Abstract
Bactrocera (Tetradacus) minax Enderlein is a major pest to wild and cultivated species of citrus. Bactrocera minax produces one generation per year with a long pupal diapause period of over 6 months, which hinders efforts to obtain vast numbers of insects under standard room conditions. Determining the mechanisms of diapause is significantly important for obtaining large quantities of these insects. To characterize the heat shock protein (Hsp) genes of B. minax and to unravel their potential contribution to diapause, we performed 3′ and 5′ RACE to isolate the complementary DNA (cDNA) sequences, bioinformatics to examine the phylogenetic relationships, and real-time quantitative PCR to detect the expression patterns of three Hsp genes during various developmental stages. These results represent the first characterization of the three Hsp genes of B. minax; the open reading frames of Bmhsp23, Bmhsp70, and Bmhsp90 were 510, 1,911, and 1,089 bp, encoding 170, 636, and 363 amino acids, respectively. BmHsp70 and BmHsp90 displayed high identity to previously identified Hsp70 and Hsp90 genes, respectively. BmHsp23 displayed varying similarity, from 28 to 83%, to previously identified small Hsps. Bmhsp23 messenger RNA (mRNA) expression was found to be upregulated during diapause initiation, maintenance, and termination. Bmhsp70 mRNA expression peaked during diapause initiation. Bmhsp90 mRNA expression remained at a relatively low level during deep diapause. Our present results suggest that Bmhsp70 might play an important role in diapause initiation, while Bmhsp23 in diapause initiation and maintenance and Bmhsp90 in diapause regulation. These results improve our understanding of the mechanism of diapause in B. minax at the molecular level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bactrocera (Tetradacus) minax Enderlein (Diptera: Tephritidae; Chinese citrus fruit fly) is unique with respect to its feeding behavior and its reproductive rate of one generation per year (van Schoubroeck 1999). Bactrocera minax has been causing considerable losses in commercial citrus for more than half a century in China (Wang & Luyi 1995). A series of strategies, including spraying of chemical pesticides and food attractants (Yang et al 1994, Wang & Luyi 1995, van Schoubroeck 1999), has been used to manage this pest. However, these measures have not effectively reduced its population density. Studies have revealed that the sterile insect/male technique (SIT) (Wang & Zhang 1993) and the genetic transformation technique play important roles in the prevention and control of fruit fly pests (Wimmer 2003, Vreysen et al 2007). The SIT and the genetic transformation technique have good potential to prevent and control B. minax, as the pest is unique with respect to its feeding behavior and its reproductive rate of one generation per year. However, B. minax exhibits a long period for pupa development (more than 6 months), which hinders the production of large quantities of these insects. Therefore, understanding and breaking diapause is a key step to obtain vast numbers of insects by producing two or more generations per year, which is important for the development of new techniques to control this pest.
Diapause is a common developmental strategy used by insects to survive winter and other periods of seasonal adversity (Xu 2008, Denlinger et al 2007). Bactrocera minax survives winter via pupa diapause from late October to late April or even early May (Wang & Luyi 1995). The mechanism of insect diapause is complex, and understanding the molecular mechanism involved in diapause initiation and regulation will provide the basic information required to break diapause. Insect diapause may be regulated by environmental, hormonal, and molecular factors, and understanding this molecular regulation remains in its infancy (Denlinger 2002, Xu 2008).
A number of diapause-specific genes have been identified based on their expression patterns during early, mid- and/or late diapause (Denlinger 2002, Xu 2008). Regulation of a subset of heat shock protein (Hsp) genes may be related to different types of insect diapause (Denlinger 2002, MacRae 2010, Xiao et al 2011). Hsps are a highly conserved superfamily of molecular chaperones that facilitate appropriate protein folding and localization while preventing protein aggregation (Feder & Hofmann 1999, Hartl & Hayer-Hartl 2002). Previous studies have demonstrated that Hsps play a major role in diapause regulation in a wide range of organisms (Yuan et al 1996, Denlinger et al 2001, Qiu & MacRae 2008a, b, MacRae 2010). During diapause, Hsps are thought to contribute to cell cycle arrest and increased stress resistance (Denlinger et al 2001, Rinehart et al 2007, MacRae 2010). Hsp gene expression patterns during diapause may be highly variable between species (Rinehart & Denlinger 2000, Denlinger et al 2001, Yocum 2001, Tungjitwitayakul et al 2008). Different classes of Hsps can play distinct roles in diapause within a species (Goto et al 1998, Rinehart & Denlinger 2000, Rinehart et al 2000, 2007, Goto & Kimura 2004, Aruda et al 2011). The characteristics of Hsp genes and their mRNA expression profiles in B. minax, as well as the relationship between Hsp gene expression and diapause, remain unknown.
