Abstract
Spirulina sp. (AlgS) was modified with the cationic surfactant hexadechyltrimethylammonium bromide (HDTMA-Br) to produce a positively charged adsorbent (AlgS-HDTMA) through a cation exchange reaction. The functional groups contained in the adsorbent were studied using an infrared (IR) spectrometer, while the surface morphology and constituent elements were studied using SEM-EDX. The AlgS-HDTMA adsorbent was applied to the mono-component and bi-component adsorption of methyl orange (MO) and crystal violet (CV) dyes. The adsorption experiments were carried out using the batch method to determine the kinetics, isotherms, mechanisms, and adsorption competition of MO and CV against the adsorbent. The amount of MO and CV adsorbed by AlgS-HDTMA was compared with the adsorbent modified by Spirulina sp. algae with Na+ ions (AlgS-Na) in the form of both mono-component and bi-component which showed greater results than AlgS-Na. MO and CV adsorption competition against AlgS-HDTMA was dominated by MO with the bi-component Langmuir isotherm constant (bj) for MO and CV of 15.60 and 2.80 × 10–2 L/mmolequiv, respectively. Surface modification of AlgS with cationic surfactant HDTMA-Br has resulted in a rich positive charge, making it more effective to adsorb anionic dyes such as MO. Data on the ability to reuse adsorbents are an important aspect in handling wastewater treatment because it is very meaningful in reducing water treatment costs. The results of adsorption of MO solution and desorption with 0.1 M hydrochloric acid solution as eluent with 1–4 repetitions were: 97.8, 94.13, 89.39, and 86.27%. These data indicate that the repetition of 4 times did not significantly reduce the adsorption capacity of MO. Lastly, the wastewater study confirms that AlgS-HDTMA adsorbents are promising as biomass for anionic/cationic dye adsorption. The review is highly beneficial for scientists, wastewater treatment, and industrial communities to support the sustainable development of natural resources.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Currently, the pollution of water bodies tends to increase. This is partly due to the increased release of wastewater containing chemicals such as dyes from the textile, cosmetic, food coloring, and paper industries. A large number of dyes that are discharged into water bodies have entered the ecosystem and have a negative impact on living things in the vicinity. Pollution by liquid waste originating from industry is a dominant environmental problem. Liquid waste that is not processed and managed will have a negative impact on waters, especially water resources [1]. One type of liquid waste that is relatively common is textile waste. Textile waste produced by the dyeing industry has the potential to pollute the environment. This is because the textile wastewater contains very complex pollutants and high color intensity [2].
Methyl orange (MO) with the molecular formula C14H14N3NaO3S and crystal violet (CV) with the molecular formula C25N3H30Cl are examples of textile waste from dyes that are often found in aquatic environments [3, 4]. Both of these dyes cause problems in the environment, if their distribution is not reduced because these substances are widely used as textile dyes, have toxic and carcinogenic properties and are not easily biodegradable; as a result, they are stable and difficult to remove in the waste treatment process [5, 6]. The impact of the dye pollution causes the dissolved oxygen in the water to decrease and triggers the activity of anaerobic organisms to produce foul-smelling products [7,8,9,10].
One of the most appropriate ways to reduce pollution and the spread of synthetic dyes in the environment is to treat the waste containing these dyes before being discharged into the environment. There are several processing processes for synthetic dye waste, among others, by degrading synthetic dyes into other substances. However, it turns out that this degradation process is not suitable for removing synthetic dyes, because synthetic dyes are designed and produced as materials that are resistant to light, biological or chemical degradation [11, 12]. Thus, most of the dyestuffs are difficult to degrade chemically or biologically. Furthermore, the dye degradation process will produce more harmful substances in the form of secondary pollution [13,14,15]. Another method that is often used to treat synthetic dye waste is the adsorption method.
One alternative method that is often used to remove synthetic dyes contained in waste is the adsorption method. This method is easy to implement because it is not complicated, the cost is affordable, and it does not produce derivative materials that are harmful to humans and the environment [16,17,18]. From several research results that have been reported, it is stated that the material used as an adsorbent must have surface characteristics as an optimal adsorption site to adsorb adsorbate both physically and chemically, chemically stable, effectively used repeatedly, and not harmful to the environment [19, 20].
Along with the increasing need for effective adsorbents to adsorb waste from synthetic dyes, algae biomass is an appropriate natural material to be used as an adsorbent. Algal biomass is easy to obtain and is available quite a lot in marine waters and can be cultivated. Naturally, algal biomass is a very effective adsorbent for adsorbing pollutants from chemical compounds such as synthetic dyes [21,22,23]. Several types of algae that have been studied show that these materials have a high enough ability to adsorb ions or molecules in solution through active adsorption sites in the form of functional groups. In addition, the modified algal biomass adsorbent is stable enough to be used in the adsorption–desorption process with several repetitions. Thus, algal biomass can be utilized as an effective adsorbent for methyl orange, chromium, and Congo red wastewater treatment [24,25,26]. Algae biomass is a natural material that is easily damaged due to the influence of microorganisms, besides that it has a low specific gravity so it is less effective as an adsorbent. Another limitation of algal biomass cannot be used directly as a filler for the adsorption column, because it is not hard and is in solid form. Therefore, to overcome the weakness of the algal biomass, it is necessary to modify it with various supporting matrices. The compounds of diethylenetriamine (DETA), alginate, and formaldehyde can be used as a modified material [27,28,29].
