Abstract
A novel, heterogeneous and homogeneous basic nanocatalysts were synthesized by grafting of lithium and cesium on zagrosian natural asphalt sulfonate (Li/Cs-Zagronas). The activity of these catalysts was examined in the Claisen–Schmidt and Knoevenagel condensations under mild reaction conditions. Li/Cs-Zagronas were characterized by FT-IR spectroscopy, scanning electron microscopy, X-ray diffraction, energy-dispersive spectroscopy, inductively coupled plasma and thermogravimetric analysis techniques. These nanocatalysts were removed by simple filtration and reused several times without any deterioration of activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent decades, carbon nanomaterials have attracted significant attention for use as catalyst substrate due to their unique surface chemistry, large specific surface area, low cost and chemical stability. Today, many substrates such as SBA-15, [1, 2] graphite [3], alumina, [4,5,6,7] zeolites [8,9,10] and silica gel [11, 12] have been used to immobilization variety alkali metals such as Cs,[13,14,15] K [16] and Li [17], but many of them require expensive material, a lot of time and tedious conditions for preparation.
The alkali metals are always used as promoter to increase the catalytic activity and lengthen the lifetime of the catalyst. The dispersion of alkali metals on the catalyst substrates has made it easier to investigate the catalytic activity of these elements. Among the alkali metals, lithium and cesium compounds due to their special properties such as conduction and thermal conductivity are used in many industrial processes [18,19,20].
In the previous works, we were able to the successful grafting of copper and potassium on natural asphalt and study of their catalytic properties [21,22,23]. Therefore, we were encouraged to grafting other metals including lithium and cesium on the same substrate and were studied their catalytic properties.
Natural asphalt is a shiny black solid hydrocarbon and a type of asphalt that easily turns into a dark brown powder. Due to the fact that natural asphalt is a natural and non-toxic substance, its use is increasing. But no studies have been conducted yet on the application of natural asphalt as a catalyst in organic reactions.
Natural asphalt with large specific surface area, excellent stability and high carbon contents is beneficial for use as catalyst substrate in the organic reactions.
The nature of functional groups on the surface of the natural asphalt, particularly carbon–carbon double bonds in aromatic and heteroaromatic rings, vinyl and hydroxyl groups can act as anchoring sites in the preparation of supported catalysts. These nanocatalysts have the advantages such as easy preparation and separation, available and inexpensive materials in comparison with other catalysts.
Claisen–Schmidt and Knoevenagel condensations are well-known strategy for carbon–carbon bond formation in organic synthesis [24, 25], because Claisen–Schmidt and Knoevenagel products play an essential role in the synthesis of remedial drugs [26], natural products [27, 28], heterocyclic compounds [29, 30] and functional polymers [31].
Herein, we reported synthesis of natural asphalt sulfonate supported with Li/ Cs as a novel, recyclable and environmentally friendly catalysts for the Claisen–Schmidt and Knoevenagel condensations in the green and mild conditions under solvent-free and use of ethanol at room temperature.
Experimental
Materials and apparatus
All reagents and solvents were purchased from Merck, Sigma-Aldrich and Fluka Companies and natural asphalt from the Kimia bitumen Zagros Cooperative, Iran. All the known compounds were identified by comparing their melting points and/or spectral data to those reported in the literature. The reactions were monitored using TLC on silica-gel Polygram SILG/UV254 plates. Proton nuclear magnetic resonance (1H NMR) spectroscopy was also performed on Bruker AVANCE DPX-400 and DPX-500 spectrometers. Chemical shifts were reported in ppm relative to TMS as the internal standard. Fourier-transform infrared spectroscopy (FT-IR) was performed using FT-IR-8300 spectrometer made by Shimadzu. The morphology of the catalyst was examined by performing scanning electron microscopy (SEM) using Mira 3-XMU. The elemental composition was determined using EDS and Mira 3-XMU. The exacted amount of Li and Cs in the catalyst was determined by inductively coupled plasma (ICP) using VISTA-PRO, Australia. X-ray diffraction (XRD) was investigated using a Holland Philips X, and the thermogravimetric analysis (TGA) curve was recorded using a PL-STA 1500 device manufactured by Thermal Sciences.
