Brazil. We expect that in both urban areas studied herein, the results for almost indices tend to be similar, since both areas have similar characteristics and a large number of bee species in common.
Material and Methods
Datasets
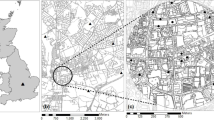
The construction of plant–visitor networks was based on the set of social apid bees netted on flowers, in two urban areas (campus of the Universidade de São Paulo (USP) (21°11′S; 47°48′W); Ribeirão Preto, state of São Paulo, southeastern Brazil; and campus of the Universidade Estadual de Londrina (UEL) (23°19′S; 51°12′W), Londrina, state of Paraná, southern Brazil). The distance between both areas is about 500 km. The method used was based on Sakagami et al (1967), with modifications. Samplings were performed biweekly and simultaneously, during a year, by one of the authors (S.H. Sofia), from June to May. Each sampling was carried out in two consecutive days from 12:00 to 18:00 p.m. (first day) and from 6:00 a.m. to 12:00 p.m. (second day; more details in Sofia 1996). During the surveys, the collector walked along a transect and sampled all plants in bloom However, trying to cover better the spatially heterogeneous distribution of the plants, throughout transect, the collector also walked in the adjacent areas to transect searching actively for bees on plants in blooming. At UEL, the extension of transect was about 2,550 m, and at USP, was about 3,650 m. The vegetation of both study areas can be considered typical of urban areas, which exhibit scarce native vegetation and a high occurrence of exotic ornamental plants, usually cultivated in residential gardens, as well as fructiferous species, such as Mangifera indica, Persea americana, and Morus spp. The similarity, based on the Morisita-Horn index, between the arrays of plant genera visited by social apid bees in both areas was of 0.487. The Morisita-Horn index was calculated through the software SAPDE (Chao & Shen 2010).
Data Analysis
Network analysis
Datasets on the plant and bees of each study area were transformed into adjacent matrices of bee and plants, with bee species plotted as B rows and plant species as P columns, for qualitative analysis. In this case, 1 represented the records and 0, the absence of records. For quantitative analysis, we constructed the matrices based on the number of records (i.e., number of bees collected from each plant species).
To compare the plant–bee networks from two urban areas the following main parameters were analyzed: (a) nestedness, a topology represented by “a core of reciprocal generalists accompanied by specialists species that interact almost exclusively with generalists” (Guimarães et al 2006); herein, nestedness was measured through both qualitative and quantitative indices, respectively, NODF (Almeida-Neto et al 2008) and WNODF (Almeida-Neto & Ulrich 2011); (b) modularity (M, Guimerà & Amaral 2005), a qualitative estimator which can be used to test for a subgroup structure and to assess this structure based on the pattern of species interactions (Mello et al 2011); (c) specialization H2′, a quantitative index used to measure the level of network specialization (Blüthgen et al 2006); connectance—C, qualitative index representing from all possible interactions, those actually observed within a network (Biesmeijer et al 2005), and niche overlap (Horn 1966) of bee and plant species; (d) robustness (R), a qualitative index representing the resistance of the network to extinctions (Burgos et al 2007); in this study was considered both random removal and first removal of generalist plant species; (e) resource range—which is a qualitative index used to measure the resources exploited by different species (Schoener 1989). All indices employed in the analyses varied from zero to 1. Particularly, for resource range “0” represents that all resources are used by the species while “1”represents that only one resource is used.
To measure the nestedness of networks, we used the qualitative NODF metric, and its significance was estimated through the Monte Carlo procedure (1,000 randomizations), using the software Aninhado (Guimarães & Guimarães 2006). There are two null models available in this software to test the significance of NODF. We used the second null model (Ce), since it takes into consideration that the probability of each cell being occupied is the average probabilities of occupancy of its column and row, which means that the probability of an interaction is proportional to the degree of generality of the animal or plant (for details, see Guimarães & Guimarães 2006). To test the significance of NODF, we compared the value of the real matrix to the indices of nestedness generated by the null model. When no random matrix had a value higher than NODF real matrix, we defined p < 0.001.
In order to compare two different indices (qualitative and quantitative), we also employed the WNODF quantitative metric. To test the significance of WNODF, we used the function “null.t.test” with 1,000 randomizations, available in Bipartite 2.0 (Dormann et al 2008) package for R 2.15.1 (R Development Core Team 2011).