The use of cationic surfactants in modifying algal biomass is one of the appropriate technologies to change the surface charge of algal biomass which is naturally negatively charged to positively charged. Negatively charged functional groups in algal biomass bound with an alkaline or alkaline earth cations can be exchanged with cationic surfactants so that the surface of the adsorbent becomes positively charged [30, 31]. Therefore, this modified adsorbent has the ability to adsorb negatively charged adsorbate through electrostatic interactions while positively charged adsorbate will be adsorbed through cation exchange reactions.
This research was conducted to produce adsorbent from algae biomass Spirulina sp. modified with HDTMA-Br surfactant (AlgS-HDTMA). The modification aims to make the surface of the adsorbent positively charged so that it is effective for adsorption of dyes both cations and anions. The compound HDTMA-Br with the chemical formula CH3(CH2)15N(CH3)3+Br is a cationic surfactant which is a cation exchanger. Thus, an effective adsorbent can be obtained to adsorb dyes such as MO and CV. In this study, it is reported about the study of the adsorption of MO and CV dyes by AlgS-HDTMA including the effect of pH, adsorption kinetics and isotherms as well as the ability to reuse adsorbents. AlgS-HDTMA can be one of the adsorbents that can be implemented to reduce the levels and distribution of toxic synthetic dyes in the aquatic environment.
Experimentals
Materials and methods
In this work, AlgS biomass is cultivated at the Center for Marine Cultivation in Lampung, Indonesia, and converted to algal biomass in the Inorganic Chemistry Laboratory of FMIPA University of Lampung. The chemicals used for adsorbent synthesis and adsorption experiments were obtained from Merck (Germany) including MO and CV dyes, HDTMA-Br, hydrochloric acid, Na2EDTA, CH3COOH, and PO43− buffers, as well as sodium hydroxide, sodium chloride, and sodium nitrate.
Adsorbent preparation and characterization
AlgS air-dried for 3 days and followed by drying using an oven at 40 ℃ for 2–3 h to obtain a constant weight. Then the dried algal biomass was ground using grinding to a size of 100 mesh [19]. AlgS-Na adsorbent was made by mixing 5 g AlgS with 100 mL of 0.1 M sodium chloride in a test tube. The solution was mixed by stirring for 60 min. The mixture was left for one night. After that, the mixture was separated by centrifugation. The precipitate obtained was neutralized with distilled water. The obtained material was dried at room temperature to constant weight.
Furthermore, the synthesis of AlgS-HDTMA was carried out by interacting 5 g of AlgS-Na with 500 mL of HDTMA-Br (15 mmol/L) solution at a temperature of 50 ℃. The mixture was stirred for 4 h using a magnetic stirrer. The precipitate and filtrate were separated using a centrifuge. The precipitate obtained was washed with distilled water until the pH was neutral. The precipitate was dried at room temperature and crushed to a size of 100 mesh (Fig. 1).
AlgS-Na and AlgS-HDTMA were characterized by scanning electron microscopy with energy-dispersive X-ray (FEI Inspect-S50, USA) to determine the surface morphology and elemental composition. Characterization with infrared (IR) spectrometer (Shimadzu Prestige-21 IR, Japan) to identify the functional groups of the Spirulina sp. algae biomass (AlgS), AlgS-Na, and AlgS-HDTMA.
Determination of the point of zero charge (pHpzc) adsorbent
A total of 0.1 g of adsorbent (AlgS-Na or AlgS-HDTMA) was added to a tube containing 20 mL of 0.1 M sodium nitrate. Then, the initial pH was adjusted to vary in the range from 3 to 12 using a standard solution of 0.1 M hydrochloric acid for an acid atmosphere and sodium hydroxide 0.1 M for alkaline conditions. The solution was mixed using a stirrer for 2 nights. Then, the final pH of the mixture was measured using a pH meter [32].
Adsorption experiment
Measurement of the concentration of MO and CV dyes was taken using a UV–Vis spectrophotometer (Agilent Cary 100, USA), at the maximum absorption wavelengths for MO and CV at = 465 and 591 nm, respectively. Dye adsorption was carried out by batch method by mixing MO or CV dye solution with adsorbent at pH varying between 3 and 12, contact time between 0 and 90 min and dye concentration varying from 0 to 1 mmol/L. Adsorption was carried out in a shaker incubator which was set at a speed of 200 rpm and a temperature of 27 ℃. The mixture was separated by centrifugation, and the filtrate was analyzed using a UV–Vis spectrophotometer. All adsorption experiments in this study were carried out with 3 repetitions. The MO or CV concentration adsorbed by AlgS-Na and AlgS-HDTMA was determined by calculating the MO and CV adsorbed per unit mass of adsorbent, and the percentage of MO or CV removal was determined using Eqs. (1, 2, and 3)
where Co is concentration in the initial state, Ce is concentration at equilibrium, Ct is concentration at a certain time t of MO or CV solution (mg/L), m is the mass of adsorbent (g), and V is the volume of the solution (L), q is the amount of MO or CV adsorbed per unit mass (mmol/g), and R is the percentage of the MO or CV removal.