Catalyst preparation
Preparation of lithium-zagrosian natural asphalt sulfonate (Li-Zagronas)
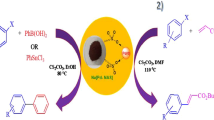
First, zagrosian natural asphalt sulfonate (Zagronas) was prepared via sulfonation of zagrosian natural asphalt in the presence of concentrated sulfuric acid according to the recently reported procedure [21]. In the next step, a mixture of zagrosian natural asphalt sulfonate (0.5 g), lithium metal (0.32 g) in water (10 mL) was stirred for 120 min at room temperature. Then, the solvent was evaporated and lithium-zagrosian natural asphalt sulfonate (Li-Zagronas) was obtained by being dried at 100 °C in oven (Scheme 1). Li-Zagronas was characterized by FT-IR, SEM, XRD, TGA and ICP techniques.
Preparation of cesium-zagrosian natural asphalt sulfonate (Cs-Zagronas)
To prepare cesium-zagrosian natural asphalt sulfonate (Cs-Zagronas), an aqueous cesium carbonate10% (10 mL) was added to the Zagronas (0.5 g) and was stirred at room temperature for 1 h. Then, the water was evaporated and cesium-zagrosian natural asphalt sulfonate (Cs-Zagronas) was obtained after drying at 100 °C in oven (Scheme 1). This catalyst was characterized by FT-IR, SEM, EDS, XRD, TGA and ICP techniques.
General procedure for Claisen–Schmidt condensation catalyzed by Li-Zagronas or Cs-Zagronas
A mixture of ketones (1 mmol), benzaldehyde derivatives (1 mmol) and Li-Zagronas or Cs-Zagronas (15 mg) in ethanol (2.0 mL) was stirred at room temperature until the reaction was completed. After completion of the reaction, the reaction mixture was cooled to room temperature and the catalyst was separated by filtration and cold water (1 mL) was added to the reaction mixture. Finally, the products were obtained through simple filtration and recrystallization with ethanol and water.
General procedure for Knoevenagel condensation catalyzed by Li-Zagronas
A reaction mixture containing benzaldehyde derivatives (1 mmol), activated methylene compounds (1 mmol) and Li-Zagronas (15 mg) in ethanol (2.0 mL) as solvent was stirred at 80 °C. When the reaction was completed, the catalyst was separated using filtration and cold water (1 mL) was added to the reaction mixture to yield the products. Finally, the pure products were obtained through recrystallization with ethanol and water.
General procedure for Knoevenagel condensation catalyzed by Cs-Zagronas
Cs-Zagronas (15 mg) was stirred with of activated methylene compounds (1 mmol) and benzaldehyde derivatives (1 mmol) at room temperature for appropriate time. After completion of the reaction, ethanol (2.0 mL) was added to the reaction mixture. Then, the catalyst was separated using filtration and cold water (1 mL) was added to the reaction mixture to yield the products. The crude products were purified using recrystallization with ethanol and water.
Selected spectral data
(E)-3-(4-chlorophenyl)-1-(p-tolyl)prop-2-en-1-one IR (KBr) (vmax/cm−1): 1406–1603 (Ar), 1656 (CO), 2850–2900(CH).1H NMR (400 MHz, CDCl3): δH = 2.47(s, 3H), 7.33 (d, J = 8 Hz, 2H), 7. 42 (d, J = 8 Hz, 2H), 7.56 (d, J = 16 Hz, 1H), 7.60 (d, J = 4 Hz, 2H), 7.80 (d, J = 16 Hz, 1H), 7.97 (d, J = 8 Hz, 2H) ppm. 13CNMR (100 MHz, CDCl3): δ = 21.7, 122.4, 128.6, 129.2, 129.4, 129.5, 133.15, 135.4, 136.3, 142.9,143.8, 189.7. ppm.
(E)-1-(4-chlorophenyl)-3-(4-methoxyphenyl) prop-2-en-1-one IR(KBr) (vmax/cm−1):
1439- 1603 (Ar), 1653 (CO). 1H NMR (400 MHz, CDCl3): δH = 2.92(s, 3H), 7.45 (d, J = 8 Hz, 2H), 7.58 (d, J = 16 Hz, 1H), 7. 67 (m, 2H), 7.84 (d, J = 16 Hz, 1H) 8.07 (d, J = 8 Hz, 2H) ppm. 13CNMR (100 MHz, CDCl3): δ = 55.5, 113.8, 121.8, 128.4, 128.9, 130.3, 130.8, 131.1, 135.1, 144.0, 163.4, 188.7 ppm.