To unravel the potential contribution of Hsp genes to diapause in B. minax, we hypothesized that different Hsp genes play distinct roles in regulating B. minax diapause. In this study, we isolated cDNA sequences from three Hsp genes, and examined the expression patterns of these Hsp genes during various developmental stages. We also examined the phylogenetic relationships between these Hsps by comparing them with Hsps from other insect taxa. This study represented the first characterization of Hsp genes in B. minax and their expression patterns during different stages of diapause. The present results improve our understanding of the mechanism of diapause in B. minax at the molecular level.
Material and Methods
Insects
Bactrocera (Tetradacus) minax eggs were collected from citrus plants in Zhangjiachong cun Jingzhou, Hubei province in August 2011. The eggs in the citrus plants were placed at 26 ± 1°C with a 12L:12D photoperiod for hatching. Newly hatched larvae were reared in citrus plants in the laboratory. The late third instars jumped into bottles filled with fine sand containing a moisture content of 10–15%. The third late instars pupated in the fine sand and were then transferred to 17 ± 1°C with a 12 L:12D photoperiod until adult eclosion.
Sampling at various developmental stages
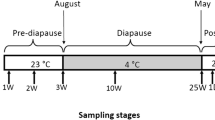
Diapause is generally separated into three stages: prediapause, diapause, and postdiapause (Denlinger 2002, Xu 2008). Prediapause includes the induction and preparation phases. First, it was generally considered based on our observations that prediapause in B. minax corresponded to organisms from second instars to 30-day pupa. We regarded the period from about the second instar to the first pupal day as the induction phase and the period from about the first to the 30th pupal day as the preparation phase. Diapause included the initiation, maintenance, and termination phases. Diapause of B. minax was about from 30- to 150-day pupa. It was considered that diapause initiation occurred at about day 30, diapause maintenance occurred from day 30 to day 120, and diapause termination occurred from day 120 to day 150. Based on these considerations, we choose every tenth day during the diapause termination phase to measure the exact termination time based on the dynamics of Hsp gene expression. Finally, postdiapause comprised organisms from approximately 150- to 160-day-old pupae. Collectively, based on the various possible time points corresponding to each diapause stage, and to reveal the exact timing of each diapause stage at the molecular level, the following time points were chosen to examine the expression profile of Hsp genes: eggs and first, second, and third instars; 1, 7, 30, 60, 90, 120, 130, 140, 150, and 160-day-old pupae; and newly emerged adults (<24 h). Three replicates were performed for each time point.
Reverse transcription PCR and rapid amplification of cDNA ends
Total RNA was isolated using the RNeasy Mini Kit (QIAGEN, Valencia, CA, USA), and 2 μg RNA was used to generate the cDNA using the oligo(dT)15 primer according to the instructions provided with the reverse transcription system (Invitrogen Life Technologies, Burlington, ON, Canada). Degenerate primers (Table 1) were used to amplify partial segments of the Hsp genes. Then, 5′ and 3′ rapid amplification of cDNA ends (RACE) were performed to obtain full-length cDNAs according to the manufacturer’s instructions (Rapid Amplification of cDNA Ends System, version 2.0, Invitrogen, Carlsbad, CA, USA) using gene-specific primers corresponding to GSP1 and GSP2 (Table 1). To ensure that the 5′ and 3′ fragments were derived from the same gene, specific primer sets flanking the open reading frames (ORFs) were designed and used to amplify the full-length cDNAs.
Sequence analysis of hsp cDNA
The hsp cDNAs from other species were used as query sequences to search for alternative insect Hsp genes in the GenBank database using the BLAST software available on the NCBI website (http://www.ncbi.nlm.gov/BLAST/). Sequence alignment and identity analyses were performed using DNAMAN (version 5.0, Lynnon BioSoft, Quebec, Canada). The ORFs were identified using ORF Finder (http://www.ncbi.nlm.nih.gov/gorf/gorf.html). The amino acid sequences and the molecular weight of the proteins were calculated using DNASTAR. The accession numbers of Bmhsp23, Bmhsp70, and Bmhsp90 are KJ541737, KJ541738, and KJ541739, respectively.
Homology and phylogenetic analyses
To evaluate the molecular evolutionary relationship of Hsps from various insects, phylogenetic trees were constructed based on their protein sequences. Sequence homology searches were performed using BLAST, and all sequences were retrieved from GenBank using BLAST-N and BLAST-X. The retrieved sequences were aligned using the multiple alignment tool of the ClustalX program. Gaps and missing data were excluded from the data analysis. MEGA 5.1 was used to perform the tree calculations. The tree constructions were performed using the maximum parsimony method. Support for the nodes was assessed as a proportion of 1,000 bootstrap replicates to derive the confidence values of the phylogeny analysis.