The modularity was measured by the index M, using the software NetCarto (Guimerà & Amaral 2005). This index ranges from 0 (no subgroups) to 1 (fully separated into subgroups). Considering that NetCarto was created for one-mode networks, and therefore, not adequate to estimate the significance of M, we opted to use the software “Combo Netcarto,” created by Flavia Marquitti. To estimate the significance of M, 1,000 random networks were first generated for each network (according to the null model 2) using the package vegan 2.0–8 (Oksanen et al 2010). After that, we applied a one-sample t test (www.graphpad.com), which provided the p values. With the information provided by NetCarto, an energized network (Kamada-Kawai, Free) was obtained for each area using the program Pajek 2.02 (Batagelj & Mrvar 1998), which includes information about the modules present in the networks and the importance of each species within its module. Also, a bipartite graph was built using a Bipartite 2.0 package (Dormann et al 2008) for R.
To compare the specialization of both networks, we accessed some indices provided by the Bipartite 2.0 package for R. We decided to use two quantitative indices H2′, niche overlap and one qualitative connectance index. All of them were obtained using the function “network level” (Dormann et al 2009) in Bipartite 2.0 for R. To test the significance of these indices, we used the function null.t.test with 1,000 randomizations, also available on bipartite 2.0 package for R.
Robustness can be defined as the ability of a network to withstand ecological extinctions. The robustness to plant species removal from both areas was estimated following Burgos et al (2007) and Memmott et al (2004) using the Bipartite 2.0 package for R. We performed two different analyses: (a) random removal of plant species (Burgos et al 2007) and (b) most generalist plant species going extinct first (Memmott et al 2004).
In order to compare bee species from both study areas (UEL and USP), we used the qualitative resource range index. However, in this analysis, we considered only those apid bee species represented by 10 or more individuals in the sample in each area (see de Mendonça Santos et al 2013). This index was obtained using the function “species level” available in the Bipartite 2.0 for R.
Results
The UEL bee–plant network included 82 plant species belonging to 69 genera and 38 different angiosperm families, as well as 15 species of corbiculate bees from 11 genera (Table S1 in Supplementary material). A total of 4,090 visits of bees to plants were recorded. Trigona spinipes was the bee specie that presented the highest degree (or number of interactions) (61), followed by Apis mellifera (59), Tetragonisca angustula (Latreille) (36), Nannotrigona testaceicornis (Lepeletier) (26), and Trigona hyalinata (Lepeletier) (22).
The plant that showed the highest degree of interaction was Malvaviscus arboreus (9), followed by Delonix regia (7), Rhododendron simsii (6), Thea sinensis (6), Cassia fistula (6), Nicotiana tabacum (6), Caesalpinia pluviosa (6) and Tibouchina granulosa (6). The whole list of plant species is shown in Tables S2 and S3 in the Online Supplementary Material.
The USP network included 105 species of plants belonging to 93 genera and 42 families and also 28 species of eusocial apid bees belonging to 18 genera (Table S4). We recorded 9,151 visits of bees to plants. As occurred at UEL, Trigona spinipes was the bee species that showed the highest number of interactions (78), followed by Apis mellifera (64), Plebeia droryana (Friese) (49), Nannotrigona testaceicornis (47), and Tetragonisca angustula (45).
The plant that showed the highest number of interaction was Pentas lanceolata (15), followed by Tecoma stans (14), Duranta repens (14), Bauhinia variegata (13), and Asystasia gangetica (12). The whole list of plant species is shown in Tables S5 and S6 in the Online Supplementary Material.
The networks of both areas were significantly nested (p < 0.001) (Table 1), with both qualitative (NODF) and quantitative (WNODF) measures of nestedness showing a slight difference between plant–bee networks from UEL and USP, as follows: UEL (NODF = 0.62 and WNODF = 0.372) and USP (NODF = 0.55 and WNODF = 0.334).
In our analyses, the two networks were significantly modular, showing very similar modularity (UEL = 0.2105; USP = 0.2151; p < 0.001), with six modules identified in each area (Fig 1). In general, comparing the groups of species that were more abundant in number of individuals and common to both areas—Trigona spinipes, Apis mellifera, Trigona hyalinata, Tetragonisca angustula, Trigona fuscipennis Friese, Nannotrigona testaceicornis, Plebeia droryana, Friesella schrotkyii Friese, Bombus morio (Swederus)—we could notice that there wasn’t a pattern for both areas, since most species were distributed in different modules in each area. For instance, Trigona spinipes and Apis mellifera, the most abundant species in both areas, shared the same module (red) at UEL but not at USP (red and purple, respectively). Particularly, regarding the larger-sized apid bees Bombus morio, Bombus pauloenis Friese and Eulaema nigrita Lepeletier, they shared the same modules (green) in both these areas (Fig 1).