Results and discussion
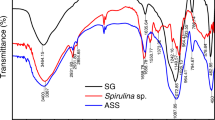
The results of adsorbent characterization using the FTIR spectrometer are shown in Fig. 2 which shows that AlgS has a C=O group characteristic of carboxylic acids which is characterized by the presence of an absorption band with a wave number of 1656 cm−1. In AlgS, the presence of primary N–H groups overlapping with –OH groups was observed with absorption at a wave number of 3394 cm−1. The absorption at wave number 2923 cm−1 indicates that there is a C–H group of a CH2 (aliphatic) carbon chain [31]. In the AlgS-Na spectrum (Fig. 2b), there is absorption at the same wave number as AlgS and the additional absorption at a wave number of 1405 cm−1 indicates the presence of an O–Na group (bond of Na cations with O in the carboxylate group) which indicates that Algae-Na has been formed [33]. Furthermore, the AlgS-HDTMA spectrum (Fig. 2c) shows that there has been a cation exchange process between Na+ ions in AlgS-Na and HDTMA+ ions with the emergence of new absorption at a wavelength of 1464 cm−1 originating from the C–H group which is a methyl group of HDTMA [34, 35].
The exchange of Na+ ions in AlgS-Na with HDTMA+ ions is also proved by the results of surface morphology and elemental composition analysis using SEM-EDX as shown in Fig. 3. Figure 3 shows that there are differences in morphology between AlgS-Na and AlgS-HDTMA. In Fig. 3a, the surface morphology of AlgS-Na looks like solid and inhomogeneous lumps, while in AlgS-HDTMA (Fig. 3b) there are small particles sticking evenly to the surface. This shows that after ion exchange occurs, HDTMA+ ions are not absorbed by AlgS-Na, but only cover the algae cell wall [33]. These data are also supported by the results of the EDX analysis which shows that in AlgS-Na (Fig. 3c) there are O, C, N, and Na atoms while in AlgS-HDTMA there are O, C, N, and Br atoms (Fig. 3d). The presence of Br atoms in AlgS-HDTMA and the absence of Na atoms indicate that the cation exchange reaction has been successfully carried out.
The surface charges of AlgS-Na and AlgS-HDTMA are known by looking at the pHPZC values. The surface of the adsorbent which has a pHPZC value close to zero is the optimum pHPZC value of the adsorbent. At pH < pHPZC, the surface charge of the adsorbent is positive, and when pH > pHPZC, the surface charge is negative [36]. Figure 4 shows that the pHPZC value of AlgS-Na and AlgS-HDTMA is 8. Thus, it can be stated that at pH < 8, the surface of the adsorbent is positively charged, so it is effective for absorbing negatively charged dyes such as MO. Meanwhile, if the pH > 8, the adsorbent is negatively charged so that it will be effective against CV dyes which are positively charged.
Figure 5 shows that AlgS-MO and AlgS-CV have very low adsorption. AlgS-HDTMA adsorbs more MO and CV than AlgS-Na. The optimum percentage of MO absorbed in AlgS-HDTMA occurred at pH 8, while CV was at pH 11. This is in line with the surface charge of the adsorbent, on AlgS-HDTMA which is positively charged at pH < 8. This indicates that the adsorption of anionic MO with AlgS-HDTMA which is positively charged occurs through electrostatic interactions. The same interaction also occurs between CV dye which is cationic with AlgS-HDTMA optimum at pH 11. In this condition, the surface charge of AlgS-HDTMA tends to be negatively charged.
The effect of the interaction time between AlgS-Na and AlgS-HDTMA with MO and CV was studied by interacting between the adsorbent and the dye at a time varying between 0 and 90 min as shown in Fig. 6. Figure 6 shows that the amount of MO and CV adsorbed by AlgS-Na is relatively small compared to AlgS-HDTMA. The amount of MO and CV dyes adsorbed by AlgS-Na was not affected by the interaction time. During the first 15 min of interaction (Fig. 6), it was observed that the amount of MO and CV adsorbed on AlgS-HDTMA increased greatly, and then an increase in interaction time did not increase the amount of MO and CV dyes adsorbed and even tended to be constant. In this situation, it can be stated that a state of equilibrium has been reached.