Real-time quantitative PCR
Total RNA from the samples was extracted using the RNeasy Mini Kit (QIAGEN, Valencia, CA, USA), and the RNase-Free Set (QIAGEN, USA) was used to remove genomic DNA. The quantity and quality of the RNA were assessed via spectrophotometry (Beckman Du 650 spectrophotometer, Fullerton, CA, USA), and the A260/A280 ratios were typically above 1.8. The RNA quality was also evaluated via 1% agarose gel electrophoresis. According to the manufacturer’s instructions, 2 μg total RNA was used to synthesize cDNAs using the SuperScriptTM III Reverse transcriptase kit (Invitrogen Life Technologies, Burlington, ON, Canada). The cDNA was stored at −80°C until further analysis.
The mRNA expression levels of hsp23, hsp70, and hsp90 from eggs, larvae, pupae, and adults were examined via quantitative real-time PCR analysis. The sequences of the primers are listed in Table 1. The reactions were performed using an iQTM 5 real-time PCR detection system (BioRad, Foster City, CA, USA). The amplification volume was 20 μL, including 0.5 μL of the forward primer (10 mM/μL), 0.5 μL of the reverse primer (10 mM/μL), 10.0 μL of SYBR Mix, 0.4 μL of Rox, 1.0 μL of the cDNA sample, and 7.6 μL of ultra-pure water. The PCR cycle conditions were as follows: 94°C for 5 min, followed by 40 cycles of amplification consisting of 94°C for 30 s, 58°C for 30 s, and 72°C for 1 min, and then 72°C for 10 min. After the amplification phase, a dissociation curve was generated to ensure that there was only one product. A control without any template was included in all batches. The amplification efficiency of each gene was validated by constructing a standard curve using five serial dilutions of cDNA. The data were analyzed based on the Cp method according to the mathematical model of Pfaffl (2001), simplified to 2△△Ct as follows:
Eggs were used as a control, and alpha-tubulin (α-TUB) was used as the reference gene based on our preliminary experiments, which revealed that α-TUB was stably expressed throughout the various developmental stages (unpublished data). The relative expression level of each hsp mRNA was defined as the fold-change normalized to the amount of α-TUB. Each sample was assessed in triplicate.
Statistical analyses
Statistical analyses were performed using the SPSS software package (version 13). Prior to all statistical analyses, the data were examined with respect to assumptions of normality using the Kolmogorov-Smirnov test. The Hsp gene expression levels on the different development stages were analyzed using one-way ANOVA followed by the least significant difference (LSD) test (p values ≤0.05).
Results and Discussion
Cloning, characterization and homology, phylogenetic, and expression profile analyses of Bmhsp23
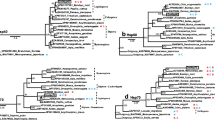
The full-length cDNA of B. minax hsp23 (Bmhsp23) is 805 bp, including a 5′-terminal UTR of 136 bp, a 3′-terminal UTR of 164 bp containing a poly(A) tail, and an ORF of 510 bp encoding a polypeptide of 170 amino acids with a predicted molecular mass of 19.03 kDa and a theoretical isoelectric point of 6.10 (Fig 1). The typical α crystal domains of sHsps were located from amino acid positions 49 to 131. In addition, homology analysis revealed that the deduced amino acid sequence of BmHsp23 displayed varying similarity, from 28 to 83%, to previously identified sHsps (Supplementary Material Table 1). Furthermore, as shown in Fig 2, Hsps in the order Diptera are differentiated into two clusters, and an orthologous cluster contained several sHsps from different insect orders, which suggested that these sHsps evolved prior to species divergence (Kokolakis et al 2008, Liu et al 2012).
The full-length cDNA sequence of Bactrocera (Tetradacus) minax hsp23 (Bmhsp23) and its deduced amino acid sequence. The character shading (ATG) indicates the translational start codon. The asterisk indicates the translational termination codon (TAA). The termination signal is in bold, and the poly(A) tail is underlined. The typical sequences characteristic of the sHspα crystal domains are double-underlined.