2-benzylidenemalononitrile IR (KBr) (vmax/cm−1): 1469–1640 (Ar), 2225(CN).1H NMR (400 MHz, CDCl3): δH = 7.57(m, 3H), 7.67 (d, J = 8 Hz, 2H), 7. 82 (s, 1H) ppm. 13CNMR (100 MHz, CDCl3): δ = 82.8, 112.6, 128.5, 130.2, 130.8, 134.7, 160.1 ppm.
2-(4-(dimethylamino) benzylidene) malononitrile IR (KBr) vmax/cm−1): 1469–1640 (Ar), 2225(CN). 1H NMR (400 MHz, CDCl3): δH = 6.84 (d, J = 8 Hz, 2H), 7.82 (d, J = 8 Hz, 2H), 8. 03 (s, 1H) ppm. 13CNMR (100 MHz, CDCl3): δ = 39.6, 68.6, 111.9, 115.5, 116.2, 118.7, 133.5, 154.3,158.8 ppm.
Results and discussion
Characterization of Li/CS-Zagronas
The prepared nanocatalysts were characterized by FT-IR, SEM, XRD, EDS, ICP and TGA techniques comprehensively.
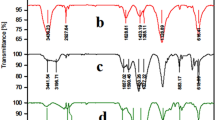
The FT-IR spectra for the zagrosian natural asphalt (a), zagrosian natural asphalt sulfonate (Zagronas) (b), Li-Zagronas (c) and Cs-Zagronas (d) are shown in Fig. 1. The FT-IR was used for the confirmation the presence of sulfuric acid moiety on the surface of zagrosian natural asphalt. The FT-IR spectrum for zagrosian natural asphalt sulfonate exhibits a stretching vibration at 800–1200 cm-1 which incorporates the contributions from both symmetrical and asymmetrical modes of O = S = O bonds (Figure b). Also, the broad band around 3400 cm−1 is related to the stretching vibrations of O–H and N–H groups on the surface of zagrosian natural asphalt. The successful graft of Li and Cs on the surface of zagrosian natural asphalt was approved by the FT-IR spectra for the Li-Zagronas (c) and Cs-Zagronas (d). Shifting some bands to lower frequencies in the spectrum of Li-Zagronas (c) and Cs-Zagronas (d) can be explained by the grafting of Li and Cs on the surface of zagrosian natural asphalt.
The morphology and size of particles was evaluated by SEM technique. Figure 2 (a–b), respectively, shows the SEM images of Li-Zagronas (a) and Cs-Zagronas (b). The SEM images showed that the nanoparticles are approximately spherical in shape and the average diameter for the Li-Zagronas and Cs-Zagronas are about 17–37 and 33–64 nm.
The XRD patterns of Li-Zagronas (a) and Cs-Zagronas(b) are shown in Fig.3. According to Fig. (3a), six peaks observed in XRD spectrum of Li-Zagronas at 2θ = 31.85°, 32.15°, 34.80°, 43.50°, 52.30° and 68.10° were related to the Li in Li-Zagronas. In addition, the peaks of the XRD spectrum of Cs-Zagronas(b) at 2ϴ = 11.00°, 19.80°, 21.25°, 24.90°, 27.30°, 34.25° and 59.45° were related to the Cs in the Cs-Zagronas.
The components of Cs-Zagronas were analyzed by using EDS analysis (Fig. 4). The EDS spectrum of catalyst shows the presence of C, O, S, N and Cs species in the Cs-Zagronas and demonstrates that the Cs successfully grafted on the zagrosian natural asphalt.
Also, the results of EDS of Li-Zagronas and Cs-Zagronas were confirmed by EDS mapping analysis. The EDS mapping was used for distributions of C, O and S in the Li-Zagronas and C, N, O, S and Cs in the Cs-Zagronas structures. Also, as shown in figures, the homogeneous distribution of all the elements is clearly observed in the EDS mapping analysis (EDS mapping figures are shown in supplementary information).
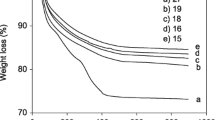
Figure 5 shows the TGA diagram of Li-Zagronas (1a) and Cs-Zagronas (1b). As shown in Fig. 5, the first weight loss below 200 °C (about 10%) is caused by the removal of the adsorbed solvents and the second weight loss between 300 and 600 °C (about 10%) in two diagrams is due to the decomposition of organic groups of the Li-Zagronas and Cs-Zagronas. As the result of this analysis, Li-Zagronas and Cs-Zagronas have high tolerance at high temperatures.