The required interaction time data between the adsorbent and the adsorbate in the adsorption process, besides being used to determine the equilibrium time for the maximum adsorption of the adsorbate, are also used to determine several parameters of adsorption kinetics. In this study, the adsorption kinetics were studied using pseudo-first-order (Eq. 4) and pseudo-second-order (Eq. 5) kinetic models [21,22,23]. The data on the effect of interaction time on the amount of MO and CV adsorbed by AlgS-Na and AlgS-HDTMA (Fig. 6) were analyzed using pseudo-kinetic models of order 1 (PKM-1) and 2 (PKM-2) to produce the rates and rate constants for the adsorption of MO and CV ions (Table 1).
where qt and qe (mmol/g) are total adsorbate (MO or CV) adsorption capacity at time t (min) and at equilibrium, respectively, and k1 (1/min) and k2 (g/ mmol·min) are the PKM-1 and PKM-2 rate constants, respectively.
The results of the analysis using the adsorption kinetic model (Eqs. 1 and 2) presented in Table 1 show that the MO and CV adsorption kinetic models by AlgS-Na and AlgS-HDTMA tend to follow PKM-2. correlation (R2) which average is 0.999 or close to 1. Table 1 shows that the PKM-2 (k2) rate constant for AlgS-HDTMA for either MO or CV is greater than that for AlgS-Na. This indicates that the exchange of Na+ ions with HDTMA+ ions has increased adsorption. On the AlgS-HDTMA adsorbent can be observed that the k2 value of MO is greater than CV. The k2 values for MO and CV were 7.043 × 10−3 and 2.205 × 10−3 g/mmol min, respectively (Table 1). The MO dye is anionic so it interacts more quickly with the AlgS-HDTMA adsorbent which has a positive surface charge.
The increase in the initial concentration of the MO and CV dyes exposed was in line with the increase in the value of q (Fig. 7). Figure 7 shows that the increase in the amount of MO adsorbed by AlgS-HDTMA is greater than that of CV. This indicates that AlgS-HDTMA has a greater adsorption affinity for MO dyes compared to CV. This is related to the increase in the number of active sites in accordance with the characteristics of the MO dye on the surface of the AlgS-HDTMA. In this case, AlgS-HDTMA has more positively charged active sites resulting from cation exchange with HDTMA-Br [37, 38].
The parameters of MO and CV adsorption isotherms on AlgS-Na and AlgS-HDTMA were determined by analyzing the data on the effect of initial concentration on the amount of MO and CV adsorbed by AlgS-Na and AlgS-HDTMA (Fig. 7) using the Langmuir adsorption model (LAM) and the Freundlich adsorption model (FAM) with respective equations presented in (Eqs. 6 and 7) [39, 40].
where qe (mmol/g) is the amount of adsorbed MO or CV per unit weight of adsorbent and Ce (mmol/ L) is unadsorbed MO or CV concentration in solution at equilibrium. The constant KL (L/mmol) is the LAM constant, KF is FAM constant, and n is FAM exponent. The FAM exponent n values were between 1 and 10 [39].
Table 2 shows that the R2 values for AlgS-Na and AlgS-HDTMA for MO adsorption are close to 1 in the LAM, while CV in the FAM. These data show that the adsorption of MO by AlgS-Na and AlgS-HDTMA tends to follow the LAM. In the LAM isotherm, it is assumed that each adsorption active site and adsorbate binding ability are equivalent and independent of the adjacent sites occupied or not [41, 42]. In this study, the adsorption process occurs through a chemical reaction involving the interaction between AlgS-HDTMA which has cationic properties and MO which is anionic. Furthermore, CV adsorption by AlgS-Na and AlgS-HDTMA tends to follow the FAM isotherm. CV adsorption on AlgS-Na and AlgS-HDTMA occurs on heterogeneous surfaces of multilayer adsorbents through physical interactions [43].
The adsorption competition experiment between MO and CV was studied by applying the LAM for a bi-component mixture (Eq. 8) [44,45,46].
where qm is the adsorption capacity of the adsorbate, and b1 (MO) and b2 (CV) are the binary LAM isotherm constants.
Table 3 shows that the qm of MO and CV in AlgS-HDTMA is greater than AlgS-Na with the qm value for the mixture of bi-component MO and CV dyes in Alg-Na and AlgS-HDTMA each of 16.940 × 10−2 and 37.030 × 10−2 mmolequiv/g. The data generated from the LAM analysis for bi-component mixtures are in accordance with those obtained from the mono-component adsorption analysis previously shown in Table 2. Furthermore, the adsorption competition between MO and CV in a bi-component solution against AlgS-Na and Alg-HDTMA showed that MO dye was more dominant than CV with the LAM isotherm bi-component constant b2 > b1. Surface modification of Spirulina sp. algal biomass with cationic surfactant HDTMA-Br has made the adsorbent rich in positive charge, making it more effective for adsorbing anionic dyes.
Several factors that affect the mechanism of adsorption of dye molecules by adsorbents in solution include: the surface charge of the adsorbent, the nature and type of interaction between the active groups of the adsorbent and the dye (such as electrostatic attraction, hydrogen bonding, ion exchange, acid–base interactions, covalent bonding, and coordination) [47, 48]. In this study, the adsorption mechanism was studied using the sequential desorption method by sequentially desorption of the material that has maximally adsorbed MO by using various types of desorbents whose strength increases sequentially to physically desorb MO (H2O), cation exchange (natrium nitrat 0.1 M), electrostatic interaction (hydrochloric acid 0.1 M), and coordination (Na2EDTA 0.1 M).