A phylogenetic tree based on the known amino acid sequences of sHsp was generated via maximum parsimony analysis, and this tree was used to determine the relationships between different insects. The numbers above the branches indicate the percentages of bootstrap replicates in which each species was grouped together. The scale bar indicates the number of substitutions per site for each unit branch length. The bootstrap values of 1,000 replicates are displayed for each branch. Apis cerana Hsp23 (AEH05930); Apis cerana Hsp24.2 (AEH05929); Bactrocera dorsalis Hsp20 (AEJ88464); Bactrocera minax Hsp23 (KJ541737); Bemisia tabaci B Hsp20 (ACH85196); Bemisia tabaci Q (ADG03464); Bemisia tabaci ZHJ1 Hsp20 (ADG03467); Bombyx mori Hsp19.9 NP_001036984; Bombyx mori Hsp20.1 (NP_001036941); Bombyx mori Hsp20.4 (NP_001037038); Bombyx mori Hsp20.8 (NP_001091794); Bombyx mori Hsp23.7 (BAD74198); Ceratitis capitat aHsp23-α (ACG58883); Ceratitis capitata Hsp23-β (ACG58884); Ceratitis capitata Hsp27 (EU700493); Drosophila melanogaster Hsp23 (AAA28637); Drosophila melanogaster Hsp26 (AAF50288); Drosophila melanogaster Hsp27 (AAF50285); Gastrophysa atrocyanea Hsp21 (BAD91164); Gastrophysa atrocyanea Hsp23 (BAD91165); Glossina morsitans Hsp23 (ADD18977); Liriomyza huidobrensis Hsp21.4 (DQ452370); Liriomyza sativae Hsp21.3 (ABE57138); Locusta migratoria Hsp20.5 (ABC84492); Locusta migratoria Hsp20.7 (ABC84494); Lucilia cuprina Hsp23 (AFA36667); Sarcophaga crassipalpis Hsp23 (AAC63387); Sarcophaga crassipalpis Hsp25 (ABL06941); Trialeurodes vaporariorum Hsp23 (ACH85200); Tribolium castaneum Hsp21 (XP_974390).
The small heat shock protein gene is an important diapause regulatory gene. A possible role for sHsp genes in diapause is their involvement in the regulation of cell cycle arrest (Tammariello & Denlinger 1998). It was reported that sHsps were upregulated during diapause in a variety of insect species (Rinehart et al 2007), which suggested that sHsps are key players in the overwintering response of many insects (Gkouvitsas et al 2008). The sHsp mRNA expression level peaked in diapause pupae of Plutella xylostella Linnaeus and Lactuca sativa Linnaeus (Sonoda et al 2006, Huang et al 2009). Aruda et al (2011) found that the hsp22 expression level was elevated during deep diapause in Calanus finmarchicus Gunnerus. The Hsp23 gene was highly upregulated during Sarcophaga crassipalpis diapause and was implicated in diapause entry (Rinehart et al 2007). Different sHsp genes displayed distinct functions in Sesamia nonagrioides Lefèbvre, and it was found that SnoHsp19.5 mRNA was consistently expressed throughout diapause, whereas SnoHsp20.8 mRNA was downregulated during mid-diapause and was upregulated upon diapause termination (Gkouvitsas et al 2008). In the present study, Bmhsp23 mRNA expression was significantly different among the developmental stages of B. minax studied (F 14,71 = 2.519, p = 0.007) (Fig 3 a). Bmhsp23 expression was upregulated during diapause initiation, maintenance, and termination, suggesting it plays a key role during diapause.
Hsp gene (a hsp23, b hsp70, and c hsp90) mRNA expression during each developmental stage in Bactrocera (Tetradacus) minax. L1, L2, and L3 represent the first, second, and third instars, respectively. P1, P7, P30, P60, P90, P120, P130, P140, P150, and P160 represent 1-, 7-, 30-, 60-, 90-, 120-, 130-, 140-, 150-, and 160-day old pupae, respectively. The results are expressed as means + SEM. The differences were considered significant for p values ≤0.05.
Cloning, characterization and homology, phylogenetic, and expression profile analyses of Bmhsp70
The full-length cDNA of the Bmhsp70 was 2262 bp long, including a 5′-UTR of 178 bp, a 3′-UTR of 180 bp containing a poly(A) tail, and an ORF of 1,911 bp encoding a polypeptide of 636 amino acids with a predicted molecular mass of 69.42 kDa and a theoretical isoelectric point of 5.36 (Fig 4). The IDLGTTYS and DLGGGTFD motifs were located at amino acid positions 6–13 and 196–203, respectively. The nonorganellar conserved motif RARFEEL was located at amino acid positions 297 to 303. The end of BmHsp70 was characteristic of the cytosolic Hsp70-specific EEVD motif. The predicted ATP-GTP binding domain was AEAYLGTT, which was located at amino acid positions 128 to 135 (Fig 4). Additionally, homology analysis revealed that the deduced amino acid sequence of BmHsp70 displayed high identity, from 70 to 96%, to the previously identified inducible Hsp70 (Supplementary Material Table 2) and that the sequence was highly conserved. Furthermore, as shown in Fig 5, the inducible Hsp70 from insects of the same order were clustered into the same group, which was consistent with traditional taxonomy.