UEL (a) and USP (b) networks. Different colors mean different modules. The most important species are drawn closer to the center of each graph. Vertex size is proportional to the network functional role of each species. Circles represent species of bees. Boxes represent species of plants. UEL codes are listed in Table S1 (bees) and S3 (plants). USP codes are listed in Table S4 (bees) and S6 (plants). UEL corresponds to Universidade Estadual de Londrina (Londrina, PR, Brazil) and USP corresponds to Universidade de São Paulo (Ribeirão Preto, SP, Brazil).
As observed for nestedness and modularity, the H2′ index, an estimate of network specialization, showed similar values for both networks (Table 1). In addition, connectance (C) showed the same pattern (Table 1). As shown in Table 1, the estimates of C were 0.2089 (UEL) and 0.1803 (USP), while the H2′ measures were 0.3983 (UEL) and 0.4533 (USP). Also, bee niche overlap (HL) and plant niche overlap (LL) were similar in both areas (Table 1).
Our findings also revealed high robustness to the analysis of random plant species removal (UEL = 0.828; USP = 0.877; p < 0.01) in apid bee–plant networks from the two studied areas. However, the robustness of both networks clearly decreased (UEL: 0.586; USP = 0.580; p < 0.01) when the analysis involved the removal of the most generalist plant species first.
The resource range index revealed that Trigona spinipes and Apis mellifera appear as the two most generalist species (i.e., showing low specialization), showing values of resource range below 0.5 (Fig 2). Figure 3 shows two mode-ordered graphs obtained for the arrays of plants and social apid bee species surveyed in two urban areas. In both graphs, Trigona spinipes, Apis mellifera, Trigona hyalinata, and Tetrahonisca angustula appear as the most abundant species at USP and UEL. It is also possible to observe that Malvaviscus arboreus, Euphorbia milli var. breonii, Rhododendron simsii, and Euphorbia milli var. milli at UEL were the plant species most visited by bees (Fig 3a). At USP, the species of plants that attracted a higher number of visitors were Asystasia gangetica, Pentas lanceolata, Euphorbia milli var. breonii and Pachystachys lutea (Fig 3b).
Scatterplots with the species level indices: resource range (0 means that the species is generalist and 1 means the opposite). T_spi = Trigona spinipes; A_mel = Apis mellifera; P_dro = Plebeia droryana; N_tes = Nannotrigona testaceicornis; T_ang = Tetragonisca angustula; P_lin = Paratrigona lineata; T_cla = Tetragona clavipes; P_hel = Partamona helleri; T_hya = Trigona hyalinata; F_sch = Friesella schrottkyi; B_pau = Bombus pauloensis; T_rec = Trigona recurs; S_lat = Scaura latitarsis; L_mue = Leurotrigona muelleri; B_mor = Bombus morio; F_var = Frieseomelitta varia; S_dep = Scaptotrigona depilis; T_fus = Trigona fuscipennis; G_inu = Geotrigona inusitata; S_bip = Scaptotrigona bipuntacta; P_eme = Plebeia emerina; and S_pos = Scaptotrogina postica.
Two mode-ordered graphs. a UEL. b USP. For both graphs, plants are black and bees are gray. We get the codes of bees retaining the first letter of the genus and the first three of the specie. UEL codes are listed in Table S1 (bees) and S3 (plants). USP codes are listed in Table S4 (bees) and S6 (plants).
Discussion
Despite the difference in number of eusocial apid bee species surveyed in each urban area (15 at UEL and 28 at USP) and the differences between the arrays of plant genera visited by bees in these areas (which were about 50% similar), our findings revealed that the two eusocial bee–plant networks were very similar in the two urban areas studied. We can attribute this result to the similar biological habits or eusocial behavior of all bee species studied herein. Besides the already mentioned social behavior, we can also include here the large period of activity of these bees, which usually forage for food resources in the tropical and subtropical areas in the Neotropical zone the whole year (Pedro & Camargo 1991, Biesmeijer & Slaa 2006).