Figure 8 shows that the adsorption mechanism of MO with AlgS-Na is dominated by physical interactions (40%), ion exchange (55%), electrostatic interactions (4%), and the other 1%. The interaction of MO with AlgS-HDTMA was dominated by electrostatic interactions (45%) followed by physical interactions (28%), ion exchange (25%), and the rest of other chemical interactions. These data are in line with the adsorption isotherm data which show that the MO adsorption by AlgS-HDTMA is dominated by chemical interactions, namely electrostatic interactions and cation exchange. This happens because the surface of AlgS-HDMTA contains a positively charged active site of HDTMA+ ions so that it can interact strongly with negatively charged MO.
Data on the ability to reuse adsorbents are an important aspect in handling wastewater treatment because it is very meaningful in reducing water treatment costs as shown in Table 4. Previous studies reported that decolorization of methylene blue using adsorbent fungus fomitopsis pinocola and durian peel waste showed 92.56% and 235.80 mg/g, respectively [49, 50]. The ability to reuse AlgS-HDTMA in absorbing MO was carried out by adsorption–desorption experiments for 5 repetitions (Fig. 9). The results of adsorption of MO solution and desorption with 0.1 M hydrochloric acid solution as eluent with 1–4 repetitions were: 97.8, 94.13, 89.39, and 86.27%. These data indicate that the repetition of 4 times did not significantly reduce the adsorption capacity of MO. 0.1 M hydrochloric acid solution was effective as an eluent to release MO dyes from adsorbents through proton substitution of MO dyes as organic cations [51].
Conclusion
The adsorbent from the modified algae biomass Spirulina sp. with cationic surfactant hexadechyltrimethylammonium bromide (HDTMA-Br) has been successfully synthesized and tested for its ability to adsorption mono-component and bi-component to MO and CV solutions. Kinetic data and adsorption isotherms of MO and CV that were adsorbed by AlgS-HDTMA showed that this adsorbent was very effective at adsorbing anionic MO dyes compared to cationic CV. The amount of MO and CV adsorbed in the mono-component solution were 17.280 × 10−2 and 8.160 × 10−2 mmol/g, while in the bi-component solution it was 15.600 and 2.800 × 10−2 L/mmolequiv. Algae surface modification Spirulina sp. with the cationic surfactant HDTMA-Br has produced an effective adsorbent to adsorb anionic dyes such as MO. AlgS-HDTMA adsorbent is very efficient to use because it has the ability to be used repeatedly.
References
P.G. Priya, V. Ramamurthi, A. Prabhu, Degradation studies of tannery effluents using electro flotation technique. J. Chem. Eng. Process Technol. 2(1), 1–4 (2011). https://doi.org/10.4172/2157-7048.1000104
S.R. Couto, Dye removal by immobilised fungi. J. Biotechnol. Adv. 27(3), 227–235 (2009). https://doi.org/10.1016/j.biotechadv.2008.12.001
T. Minmin, Q. Junlian, L. Fengting, P.K. Bera, Electrospun mesoporous carbon nanofibers produced from phenolic resin and their use in the adsorption of large dye. Mol. J. Carbon 50(8), 2877–2886 (2012). https://doi.org/10.1016/j.carbon.2012.02.056
R. Senthilkumar, K. Vijayaraghavan, M. Thilakavathi, P.V.R. Iyer, M. Velan, Seaweeds for the remediation of wastewaters contaminated with zinc(II) ions. J. Hazard. Mater. 136(3), 791–799 (2006). https://doi.org/10.1016/j.jhazmat.2006.01.014
T.A. Khan, E.A. KhanShahjahan, Removal of basic dyes from aqueous solution by adsorption onto binary iron-manganese oxide coated kaolinite: non-linear ishoterm and kinetics modelling. Appl. Clay Sci. 107, 70–77 (2015). https://doi.org/10.1016/j.clay.2015.01.005
V.K. Gupta, S. Khamparia, I. Tyagi, D. Jaspal, A. Malviya, Decolorization of mixture of dyes: a critical review. Glob. J. Environ. Sci. Manag. 1(1), 71–94 (2015). https://doi.org/10.7508/gjesm.2015.01.007
S. Kittappa, S. Pichiah, J.R. Kim, Y. Yoon, S.A. Snyder, M. Jang, Magnetised nanocomposite mesoporous silica and its application for effective removal of methylene blue from aqueous solution. Sep. Purif. Technol. 153, 67–75 (2015). https://doi.org/10.1016/j.seppur.2015.08.019