The full-length cDNA sequence of Bactrocera (Tetradacus) minax hsp70 (Bmhsp70) and its deduced amino acid sequence. The character shading (ATG) indicates the translational start codon. The asterisk indicates the translational termination codon (TAA). The termination signal is in bold, and the poly(A) tail is underlined. The characteristic Hsp70 motifs are boxed, and the cytosolic Hsp70-specific motif is double-underlined.
A phylogenetic tree based on the known amino acid sequences of inducible Hsp70 was generated via maximum parsimony analysis, and this tree was used to determine the relationships between different insects. The numbers above the branches indicate the percentages of bootstrap replicates in which each species was grouped together. The scale bar indicates the number of substitutions per site for each unit branch length. The boots trap values of 1,000 replicates are displayed for each branch. Aedes aegypti Hsp70Aa (ACJ64193); Aedes aegypti Hsp70Ab (ACJ64194); Aedes aegypti Hsp70Ba (ACJ64195); Aedes aegypti Hsp70Bb (ACJ64196); Aedes aegypti Hsp70Ca (ACJ64197); Aedes aegypti Hsp70Cb (ACJ64198); Anatolica polita borealis Hsp70 (ABQ39970); Anopheles albimanus Hsp70A2 (AAC41543); Antheraea pernyi Hsp70 (ADI50267); Antheraea yamamai Hsp70 (BAD18974); Bactrocera dorsalis Hsp70BD2 (ADQ12986); Bactrocera minax Hsp70 (KJ541738); Bactrocera oleae Hsp70 (CAI44197); Bemisia tabaci Hsp70 (ACH85197); Bemisia tabaci Hsp70 (ADG03465); Bemisia tabaci ZHJ1 Hsp70 (ADG03468); Bemisia tabaci ZHJ2 Hsp70 ADO14473; Bombyx mori Hsp70B (AEI58996); Ceratitis capitata Hsp70 (AAC23392.1); Culex pipiens Hsp70 (AAX84696); Cydia pomonella Hsp70-1 (AFK93489); Cydia pomonella Hsp70-2 (AFK93490); Delia antiqua Hsp70 (AAY28732); Drosophila auraria Hsp70 (CAA55168); Drosophila melanogaster Hsp70Aa (AAN13535); Drosophila melanogaster Hsp70Ba (AAN13545); Drosophila melanogaster Hsp70Bb (AAN13546); Drosophila Montana Hsp70 (ACB59072); Glossina morsitans Hsp70 (ADD19447); Helicoverpa armigera Hsp70 (ADP37711); Helicoverpa zea Hsp70 (ACV32640); Heliothis viriplaca Hsp70 (ACS72236); Liriomyza sativae Hsp70 (AAW32099); Macrocentrus cingulum Hsp70 (ACD84944); Microdera dzhungarica punctipennis Hsp70 (AEB52075); Microplitis mediator Hsp70 (ABV55505); Oxya chinensis Hsp70 (AFN08643); Paratlanticus ussuriensis (Hsp70 AEP68850); Plutella xylostella Hsp70 (ADV58255); Quadrastichus erythrinae Hsp70 (AFC76151); Rhagoletis pomonella Hsp70 (ABL06948); Sesamia nonagrioides Hsp70 (ABZ10939); Spodoptera litura Hsp70 (ADV03160); Stratiomys singularior Hsp70 (ACB59073); Tenebrio molitor Hsp70 (AFE88580); Trialeurodes vaporariorum Hsp70 (ACH85201); Tribolium castaneum Hsp70 (XP_974442).
The role of hsp70 in mediating diapause varied greatly among insect taxa. For example, changes in hsp70 expression were not a factor in Lucilia sericata Wiedemann larval diapauses (Tachibana et al 2005), Helicoverpa zea Boddie pupal diapause (Zhang & Denlinger 2009) or Drosophila triauraria Bock & Wheeler adult diapause (Goto et al 1998).