Catalytic studies
Claisen–Schmidt condensation
After characterization of the catalyst, catalytic activity of Li-Zagronas and Cs-Zagronas was investigated in the Claisen–Schmidt condensations. First, we examined the activity of Li-Zagronas and Cs-Zagronas for the synthesis of α,β-unsaturated carbonyl compounds via Claisen–Schmidt condensation. In this respect, reaction of acetone and benzaldehyde was selected as model reaction and the reaction conditions were optimized with respect to the solvent and the amount of catalyst.
The obtained results for Li-Zagronas and Cs-Zagronas are listed in Table 1. First, the effect of catalyst loading on the model reaction was studied (Table 1, Entry 1–5). The best results were obtained with 0.015 g Li-Zagronas and Cs-Zagronas in ethanol (Table 1, entry 4). Then, under catalytic amount of Li-Zagronas and Cs-Zagronas (15 mg), the effect of solvent in the model reaction was investigated. This process was performed in the absence of solvent and a series of solvents such as PhCH3, DMF, THF, H2O and EtOH at room temperature. The remarkable point is that these nanocatalysts dissolve in organic solvents such as PhCH3, DMF and EtOH while insoluble in water (Fig. 6). Therefore, they can act as heterogeneous and homogeneous catalysts.

After optimizing the reaction conditions, to explore the activity of these catalysts, reaction of various benzaldehyde derivatives with electron-donating and electron-withdrawing functional groups and a wide range of aliphatic and aromatic ketones were investigated under optimum conditions. The results are listed in Tables 2 and 3. Various benzaldehyde derivatives afforded the relevant products in short times and high yields.
Knoevenagel condensation
In the second part of our research, we studied the activity of Li-Zagronas and Cs-Zagronas nanocatalyst in the Knoevenagel condensation. In this respect, the condensation between benzaldehyde and malononitrile was chosen as the model reaction. The effect of different parameters including solvent, the amount of catalyst and temperature on the model reaction, was investigated and the results summarized in Table 4. As seen on Table 4, optimum conditions for the Li-Zagronas were obtained with 15 mg of Li-Zagronas in ethanol at room temperature and for the Cs-Zagronas using 15 mg of the catalyst under solvent-free condition.

Subsequently, under the optimized reaction conditions, Knoevenagel condensation of various aromatic aldehydes and two types of active methylene compounds (malononitrile and ethyl cyanoacetate) were investigated. The results are summarized in Table 5.
Li-Zagronas and Cs-Zagronas were found to be a highly effective catalyst for the Knoevenagel condensation; all substituents on the various benzaldehyde derivatives, including methoxy, hydroxyl, halide and nitro on ortho, meta and para positions, afforded the relevant products in short reaction times with high yields.
Based on results in Tables 2, 3 and 5, both catalysts have a good to excellent yields with a short time, but in comparison between the two catalysts, Cs-Zagronas has more activity and efficiency in the Claisen–Schmidt and Knoevenagel condensations.
Recyclability of the catalyst
Recovery and reusability are an important factor in catalytic processes. For this purpose, we checked the recycling of the Li-Zagronas and Cs-Zagronas by choosing the reaction of benzaldehyde with acetophenone and benzaldehyde with malononitrile as model reactions. In these experiments, after completion of the reactions, the catalysts were easily separated from the reaction mixture using filtration and the recyclability of Li-Zagronas and Cs-Zagronas was examined in the model reactions for five times in the Claisen–Schmidt and Knoevenagel condensations. As shown in Figs. 7 and 8, no significant loss of activity was observed in the separated catalysts.
Characterization of recycled catalyst
In order to show the structure stability of Li-Zagronas and Cs-Zagronas after recycling, recovered catalysts were characterized by SEM and FT-IR techniques (Figs. 9, 10, 11). The SEM images of the recovered catalysts are shown in Fig. 9. The FT-IR spectrum and SEM images of the recovered Li-Zagronas and Cs-Zagronas indicate that these catalysts can be recycled without any change in their structure. Furthermore, the exact amount of lithium and cesium in fresh catalysts are 1.9 mmol g−1 and 2.2 mmol g−1, respectively. Based on ICP analysis, after five reuse runs, the lithium and cesium concentration is found to be 5.03 and 6.08%; in addition, the catalyst leaching for two catalysts after recovery is approximately 0.13%.