S. Dardouri, J. Sghaier, A comparative study of adsorption and regeneration with different agricultural wastes as adsorbents for the removal of methylene blue from aqueous solution. Chin. J. Chem. Eng. 25(9), 1282–1287 (2017). https://doi.org/10.1016/j.cjche.2017.01.012
M. Sharma, K. Anubha, C.P. Kaushik, Waste biomass of Nostoc linckia as adsorbent of crystal violet dye: optimization based on statistical model. Int. Biodeterior. Biodegrad. 65(3), 513–521 (2011). https://doi.org/10.1016/j.ibiod.2011.02.002
C.S. Umpierres, L.T.D. Prola, M.A. Adebayo, E.C. Lima, G.S.D. Reis, D.D.F. Kunzler, G.L. Dotto, L.T. Arenas, E.V. Benvenutti, Mesoporous Nb2O5/SiO2 material obtained by sol-gel method and applied as adsorbent of crystal violet dye. Environ. Technol. 38(5), 566–578 (2016). https://doi.org/10.1080/09593330.2016.1202329
T. Robinson, G. McMullan, R. Marchant, P. Nigam, Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Biores. Technol. 77(3), 247–255 (2001). https://doi.org/10.1016/S0960-8524(00)00080-8
B. Saba, B.V. Kjellerup, A.D. Christy, Eco-friendly bio-electro-degradation of textile dyes wastewater. Bioresour. Technol. Rep. 15, 100734 (2021). https://doi.org/10.1016/j.biteb.2021.100734
I.S. Saputra, Y. Yulizar, Y.K. Krisnandi, D. Annas, K.C. Sembiring, A.H. Saputro, A.G. Fahmi, Green fabrication of Au nanoparticles as SERS-active substrate for enhancement hydroquinone detection in cosmetics. Vib. Spectrosc. 126, 103543 (2023). https://doi.org/10.1016/j.vibspec.2023.103543.
H.Y. Rong, B.Y. Gao, R.H. Li, Y. Wang, Q.Y. Yue, Q. Li, Effect of dose methods of a synthetic organic polymer and PFC on floc properties in dyeing wastewater coagulation process. Chem. Eng. J. 243, 169–175 (2014). https://doi.org/10.1016/j.cej.2013.12.005
W. Wang, J.J. Hu, R.D. Zhang, C. Yan, L. Cui, J.J. Zhu, A pH-responsive carboxymethyl cellulose/chitosan hydrogel for adsorption and desorption of anionic and cationic dyes. Cellulose 28(2), 897–909 (2021). https://doi.org/10.1007/s10570-020-03561-4
Buhani, T.A. Wijayanti, Suharso, Sumadi, M. Ansori, Application of modified green algae Nannochloropsis sp. as adsorbent in the simultaneous adsorption of methylene blue and Cu(II) cations in solution. Sustain. Environ. Res. 31, 17 (2021). https://doi.org/10.1186/s42834-021-00090-y
S. Hussain, M. Kamran, S.A. Khan, K. Shaheen, Z. Shah, H. Suo, Q. Khan, A.B. Shah, W.U. Rehman, Y.O. Al-Ghamdi, U. Ghani, Adsorption, kinetics and thermodynamics studies of methyl orange dye sequestration through chitosan composites films. Int. J. Biol. Macromol. 168, 383–394 (2021). https://doi.org/10.1016/j.ijbiomac.2020.12.054
M.T. Yagub, T.K. Sen, S. Afroze, H.M. Ang, Dye and its removal from aqueous solution by adsorption: a review. Adv. Coll. Interface. Sci. 209, 172–184 (2014). https://doi.org/10.1016/j.cis.2014.04.002
S. Buhani, F. Luziana, M. Rilyanti, Sumadi., Production of adsorbent from activated carbon of palm oil shells coated by Fe3O4 particle to remove crystal violet in water. Desalin. Water Treat. 171(1), 281–293 (2019). https://doi.org/10.5004/dwt.2019.24776
Y. Aruna, N. Bagotia, S. Yadav, A.K. Sharma, S. Kumar, Adsorptive studies on the removal of dyes from single and binary systems using Saccharum munja plant-based novel functionalized CNT composites. Environ. Technol. Innov. 24, 102015 (2021). https://doi.org/10.1016/j.eti.2021.102015
M.M. Montazer-Rahmati, P. Rabbani, A. Abdolali, A.R. Keshtkar, Kinetics and equilibrium studies on biosorption of cadmium, lead, and nickel ions from aqueous solutions by intact and chemically modified brown algae. J. Hazard. Mater. 185(1), 401–407 (2011). https://doi.org/10.1016/j.jhazmat.2010.09.047
R. Angelova, E. Baldikova, K. Pospiskova, Z. Maderova, M. Safarikova, I. Safarik, Magnetically modified Sargassum horneri biomass as an adsorbent for organic dye removal. J. Clean. Prod. 137, 189–194 (2016). https://doi.org/10.1016/j.jclepro.2016.07.068
E. Daneshvar, A. Vazirzadeh, A. Niazi, M. Kousha, M. Naushad, A. Bhatnagar, Desorption of methylene blue dye from brown macroalga: effects of operating parameters, isotherm study and kinetic modelling. J. Clean. Prod. 152, 443–453 (2017). https://doi.org/10.1016/j.jclepro.2017.03.119