Hsp70 mRNA expression decreased upon diapause initiation in Omphisa fuscidentalis Hampson and remained lower at the pupal stage, which indicated that hsp70 was not associated with O. fuscidentalis diapause (Tungjitwitayakul et al 2008). In S. nonagrioides, hsp70 expression was downregulated during larval diapause (Gkouvitsas et al 2009). However, hsp70 expression was strongly induced during Megachile rotundata Fabricius pupal diapause (Yocum et al 2005). In the present study, we found that Bmhsp70 expression was significantly different between various developmental stages (F 14,69 = 12.225, p < 0.001) (Fig 3 b). Bmhsp70 expression peaked during diapause initiation in third instars, which suggested that Bmhsp70 might play an important role in diapause induction in B. minax.
Cloning, characterization and homology, phylogenetic, and expression profile analyses of Bmhsp90
The partial cDNA sequence of B. minax hsp90 (Bmhsp90) was 1,720-bp long (Fig 6). It was found that the present Hsp90 sequence may represent the second half of the full-length cDNA (including the 3′ end) and consisted of about half of the full-length cDNA. The present Hsp90 cDNA sequence included a 3′-terminal UTR of 180 bp containing a poly(A) tail and an ORF of 1,089 bp encoding a polypeptide of 363 amino acids. The end motif of MEEVD was identified. One characteristic Hsp90 family sequence, GVVDSEDLPLNISRE, was detected. In addition, homology analysis revealed that compared to previously identified Hsp90 genes, the identity of the deduced amino acid sequence of BmHsp90 varied from 76 to 98% (Supplementary Material Table 3), indicating that the Hsp90 sequence is highly conserved. Hsp90 from Orthoptera, Hymenoptera, Hemiptera, Diptera, and Coleoptera were clustered into the same large group, which revealed that Hsp90 from these orders is highly conserved (Fig 7). However, the genetic distance of Hsp90 from Lepidoptera indicated several evolutionary divergences.
The partial cDNA sequence of Bactrocera (Tetradacus) minax hsp90 (Bmhsp90) and its deduced amino acid sequence. The character shading (ATG) indicates the translational start codon. The asterisk indicates the translational termination codon (TAA). The termination signal is in bold, and the poly(A) tail is underlined. The C-terminal end motif of Hsp90 is double-underlined.
A phylogenetic tree based on the known amino acid sequences of Hsp90 was generated via maximum parsimony analysis, and this tree was used to determine the relationships between different insects. The numbers above the branches indicate the percentages of bootstrap replicates in which each species was grouped together. The scale bar indicates the number of substitutions per site for each unit branch length. The bootstrap values of 1,000 replicates are displayed for each branch. Antheraea pernyi (ADD91573); Apis mellifera (NP-001153536); Bactrocera dorsalis (AEJ88466); Bactrocera minax Hsp90 (KJ541739); Bemisia tabaci B (ACH85198); Bemisia tabaci Q (ADG03466); Bemisia tabaci ZHJ1 (ADG03469); Bombyx mori (ADG57739); Chilo suppressalis (BAE44307); Cydia pomonella (AFA35118); Delia antiqua (CAI64494); Drosophila melanogaster (AAF47734); Exorista civilis (ACD63052); Gryllus firmus (ADK64952); Harmonia axyridis (ACL50550); Helicoverpa armigera (ADP37710); Helicoverpa assulta (ADM26742); Helicoverpa zea (ACV32639); Liriomyza huidobrensis (AAW49252); Liriomyza sativae (AAW49253); Locusta migratoria (AAS45246); Lucilia cuprina (ABQ42553); Nilaparvata lugens (ADE34169); Oxya chinensis (AFN08644); Paratlanticus ussuriensis (AFP54306); Plutella xylostella (BAE48742); Polypedilum vanderplanki (ADM13380); Polyrhachis vicina (AEM76721); Quadrastichus erythrinae (AFC76152); Sogatella furcifera (AFK64820); Spodoptera exigua (ACL77779); Spodoptera litura (ADM26738); Tenebrio molitor (AFN02497); Trialeurodes vaporariorum (ACH85202); Tribolium castaneum (NP-001094067).
Hsp90 displayed a different expression pattern during insect diapause. In the present study, Bmhsp90 mRNA expression was not significantly different between the various diapause stages (F 14,72 = 1.013, p = 0.453) (Fig 3 c). Bmhsp90 mRNA was expressed throughout life and remained at a relatively low level during deep diapause of B. minax. The low expression level of Bmhsp90 mRNA might be attributed to a reduction in the level of ecdysone, leading to an increase in Bmhsp23 mRNA expression (MacRae 2010). It was reported that the hsp90 expression level was downregulated during pupal diapause of S. crassipalpis Macquart (Rinehart & Denlinger 2000), while it was upregulated during diapause termination of L. sericata (Tachibana et al 2005), and it was constantly expressed throughout pupal diapause of M. rotundata (Yocum et al 2005). Fan et al (2013) found that Hsp90, Hsp70, Hsp20.8, and Hsp20.4 were highly expressed in both diapause and nondiapause eggs of Bombyx mori Linnaeus and suggested they may play an important role in initial embryonic development regardless of the occurrence of diapause.