The eusocial apid bees are recognized as generalist in their use of plant sources, since they frequently exploit a wide array of food sources (Biesmeijer & Slaa 2006). However, among the large set of social apid bees analyzed by these authors, Trigona spinipes and Apis mellifera showed the broadest niches of all social Apidae. Thus, these species have been considered by different authors as super-generalists in terms of food plant use (Biesmeijer et al 2005, Biesmeijer & Slaa 2006, de Mendonça Santos et al 2012).
In different analyses involving bee–plant networks in Brazil, Apis mellifera and Trigona spinipes are among the most generalist species (Pigozzo & Viana 2010, de Mendonça Santos et al 2010, 2012, 2013, Kleinert & Giannini 2012, Mouga et al 2012). Also, within the set of social apid bees visiting flowers in Caatinga biome, Trigona spinipes and Apis mellifera were among the six apid species that were more generalist and which were particularly important in structuring the bee–plant network (de Mendonça Santos et al 2010). In our study, these two species also behaved as super-generalist in both bee–plant networks and developed an important role in structuring these networks. The super-generalist behavior of both Apis mellifera and Trigona spinipes were also corroborated by the resource range index, since they showed the lower values of this index in both urban areas.
With respect to connectance (C), the C values found for the networks of the UEL (0.2089) and USP (0.1803) were similar to estimates of connectance described by Biesmeijer et al (2005) (0.2426 ± 0.0470) also for social bee–plant networks of urban areas. According to Blüthgen et al (2006), although network analyses of plant–animal interactions provide useful biological information, when only qualitative indices, such as “connectance” or “number of links,” are used to estimate the degree of specialization in these networks part of the information can be lost. Thus, these authors proposed the use of H2′, a quantitative network-level index that is useful for comparison across different interaction webs. Comparing the values of C and H2′ (UEL: 0.3983; USP: 0.4533) obtained for the social bee–plant networks in the current study, and considering that both indices herein applied ranged from 0 to 1, we can notice that H2′ show a tendency toward higher specialization, and consequently a lower niche overlap, in both networks than that revealed by the connectance index. Reinforcing H2′ estimates, bee niche overlap (HL) was low in both areas, indicating that there is a division in the exploration of floral resources between bee species. Diversely, the overlap (LL) values of the plant niches found, which were from three to four times higher than those measured for bees, reflecting that both networks have a greater number of plant species than of bee species. So, the chance of two plants sharing the same bee is greater than the chance of two or more bees sharing the same plant species.
Nestedness is the most common pattern of structure in mutualistic networks (Bascompte et al 2003). Krishna et al (2008) suggest that from 60% to 70% of nestedness in the plant–animal mutualistic networks can be explained by the relative abundance of the species. Guimarães et al (2006) stated that nested networks are characterized by “(i) generalist species that interact with each other, composing a core of interacting species, (ii) specialist species that usually interact only with generalist and, (iii) the absence of specialist that interact only with other specialists.” This pattern can be seen in Fig 3. Comparing our measures of nestedness for both networks with the estimates found in several studies on bee–plant networks in different natural ecosystems in Brazil (Pigozzo & Viana 2010, Mouga et al 2012, de Mendonça Santos et al 2012), we can notice that our measures of nestedness were very higher. Such difference was somehow expected, since our analyses included only eusocial apid species, while these authors studied all Apoidea assemblage present in the natural areas. Despite the peculiar differences among different species of eusocial apid bees, they are recognized for exploring a broader plant species spectrum than solitary bee species. Pigozzo & Viana (2010) suggest that to a certain extent, nestedness can be a consequence of the difference in attractiveness of plant species. These authors also suggest that nestedness could be related to the ability of different species to exploit the different range of resources (generalist × specialist). Similarly, Lewinsohn et al (2006) propose that the nested pattern could be related to the abundance of species, with the most abundant being more generalist, and the less abundant showing a more restricted range of spectrum, fact that occurred in our two networks (data not shown).