Sh. Husien, M.E. Reem, M. Nora, A.B. Abdel-aziz, A. Khlood, O.E. Salma, G.M. Nagwan, A.S. Lobna, S.F. Irene, G.R. Ahmed, Potentials of algae-based activated carbon for the treatment of M.orange in wastewater. Case Stud. Chem. Environ. Eng. 7, 100330 (2023). https://doi.org/10.1016/j.cscee.2023.100330
B.C. Talles, L.S. Thiago, S.D.C. Camila, G.C.S. Meuris, G.A.V. Melissa, Chromium adsorption using Sargassum filipendula algae waste from alginate extraction: batch and fixed-bed column studies. Chem. Eng. J. Adv. 11, 100341 (2022). https://doi.org/10.1016/j.ceja.2022.100341
N. Piyushi, K.A. Subramanian, M.G. Dastidar, Adsorptive removal of dye using biochar derived from residual algae after in-situ transesterification: alternate use of waste of biodiesel industry. J. Environ. Manag. 182, 187–197 (2016). https://doi.org/10.1016/j.jenvman.2016.07.063
K.Z. Elwakeel, A.A. El-Bindary, A.Z. El-Sonbati, A.R. Hawas, Adsorption of toxic acidic dye from aqueous solution onto diethylenetriamine functionalized magnetic glycidyl methacrylate-N, N′-methylenebisacrylamide. RSC Adv. 4(6), 3350–3361 (2016)
K.Z. Elwakeel, A.A. El-Bindary, A.Z. El-Sonbati, A.R. Hawas, Magnetic alginate beads with high basic dye removal potential and excellent regeneration ability. Can. J. Chem. 95, 807–815 (2017)
K.Z. Elwakeel, A.M. Elgarahy, G.A. Elshoubaky, S.H. Mohammad, Microwave assist sorption of crystal violet and Congo red dyes onto amphoteric sorbent based on upcycled Sepia shells. J. Environ. Health Sci. Eng. 18, 35–50 (2020). https://doi.org/10.1007/s40201-019-00435-1
Buhani, Suharso, Z. Sembiring, Immobilization of Chetoceros sp microalgae with silica gel through encapsulation technique as adsorbent of Pb metal from solution. Orient. J. Chem. 28(1), 271–278 (2012). https://doi.org/10.13005/ojc/280133
Buhani, Suharso, I. Aditiya, R.A. Kausar, Sumadi, Rinawati, Production of a Spirulina sp. algae hybrid with a silica matrix as an effective adsorbent to absorb crystal violet and methylene blue in a solution. Sustain. Environ. Res. 29(1), 27 (2019). https://doi.org/10.1186/s42834-019-0027-2
Buhani, Narsito, Nuryono, E.S. Kunarti, Production of metal ion imprinted polymer from mercapto-silica through sol-gel process as selective adsorbent of cadmium. Desalination 251(1–3), 83–89 (2010). https://doi.org/10.1016/j.desal.2009.09.139
U.A. Guler, M. Ersan, E. Tuncel, F. Dügenci, Mono and simultaneous removal of crystal violet and safranin dyes from aqueous solutions by HDTMA-modified Spirulina sp. Process Saf. Environ. Prot. 99, 194–206 (2016). https://doi.org/10.1016/j.psep.2015.11.006
Z. Li, W.T. Jiang, H. Hong, An FTIR investigation of hexadecyltrimethylammonium intercalation into rectorite. Spectrochim. Acta, A: Mol. Biomol. Spectrosc. 71(4), 1525–1534 (2008). https://doi.org/10.1016/j.saa.2008.05.015
A.G. Plaska, M. Majdan, S. Pikus, D. Sternik, Simultaneous adsorption of chromium(VI) and phenol on natural red clay modified by HDTMA. Chem. Eng. J. 179, 140–150 (2012). https://doi.org/10.1016/j.cej.2011.10.071
L. Ai, C. Zhang, F. Liao, Y. Wang, M. Li, L. Meng, J. Jiang, Removal of methylene blue from aqueous solution with magnetite loaded multi-wall carbon nanotube: kinetic, isotherm and mechanism analysis. J. Hazard. Mater. 198, 282–290 (2011). https://doi.org/10.1016/j.jhazmat.2011.10.041
N. Atar, A. Olgun, S. Wang, Adsorption of cadmium(II) and zinc(II) on boron enrichment process waste in aqueous solutions: batch and fixed-bed system studies. Chem. Eng. J. 192, 1–7 (2012). https://doi.org/10.1016/j.cej.2012.03.067
A.H. AbdEl-Salam, H.A. Ewais, A.S. Basaleh, Silver nanoparticles immobilised on the activated carbon as efficient adsorbent for removal of crystal violet dye from aqueous solutions. A kinetic study. J. Mol. Liq. 248, 833–841 (2017). https://doi.org/10.1016/j.molliq.2017.10.109
Y.S. Ho, J.F. Porter, G. McKay, Equilibrium isotherm studies for the sorption of divalent metal ions onto peat: copper, nickel, and lead single component systems. Water Air Soil Pollut. 141, 1–33 (2002). https://doi.org/10.1023/A:1021304828010