In summary, different Hsp genes play distinct roles in B. mina during the various diapause stages and that these Hsp genes interact with one another. This study represented the first characterization of Hsp genes in B. minax and their mRNA expression profiles during different diapause stages. The unique physiological expression patterns suggested that the Hsp genes play distinct roles in the regulation of diapause in B. minax. Bmhsp70 might play an important role in initiation diapause, Bmhsp23 might play a key role in diapause initiation and maintenance, and Bmhsp90 might play a minor role in the regulation of diapause. Our data improve our understanding of the mechanisms of diapause in B. minax at the molecular level. However, the precise physiological function of these Hsp genes during diapause in B. minax warrants further investigation.
References
Aruda AM, Baumgartner MF, Reitzel AM, Tarrant AM (2011) Heat shock protein expression during stress and diapause in the marine copepod Calanus finmarchicus. J Insect Physiol 57:665–675
Denlinger DL (2002) Regulation of diapause. Annu Rev Entomol 47:93–122
Denlinger DL, Rinehart JP, Yocum GD, Denlinger DL, Giebultowicz JM, Saunders DS (2001) Stress proteins: a role in insect diapause? In: Denlinger DL, Giebultowicz JM, Saunders DS (eds) Insect timing: circadian rhythmicity to seasonality. Elsevier Science, Netherlands, pp 155–171
Denlinger D, Li A, Rinehart J (2007) Heat shock proteins are critical for insect winter survival. Comp Biochem Physiol A 146:149–157
Fan LF, Lin JR, Zhong YS, Liu JY (2013) Shotgun proteomic analysis on the diapause and non-diapause eggs of domesticated silkworm Bombyx mori, PLoS One doi: 10.1371/journal.pone.0060386
Feder ME, Hofmann GE (1999) Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol 61:243–282
Gkouvitsas T, Kontogiannatos D, Kourti A (2008) Differential expression of two small Hsps during diapause in the corn stalk borer Sesamia nonagrioides (Lef.). J Insect Physiol 54:1503–1510
Gkouvitsas T, Kontogiannatos D, Kourti A (2009) Cognate Hsp70 gene is induced during deep larval diapause in the moth Sesamia nonagriodes. Insect Mol Biol 18:253–264
Goto SG, Kimura MT (2004) Heat-shock-responsive genes are not involved in the adult diapause of Drosophila triauraria. Gene 326:117–122
Goto SG, Yoshida KM, Kimura MT (1998) Accumulation of Hsp70 mRNA under environmental stresses in diapausing and nondiapausing adults of Drosophila triauraria. J Insect Physiol 44:1009–1015
Hartl FU, Hayer-Hartl M (2002) Protein folding-molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295:1852–1858
Huang LH, Wang CZ, Kang L (2009) Cloning and expression of five heat shock protein genes in relation to cold hardening and development in the leafminer, Liriomyza sativa. J Insect Physiol 55:279–285
Kokolakis G, Tatari M, Zacharopoulou A, Mintzas AC (2008) The hsp27 gene of the Mediterranean fruit fly, Ceratitis capitata: structural characterization, regulation and developmental expression. Insect Mol Biol 17:699–710
Liu ZH, Xi DM, Kang MJ, Guo XQ, Xu BH (2012) Molecular cloning and characterization of Hsp27.6: the first reported small heat shock protein from Apis cerana cerana. Cell Stress Chaperones 17:539–551
MacRae TH (2010) Gene expression, metabolic regulation and stress tolerance during diapause. Cell Mol Life Sci 67:2405–2424
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9):e45
Qiu ZJ, MacRae TH (2008a) ArHsp21, a developmentally regulated small heat shock protein synthesized in diapausing embryos of Artemia franciscana. Biochem J 411:605–611
Qiu ZJ, MacRae TH (2008b) ArHsp22, a developmentally regulated small heatshock protein produced in diapause-destined Artemia embryos, is stress inducible in adults. FEBS J 275:3556–3566
Rinehart JP, Denlinger DL (2000) Heat-shock protein 90 is down-regulated during pupal diapause in the flesh fly, Sarcophaga crassipalpis, but remains responsive to thermal stress. Insect Mol Biol 9:641–645
Rinehart JP, Yocum GD, Denlinger DL (2000) Developmental upregulation of inducible hsp70 transcripts, but not the cognate form, during pupal diapause in the flesh fly, Ssarcophaga crassipalpis. Insect Biochem Mol Biol 30(6):515–521