Comparison of the catalysts
The efficiency of Li-Zagronas and Cs-Zagronas as catalysts was demonstrated by comparing them with the catalysts reported in literature. Tables 6 and 7 present the results associated with the reaction of benzaldehyde with acetophenone and malononitrile in the presence of Li-Zagronas and Cs-Zagronas. As is evident, these catalysts show shorter reaction time and better yield than the other catalysts. Also, Li-Zagronas and Cs-Zagronas are comparable in terms of cost, non-toxicity, simple preparation and stability.
Conclusions
In summary, we have demonstrated that Li-Zagronas and Cs-Zagronas are a novel and efficient reusable heterogeneous and homogeneous nanocatalysts for the Claisen–Schmidt and Knoevenagel condensations. This is the first report for the use of lithium and cesium grafted on zagrosian natural asphalt sulfonate as a basic separable nanocatalysts.
Notable advantages of these protocols are good to excellent yields, the mild reaction conditions and eco-friendly conditions. Also, the nanocatalysts can be reused for many times without any significant loss in its activity. More importance, Li-Zagronas and Cs-Zagronas have several advantages such as low toxicity, thermal and mechanical stability, simple and inexpensive procedure of preparation. Also, these catalysts, being soluble in water and insoluble in other organic solvents, can be used as heterogeneous and homogeneous catalysts in organic reactions.
References
A. Infantes-Molina, A. Romero-Perez, V. Sanchez-Gonzalez, B. Pawelec, J.L. Fierro, A. Jimenez-Lopez, E. Rodriguez-Castellon, ACS Catal. 1, 175 (2011)
Y. Wang, R. Yan, Z. Lv, H. Wang, L. Wang, Z. Li, S. Zhang, Catal. Letters. 146, 1808 (2016)
W.P. Leung, Y.C. Chan, Ency. Inorg. Bioinorg. Chem. 15, 1 (2011)
C. Xiong, N. Liang, H. An, X. Zhao, Y. Wang, RSC Adv. 5(125), 103523 (2015)
G. Zhang, H. Zhang, D. Yang, C. Li, Z. Peng, S. Zhang, Catal. Sci. Technol. 6, 6417 (2016)
Z. Guo, G. Zhang, L. Wang, P. Li, C. Li, Ind. Eng. Chem. Res. 59, 3334 (2020)
M. Yao, W. Liang, H. Chen, X. Zhang, Catal. Letters. 17, 1 (2020)
C.J. Reinhold, P.A. Anderson, P.P. Edwards, V.V. Terskikh, C.I. Ratcliffe, J.A. Ripmeester, J. Phys. Chem. 112, 17796 (2008)
H. Han, M. Liu, F. Ding, Y. Wang, X. Guo, C. Song, Ind. Eng. Chem. 55, 1849 (2016)
C. Zuo, T. Ge, X. Guo, C. Li, S. Zhang, Microporous and Mesoporous Mater. 256, 58 (2018)
Y. Wang, X. Lang, G. Zhao, H. Chen, Y. Fan, L. Yu, X. Ma, Z. Zhu, RSC Adv. 5, 32826 (2015)
T. He, Y. Qu, J. Wang, Catal. Lett. 149, 373 (2019)
I.A. Ibarra, E. Lima, S. Loera, P. Bosch, S. Bulbulian, V. Lara, J. Phys. Chem. B. 110, 21086 (2006)
B. Li, R. Yan, L. Wang, Y. Diao, Z. Li, S. Zhang, Ind. Eng. Chem. 53, 1386 (2014)
H. Ma, Y. Guan, W. Chen, Z. Sui, G. Qian, D. Chen, X. Zhou, X. Duan, Cata. Today 365, 310 (2020)
E. Vance, M. Stewart, G. Lumpkin, J. Mater. Sci. 26, 2694 (1991)
K. Chen, Z. Sun, R. Fang, Y. Shi, H. Cheng, Adv. Funct. Mater. 28, 1707592 (2018)
T. Yi, L. Jiang, J. Shu, C. Yue, R. Zhu, H. Qiao, J. Phys. Chem. Solids. 71, 1236 (2010)