R. Foroutan, H. Esmaeili, M. Abbasi, M. Rezakazemi, M. Mesbah, Adsorption behavior of Cu(II) and Co(II) using chemically modified marine algae. Environ. Technol. 39(21), 2792–2800 (2018). https://doi.org/10.1080/09593330.2017.1365946
X. Xin, Q. Wei, J. Yang, L. Yan, R. Feng, G. Chen, B. Du, H. Li, Highly efficient removal of heavy metal ions by amine-functionalized mesoporous Fe3O4 nanoparticles. Chem. Eng. J. 184, 132–140 (2012). https://doi.org/10.1016/j.cej.2012.01.016
I. Larraza, M. Lŏpez-Gŏnzales, T. Corrales, G. Marcelo, Hybrid materials: magnetite polyethylenimine-montmorillonite, as magnetic adsorbents for Cr(VI) water treatment. J. Colloid Interface Sci. 385(1), 24–33 (2012). https://doi.org/10.1016/j.jcis.2012.06.050
Y. Shao, L. Zhou, C. Bao, J. Ma, M. Liu, F. Wang, Magnetic responsive metal-organic frameworks nanosphere with core-shell structure for highly efficient removal of methylene blue. Chem. Eng. J. 283, 1127–1136 (2016). https://doi.org/10.1016/j.cej.2015.08.051
M.R. Fagundes-Klen, P. Ferri, T.D. Martins, C.R.G. Tavares, E.A. Silva, Equilibrium study of the binary mixture of cadmium–zinc ions biosorption by the Sargassum filipendula species using adsorption isotherms models and neural network. Biochem. Eng. J. 34(2), 136–146 (2007). https://doi.org/10.1016/j.bej.2006.11.023
S.J. Kleinübing, E.A. da Silva, M.G.C. da Silva, E. Guibal, Equilibrium of Cu(II) and Ni(II) biosorption by marine alga Sargassum filipendula in a dynamic system: competitiveness and selectivity. Biores. Technol. 102(7), 4610–4617 (2011). https://doi.org/10.1016/j.biortech.2010.12.049
N. Singh, C. Balomajumder, Simultaneous removal of phenol and cyanide from aqueous solution by adsorption onto surface modified activated carbon prepared from coconut shell. J. Water Process Eng. 9, 233–245 (2016). https://doi.org/10.1016/j.jwpe.2016.01.008
Y.Y. Zhang, Q. Liu, C. Yang, S.C. Wu, J.H. Cheng, Magnetic aluminum-based metal organic framework as a novel magnetic adsorbent for the effective removal of minocycline from aqueous solutions. Environ. Pollut. 255(2), 113226 (2019). https://doi.org/10.1016/j.envpol.2019.113226
Buhani, Suharso, M. Rilyanti, Sumadi, Implementation of sequential desorpsion in determining Cd(II) ion interaction with adsorbent of ionic imprinting amino-silica hybrid. Rasayan J. Chem. 11(2), 865–870 (2018). https://doi.org/10.7324/RJC.2018.1122084
S.P. Adi, D.R. Hamdan, U. Aulia, N. Refdinal, S.P. Herdayanto, Decolorization and transformation of synthetic dye methylene blue by brown-rot fungus fomitopsis pinicola. Indones. J. Chem. 22(2), 557–564 (2022). https://doi.org/10.22146/ijc.69834
T.T. Nguyen, T.T.N. Nguyen, T.C.N. Vo, N.B. Hoang, T.P.Q. Bui, G.B. Long, D.N. Trinh, A fixed-bed column study for removal of organic dyes from aqueous solution by pre-treated durian peel waste. Indones. J. Chem. 19(2), 486–494 (2019). https://doi.org/10.22146/ijc.39712
S.P. Varghese, A.T. Babu, B. Babu, R. Antony, γ-MnOOH nanorods: efficient adsorbent for removal of methylene blue from aqueous solutions. J. Water Process Eng. 19, 1–7 (2017). https://doi.org/10.1016/j.jwpe.2017.06.001
Acknowledgements
The authors are grateful to Higher Education for Technology and Innovation (HETI) Project Universitas Lampung for funding this study based on Research innovation and collaboration program 2022 with contract numbers: 6018/UN26/HK.01.03/2022.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this manuscript. In addition, the ethical issues, including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, and redundancies have been completely observed by the authors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Buhani, Nadia, P.O., Suharso et al. Modification of Spirulina sp. algae by exchanging Na+ cations and hexadechyltrimethylammonium+ (HDMTA)+ for removal of methyl orange and crystal violet dyes from solution. J IRAN CHEM SOC 20, 2363–2372 (2023). https://doi.org/10.1007/s13738-023-02844-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-023-02844-4