Rinehart JP, Li A, Yocum GD, Robich RM, Hayward SAL, Denlinger DL (2007) Up-regulation of heat shock proteins is essential for cold survival during insect diapause. PNAS 104:11130–11137
Sonoda S, Ashfaq M, Tsumuki H (2006) Cloning and nucleotide sequencing of three heat shock protein genes (hsp90, hsc70, and hsp19.5) from the diamondback moth, Plutella xylostella (L.) and their expression in relation to developmental stage and temperature. Arch Insect Biochem Physiol 62:80–90
Tachibana SI, Numata H, Goto SG (2005) Gene expression of heat-shock proteins (Hsp23, Hsp70 and Hsp90) during and after larval diapause in the blow fly Lucilia sericata. J Insect Physiol 51:641–647
Tammariello SP, Denlinger DL (1998) G0/G1 cell cycle arrest in the brain of Sarcophaga crassipalpis during pupal diapause and the expression pattern of the cell cycle regulator, proliferating cell nuclear antigen. Insect Biochem Mol Biol 28:83–89
Tungjitwitayakul J, Tatun N, Singtripop T, Sakurai S (2008) Characteristic expression of three heat shock-responsive genes during larval diapause in the bamboo borer Omphisa fuscidentalis. Zool Sci 25:321–333
Van Schoubroeck F (1999) Learning to fight a fly: developing citrus IPM in Bhutan. PhD thesis. Wageningen University and Research Centre, Wageningen, The Netherlands, p 200
Vreysen MJB, Robinson AS, Hendrichs J (2007) Area-wide control of insect pests: from research to field implementation. Alphey LS: Engineering Insects for the Sterile Insect Technique, pp 51–60
Wang XJ, Luyi L (1995) Research progress in the Chinese citrus fruit fly. Entomol Knowl 32:310–315
Wang H, Zhang H (1993) Control of the Chinese citrus fly, Dacus citri (Chen), using the sterile insect technique, pp 505–512. In: Proceedings of an International Symposium on Management of Insect Pests, Vienna, 19–23 October 1992. International Atomic Energy Agency, Vienna
Wimmer EA (2003) Applications of insect transgenesis. Nat Rev 4:225–233
Xiao HJ, Wei ZJ, Xue FS (2011) Progress in heat shock proteins (Hsps) related to insect diapauses. Acta Entomol Sin 54(9):1068–1075
Xu WH (2008) Progress in insect diapauses. Acta Entomol Sin 45(4):512–518
Yang P, Carey JR, Dowell RV (1994) Tephritid fruit flies in China: historical background and current status. Pan Pac Entomol 70:159–167
Yocum GD (2001) Differential expression of two HSP70 transcripts in response to cold shock, thermoperiod, and adult diapause in the Colorado potato beetle. J Insect Physiol 47:1139–1145
Yocum GD, Kemp WP, Bosch J, Knoblett JN (2005) Temporal variation in overwintering gene expression and respiration in the solitary bee Megachile rotundata. J Insect Physiol 51:621–629
Yuan Y, Crane DD, Barry CE (1996) Stationary phase-associated protein expression in Mycobacterium tuberculosis: function of the mycobacterial alpha-crystallin homolog. J Bacteriol 178(15):4484–4492
Zhang QR, Denlinger DL (2009) Molecular characterization of heat shock protein 90, 70 and 70 cognate cDNAs and their expression patterns during thermal stress and pupal diapause in the corn earworm. J Insect Physiol 56:138–150
Acknowledgments
We thank Fu-Lian Wang for her help in the collection of insects. This study was supported by the National Basic Research and Development Program (Grant No. 2009CB119200), the Ministry of Science and Technology, China, and the Commonwealth Special Fund for the Agricultural Industry (no. 200903047).
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Anne-Nathalie Volkoff – INRA / Univ of Montpellier
Z. C. Lü and L. H. Wang contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table 1
sHsp sequences used for homology and phylogenetic analysis (DOCX 19 kb)
Table 2
Inducible Hsp70 sequences used for homology and phylogenetic analysis (DOCX 21 kb)
Table 3
Hsp90 sequences used for homology and phylogenetic analysis (DOCX 20 kb)
Rights and permissions
About this article
Cite this article
Lü, Z.C., Wang, L.H., Zhang, G.F. et al. Three Heat Shock Protein Genes from Bactrocera (Tetradacus) minax Enderlein: Gene Cloning, Characterization, and Association with Diapause. Neotrop Entomol 43, 362–372 (2014). https://doi.org/10.1007/s13744-014-0216-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-014-0216-y