The measures of modularity detected in both apid bee–plant networks were comparable to those described for bee–plants showing a high degree of specialization, such as oil flower networks (Bezerra et al 2009, Mello et al 2013). These latter authors stated that even in highly specialized mutualistic networks (e.g., oil flower–bee networks), local conditions can play an important role in network structuring. Also, the modularity in both urban areas studied herein were consistently low compared to the average modularity of 29 pollinator–plant networks (\( \overline{M}=0.52\pm 0.068 \); p < 0.05) analyzed by Olesen et al (2007). These authors suggest that the level of modularity can be reduced if the size of the core of links between generalist species is dense, a fact that was also observed in our analyses (Fig 3). Thus, in our study, the low modularity found could be reflecting different factors, including the low phylogenetic distance between corbiculate bees and the broad dietary habits (wide trophic niches) of the social apid bees (Cortopassi-Laurino & Ramalho 1988, Biesmeijer et al 2005, Biesmeijer & Slaa 2006). Furthermore, the simplification or homogenization of the array of plants commonly present in urban areas (Schwartz et al 2006, Gong et al 2013) can also play a role in the network structure.
Our two different analyses concerning the networks robustness, i.e., considering random plant removal and most generalist plant species going extinct first (showing higher degrees) were important, since they showed very distinctive results. While in the first condition, bees were apparently resistant to extinction of plants, in the second case, they were much less resistant to extinction. Since changes in the vegetation of urban areas are usually frequent and highly determined by anthropic interference, we can expect that if the changes in plant assemblages affect more directly the array of generalist plants, the social apid bees will probably be more negatively affected. Thus, despite the wide trophic niche of social apid bees, such bees are not free from the risk of experiencing a decline in their populations in urban areas. Based on this and considering both the declines in bee populations that have been reported by different authors in the last decade (Potts et al 2010), as well as the recognized importance of urban areas as potential refuges for different species of bees, we highlight the relevance of an adequate management of the flora of these areas for a better conservation of eusocial apid bees and probably other groups of bees.
Overall, regarding biodiversity conservation in urban areas, our study revealed two main findings: (1) the usefulness of the network analysis to better understand the structure of bee–plant interactions in urban areas and (2) that changes in the array of plants can negatively affect the network structure in urban environments. Furthermore, based on the similar pattern of the network structure of both areas found in the current study, we cannot discard the existence of a pattern in bee–plant networks for urban areas. Taken together, these findings highlight the need of new and broader approaches on bee–plant networks from urban areas, involving the entire bee community present in these environments as well as a larger number of study sites or cities. In this scenario, the information obtained could help us to manage urban areas and their bee and plant assemblages more properly.
References
Aguiar CML, de Mendonça Santos GM (2007) Compartilhamento de recursos florais por vespas sociais (Hymenoptera: Vespidae) e abelhas (Hymenoptera: Apoidea) em uma área de Caatinga. Neotropical Entomol 36:836–842
Almeida-Neto M, Ulrich W (2011) A straightforward computational approach for measuring nestedness using quantitative matrices. Environ Model Softw 26:173–178
Almeida-Neto M, Guimarães P, Guimarães PR Jr, Loyola RD, Ulrich W (2008) A consistent metric for nestedness analysis in ecological systems: reconciling concept and quantification. Oikos 117:1227–1239
Bascompte J, Jordano P, Melián CJ, Olesen JO (2003) The nested assembly of plant–animal mutualistic networks. Proc Natl Acad Sci 100:9383–9387
Batagelj V, Mrvar A (1998) Pajek—a program for large network analysis. Connections 21:47–57
