Abstract
In this study, a hybrid material was successfully fabricated through the hybridization of zinc-based metal–organic framework (Zn-MOF) and covalent organic framework (COF) based on melamine and terephthaldehyde (Zn-MOF/COF) and characterized by FT-IR, SEM, XRD, and STA analysis. Then, the resultant hybrid was employed as an amazing and cost-effective catalyst in the condensation of a variety of aldehydes with malononitrile under solvent-free conditions at 25 °C in a short time (5–60 min) to offer benzylidenemalononitrile derivatives in high yields (82–100%). The catalyst could be reused without a noteworthy drop in catalytic activity at least eight times. The use of Zn-MOF/COF catalyst outcomes under mild reaction conditions in very short reaction time, exceptional catalytic activity, high recyclability and an easy work-up process for the Knoevenagel condensation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Knoevenagel reaction of the active methylene compounds and aromatic carbonyl materials using a weak base is the well-known nucleophilic addition reaction for the construction of C–C double bonds, which has been extensively employed in the construction of intermediates or products for pharmaceuticals, polymers and bioactive materials [1,2,3,4,5]. So far, the various catalysts have been recognized to improve Knoevenagel reaction such as polytriazine [6], potassium salt-loaded MgAl hydrotalcites [7], quinine [8], 2D MOF-5 [9], fluorinated MOFs [10], cyclotriveratrylene MOFs [11], Au@Cu(II)-MOF [12], imine-linked COFs [13] and so on. Thus, the development of appropriate catalysts for this process is an enhancing request.
Metal–organic frameworks (MOFs) are developing as a notable class of porous materials with an extensive range of valuable applications such as separation, storage, medicinal, sensors and catalysis [14,15,16,17,18,19]. They are inorganic–organic hybrid materials, constructed via the self-assembly of metal ions and organic components [20]. The key features of MOFs are ultrahigh porosity, high surface areas and outstanding chemical and thermal stability, which could make these materials exceptional heterogeneous catalysis [21]. Further than MOFs, scientists are more and more attracted to developing hybrid materials based on MOFs through linking MOFs with other compounds including metal nanoparticles, other MOFs, polymers and covalent organic frameworks (COFs) to attain benefits both materials [22,23,24,25].
COFs have developed as a novel class of ordered polyporous crystalline polymers constructed from organic building moieties connected via reversible covalent bonds and commonly comprise nanometer-sized uniform pores [26,27,28,29,30]. COFs owing to their outstanding features including high porosity, large surface area, high adsorption capacities, excellent chemical stability, high thermal stability, low density and highly ordered structures have drawn remarkable research interests in gas storage and separation, energy storage, proton conduction, chemical sensors, optoelectronics, semiconductors, drug delivery and catalysis [31,32,33,34,35,36,37,38]. Thomas et al. developed a novel series of porous COFs, i.e., Schiff base network (SNW) series, constructed from melamine and di- or trialdehydes [39]. For example, a melamine-based polymeric network SNW with microporosity could be synthesized by a polycondensation reaction of melamine and terephthalaldehyde in dimethyl sulfoxide [40]. To date, the amalgamation of COFs and MOFs to fabricate a promising type of hybrid materials with more functionality has been established [41,42,43,44].
In this work, Zn-MOF/COF was hydrothermally synthesized. The prepared Zn-MOF/COF can be an excellent heterogeneous catalyst for the Knoevenagel reaction under solvent-free conditions. The astonishing activity was observed, and the catalyst reused eight times without a noteworthy decrease in activity.
Experimental
Materials
All reagents and chemicals including 1,4-benzenedicarboxylate (BDC, terephthalic acid), N,N-dimethylformamide (DMF), ethanol, Zinc acetate dehydrate (Zn(CH3CO2)2·2H2O), ethylenediamine, melamine, terephthaldehyde, dimethyl sulfoxide (DMSO), malononitrile, 4-nitrobenzaldehyde, 4-chlorobenzaldehyde, 2-chlorobenzaldehyde, 4-methoxybenzaldehyde, 4-methylbenzaldehyde and 2-hydroxybenzaldehyde were purchased from Merck Company (Darmstadt, Germany) and used without further purification.