J. Hadermann, A. Abakumov, S. Turner, Z. Hafideddine, N. Khasanova, E. Antipov, G. Van Tendeloo, Chem. Mater. 9, 3540 (2011)
I. Zuraida, S. Budijanto, Int. Food Res. 18, 405 (2011)
H. Kohzadi, M. Soleiman-Beigi, New J. Chem. 44, 12134 (2020)
S. Falah, M. Soleiman-Beigi, H. Kohzadi, Appl. Organomet. Chem. 34, e5840 (2020)
H. Kohzadi, M. Soleiman-Beigi, React. Kinet. Mech. Catal. 30, 1 (2021)
Z. Rahmatizadeh-Pashaki, N. Daneshvar, F. Shirini, J. Iran. Chem. Soc. 25, 5 (2021)
V. Ashokkumar, A. Siva, B. Sankar, J. Iran. Chem. Soc. 16, 1939 (2019)
G.A. Kraus, M.E. Krolski, J. Org. Chem. 51, 3347 (1986)
L.F. Tietze, N. Rackelmann, Pure Appl. Chem. 76, 196 (2004)
M.M. Heravi, F. Janati, V. Zadsirjan, Monatsh. Chem. 151, 439 (2020)
L.F. Tietze, Chem. Rev. 96, 115 (1996)
K. van Beurden, S. de Koning, D. Molendijk, J. van Schijndel, Green Chem Lett Rev. 13, 349 (2020)
F. Liang, Y.-J. Pu, T. Kurata, J. Kido, H. Nishide, Polymer 46, 3767 (2005)
J.K. Rajput, G, Kaur. Tetrahedron Lett. 53, 646 (2012)
Y. Cao, L. Liu, T. Huang, T. Chen, New J Chem. 44, 8697 (2020)
C. Niu, A. Tuerxuntayi, G. Li, M. Kabas, C.Z. Dong, H.A. Aisa, Chin Chem Lett. 28, 1533 (2017)
Y.Q. Cao, Z. Dai, R. Zhang, B.H. Chen, Synth. Commun 35, 1045 (2005)
M. Ghanei, M.A. Khalilzadeh, M.M. Hashemi, Orient. J. Chem 32, 665 (2016)
F. Tamaddon, D. Azadi, J. Iran. Chem. Soc. 14, 2077 (2017)
F. Hajizadeh, B. Maleki, F.M. Zonoz, A. Amiri, J. Iran. Chem. Soc. 27, 1 (2020)
F. Shirini, N. Daneshvar, RSC Adv. 6, 190 (2016)
L. Muralidhar, C.R. Girija, J. Saudi Chem. Soc. 18, 541 (2014)
D. Khalili, S. Lavian, M. Moayyed, Tetrahedron Lett. 61, 151470 (2020)
S. Mohammad, F.A.G. Khoerunnisa, S. Rigolet, T.T.J. Jean Daou, T.C. Ling, E.P. Ng, Process. Chem. 8, 1 (2020)
F. Zhong, Q.Y. Xue, L. Yin, Angew. Chem. Int. Ed. Engl. 59, 1562 (2020)
S. Xing, J. Li, G. Niu, Q. Han, J. Zhang, H. Liu, J. Mol. Catal. 458, 83 (2018)
B. Sakthivel, A. Dhakshinamoorthy, J. Colloid Interface Sci. 485, 75 (2017)
X. Li, B. Lin, H. Li, Q. Yu, Y. Ge, X. Jin, X. Liu, Y. Zhou, J. Xiao, Appl. Catal. B 239, 254 (2018)
A. Das, N. Anbu, A. Dhakshinamoorthy, S. Biswas, Micropor. Mesopor. Mat. 284, 459 (2019)
T.I. Reddy, R.S. Varma, Tetrahedron Lett. 38, 1721 (1997)
S. Saravanamurugan, M. Palanichamy, M. Hartmann, V. Murugesan, Appl. Catal. A Gen. 298, 8 (2006)
R.M. Kumbhare, M. Sridhar, Catal. Commun. 9, 403 (2008)
Y. Zhang, T. Dai, F. Zhang, J. Zhang, G. Chu, C. Quan, Chin. J. Catal. 37, 2106 (2016)
G. Postole, B. Chowdhury, B. Karmakar, K. Pinki, J. Banerji, A. Auroux, J. Catal. 269, 110 (2010)
Acknowledgements
Authors thank of Ilam University, Ilam, Iran, for financial support of this research project.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Soleiman-Beigi, M., Ghalavand, S., Venovel, H.G. et al. Synthesis of lithium/cesium-Zagronas from zagrosian natural asphalt and study of their activity as novel, green, heterogeneous and homogeneous nanocatalysts in the Claisen–Schmidt and Knoevenagel condensations. J IRAN CHEM SOC 18, 3267–3279 (2021). https://doi.org/10.1007/s13738-021-02270-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-021-02270-4