Bezerra ELS, Machado IC, Mello MAR (2009) Pollination networks of oil-flowers: a tiny world within the smallest of all worlds. J Anim Ecol 78:1096–1101
Biesmeijer JC, Slaa J (2006) The structure of eusocial bee assemblages in Brazil. Apidologie 37:240–258
Biesmeijer JC, Slaa J, Castro MS, Viana BF, Kleinert MP, Imperatriz-Fonseca VL (2005) Connectance of Brazilian social bee—food plant networks is influenced by habitat, but not by latitude, altitude or network size. Biota Neotrop 5:1–9
Blüthgen N, Menzel F, Blüthgen N (2006) Measuring specialization in species interaction networks. BMC Ecol 6:9
Bortoli C, Laroca S (1997) Melissocenologia no Terceiro Planalto Paranaense. I. Abundância relativa das abelhas silvestres (Apoidea) de um biótopo urbano de Guarapuava (PR, Brasil). Acta Biol Par 26(1,2,3,4):51–86
Burgos E, Ceva H, Perazzo RPJ, Devoto M, Medan D, Zimmermannd M, Delbuee AM (2007) Why nestedness in mutualistic networks? J Theor Biol 249:307–313
Chao A, Shen TJ (2010) Program SPADE (species prediction and diversity estimation). program and user’s guide published at http://chao.stat.nthu.edu.tw
Cortopassi-Laurino M, Ramalho M (1988) Pollen harvest by Africanized Apis mellifera and Trigona spinipes in São Paulo: botanical and ecological views. Apidologie 19:1–24
de Mendonça Santos GM, Aguiar CML, Mello MAR (2010) Flower-visiting guild associated with the Caatinga flora: trophic interaction networks formed by social bees and social wasps with plants. Apidologie 41:466–475
de Mendonça Santos GM, Aguiar CML, Genini J, Martins CF, Zanella FCV, Mello MAR (2012) Invasive Africanized honeybees change the structure of native pollination networks in Brazil. Biol Invasions 14:2369–2378
de Mendonça Santos GM, Carvalho CAL, Aguiar CML, Macêdo LSSR, Mello MAR (2013) Overlap in trophic and temporal niches in the flower-visiting bee guild (Hymenoptera, Apoidea) of a tropical dry forest. Apidologie 44:64–74
Dormann CF, Gruber B, Fründ J (2008) Introducing the bipartite package: analyzing ecological networks. R News 8:8–11
Dormann CF, Fründ J, Blüthgen N, Gruber B (2009) Indices, graphs and null models: analyzing bipartite ecological networks. Open Ecol J 2:7–24
Ernstson H, Barthel S, Andersson E (2010) Scale-c brokers and network governance of urban ecosystem services: the case of Stockholm. Ecol Soc 15:28
Frankie GW, Thorp RW, Schindler M, Hernandez J, Ertter B, Rizzardi M (2005) Ecological patterns of bees and their host ornamental flowers in two northern California cities. J Kansas Entomol Soc 78:227–246
Frankie GW, Thorp RW, Hernandez J, Rizzardi M, Ertter B, Pawelek JC, Witt SL, Schindler M, Coville R, Wojcik VA (2009) Native bees are a rich natural resource in urban California gardens. Calif Agric 63:113–120
Gong C, Chen J, Yu S (2013) Biotic homogenization and differentiation of the flora in artificial and near-natural habitats across urban green spaces. Landsc Urban Plan 120:158–169
Guimarães PR, Guimarães PR (2006) Improving the analyses of nestedness for large sets of matrices. Environ Model Softw 21:1512–1513
Guimarães PR Jr, Rico-Gray V, dos Reis SF, Thompson JN (2006) Asymmetries in specialization in ant-plant mutualistic networks. Proc R Soc Lond B Biol Sci 273:2041–2047
Guimerà R, Amaral LAN (2005) Functional cartography of complex metabolic networks. Nature 433:895–900
Horn HS (1966) Measurement of “overlap” in comparative ecological studies. Am Nat 100:419–424
Kleinert AMP, Giannini TC (2012) Generalist bee species on Brazilian bee–plant interaction networks. Psyche Article ID 291519, 7 pages
Knoll FRN, Rego LR, Imperatriz-Fonseca VL (1993) As abelhas em áreas urbanas—um estudo no Campus da Universidade de São Paulo. In: Pirani JR, Cortopassi-Laurino M (eds) Flores e abelhas em São Paulo. Edusp/Fapesp, São Paulo, pp 31–42
Krishna A, Guimarães PR Jr, Jordano P, Bascompte J (2008) A neutral-niche theory of nestedness in mutualistic networks. Oikos 117:1609–1618
Laroca S, Cure JR, Bortoli C (1982) A associação de abelhas silvestres (Hymenoptera, Apoidea) de uma área restrita no interior da cidade de Curitiba (Brasil): uma abordagem biocenótica. Dusenia 13:93–117
Lewinsohn T, Prado PI, Jordano P, Bascompte J, Olesen JM (2006) Structure in plant–animal interaction assemblages. Oikos 113:174–184
Liow LH, Sodhi NS, Elmqvist T (2001) Bee diversity along a disturbance gradient in tropical lowland forests of south-east Asia. J Appl Ecol 38:180–192
McKinney ML (2006) Urbanization as a major cause of biotic homogenization. Biol Conserv 127:247–260
Mello MAR, Marquitti FMD, Guimarães PR Jr, Kalko EKV, Jordano P, Aguiar MA (2011) The missing part of seed dispersal networks: structure and robustness of bat–fruit interactions. PLoS ONE 6:e17395
Mello MAR, Bezerra ELS, Machado IC (2013) Functional roles of centridini oil bees and Malpighiaceae oil flowers in biome-wide pollination networks. Biotropica 45:45–53
Memmott J, Waser NM, Price MV (2004) Tolerance of pollination networks to species extinctions. Proc R Soc Lond B Biol Sci 271:2605–2611
Michener CD (2000) The bees of the world. John Hopkins University Press, Baltimore, 992p
Mouga DMDS, Noble CF, Bussmann DBG, Krug C (2012) Bees and plants in a transition area between Atlantic rainforest and Araucaria forest in southern Brazil. Rev Ecol Terre Vie 67:313–327
Nieh JC (2004) Recruitment communication in stingless bees (Hymenoptera, Apidae, Meliponini). Apidologie 35:159–182
Oksanen J, Blanchet FG, Kindt R, Legendre P, O’Hara RG, Simpson GL, Solymos P, Stevens MHH, Wagner H (2010) Vegan: community ecology package. R package version 1.17-0. http://cran.r-project.org/web/packages/vegan
Olesen JM, Bascompte J, Dupont YL, Jordano P (2007) The modularity of pollination networks. Proc Natl Acad Sci U S A 104:19891–19896
Pedro SEM, Camargo JMF (1991) Interactions on floral resources between the africanized honey bee Apis mellifera L. and the native bee community (Hymenoptera: Apoidea) in a natural “cerrado” ecosystem in southeast Brazil. Apidologie 22:397–415
Pigozzo CM, Viana BF (2010) Estrutura da rede de interações entre flores e abelhas em ambiente de Caatinga. Oecologia Aust 14:100–114
Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE (2010) Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol 25:345–353
R Development Core Team (2011) R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. ISBN 3-900051-07-0, URL: http://www.R-project.org
Roubik DW (1989) Ecology and natural history of tropical bees. Cambridge University Press, New York, 526p
Sakagami SF, Laroca S, Moure JS (1967) Wild bees biocenotics in São José dos Pinhais (PR), South Brazil—preliminary report. J Fac Sci Hokkaido Univ (Ser 6, Zool) 18:57–127
Schoener TW (1989) Food webs from the small to the large. Ecology 70:1559–1589
Schwartz MW, Thorne JH, Viers JH (2006) Biotic homogenization of the California flora in urban and urbanizing regions. Biol Conserv 127:282–291
Sofia SH (1996) As abelhas e suas visitas às flores em duas áreas urbanas. PhD thesis Instituto de Biociências do campus de Rio Claro, Universidade Estadual Paulista, Brazil. 236p
Stouffer DB, Sales-Pardo M, Sirer MI, Bascompte J (2012) Evolutionary conservation of species’ roles in food webs. Science 335:1489–1492
Taura HM, Laroca S (2001) A associação de abelhas silvestres de um biótopo urbano de Curitiba (Brasil), com comparações espaço-temporais: abundância relativa, fenologia, diversidade e exploração de recursos. Acta Biol Par 30:35–137
Taura HM, Laroca S, Barbosa JF, Rodrigues J (2007) Melissocenótica (Hymenoptera, Anthophila) no Parque Florestal dos Pioneiros, Maringá, PR. (sul do Brasil)—I. Abundância relativa e diversidade. Acta Biol Par 36(1–2):47–65
Wojcik VA, McBride JR (2012) Common factors influence bee foraging in urban and wildland landscapes. Urban Ecosyst 15:581–598
Zanette LRS, Martins RP, Ribeiro SP (2005) Effects of urbanization on Neotropical wasp and bee assemblages in a Brazilian metropolis. Landsc Urban Plan 71:105–121
Acknowledgments
The authors would like to thank PROCAD/CAPES (158/2007) for their financial support. H. Zotarelli received a scholarship from Coordination of Improvement of Higher Education Personnel (CAPES). The authors also thank the two anonymous reviewers for their valuable comments. Silvia H. Sofia is research fellows from the Brazilian Council for Scientific and Technological Development (CNPq).
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Fernando B Noll – UNESP
Rights and permissions
About this article
Cite this article
Zotarelli, H.G.S., Evans, D.M., Bego, L.R. et al. A Comparison of Social Bee–Plant Networks between Two Urban Areas. Neotrop Entomol 43, 399–408 (2014). https://doi.org/10.1007/s13744-014-0227-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-014-0227-8