Apparatus
Fourier transform infrared (FT-IR) spectra of samples were recorded with a Jasco-680 spectrometer (Japan) in the range of 4000–400 cm−1. The X-ray diffraction (XRD) patterns of samples were recorded in the reflection mode using a Bruker, D8 Advance diffractometer. The surface morphology of the resulting materials was investigated using field emission‐scanning electron microscopy (FE-SEM; EM10C-ZEISS, 80 kV, Zeiss Co., Germany).
Preparation of Zn-MOF
Terephthalic acid (0.498 g, 3.00 mmol) was dissolved in DMF (40 mL) and stirred for 15 min till a clear solution was formed. In another vessel, Zn(CH3CO2)2·2H2O (2.19 g, 10.00 mmol) was dissolved in DMF (20 mL) with stirring for 10 min at room temperature. The solutions were mixed, and then the mixture was transferred to a Teflon‐lined autoclave (150 mL) and placed in an oven at 100 °C for 12 h. After cooling, the resultant white crystalline solid was collected, washed with ethanol several times and dried overnight at room temperature [45].
Preparation of Zn-MOF-NH2
A mixture of Zn-MOF (0.20 g), ethanol (25 mL) and ethylenediamine (5 mL) was refluxed for 12 h under argon gas. After cooling, the reaction mixture was filtered to produce a white precipitate which was washed with ethanol and dried at 80 °C under vacuum.
Preparation of Zn-MOF/COF
Zn-MOF-NH2 (0.2 g), melamine (0.5 g, 3.96 mmol), terephthaldehyde (0.5 g, 3.73 mmol), DMSO (25 mL) and distilled water (5 mL) were mixed and transferred to a 150 mL Teflon‐lined autoclave, sealed and heated in an oven at 180 °C for 12 h. After cooling, the resultant yellow precipitate was collected through filtration, washed with ethanol and dried overnight at room temperature.
General procedure for the Knoevenagel condensation using Zn-MOF/COF as a catalyst
A mixture of various aldehydes (1 mmol), malononitrile (1.5 mmol) and Zn-MOF/COF (15 mg) was stirred at 25 °C under solvent-free conditions. TLC was utilized to monitor the progress of the reaction. After completion of the reaction, warm ethanol (10 mL) was added to the reaction mixture, and Zn-MOF/COF was separated and washed with ethanol. The solvent was evaporated, and the attained solid was recrystallized from ethanol to produce the pure product. Then, the recovered catalyst was reused in eight runs under similar conditions as the first run to exhibit the recyclability and stability of the prepared catalyst.
Results and discussion
Fabrication of Zn-MOF/COF
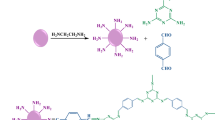
Zn-MOF hybrid material was prepared using the solvothermal technique [45]. After the preparation of Zn-MOF, it was functionalized with ethylenediamine, to form amine-functionalized Zn-MOF-NH2. The COF was then synthesized on the surface of Zn-MOF-NH2 by covalently linking melamine and terephthaldehyde through a condensation reaction to form the Zn-MOF/COF hybrid material (Fig. 1).
Characterization of Zn-MOF/COF
Figure 2 designated the FT-IR spectra of Zn-MOF, Zn-MOF–NH2 and Zn-MOF/COF, the absorption band around 3416 cm−1 in Zn-MOF is contributed to O–H stretching vibration in adsorbed H2O molecules in Zn-MOF structure. The two absorption peaks that appeared at 1662 and 1392 cm−1 correspond to the asymmetric and symmetric stretching vibrations of the O=C–O bonded to Zn. The absorption band at 532 cm−1 is assigned to Zn–O stretching vibration [46]. In the FT-IR spectrum of functionalized Zn-MOF, the absorption peaks at 3324 and 3270 cm−1 are assignable to stretching vibrations of NH2 groups and the absorption bands observed at 2958, 2904 and 2873 cm−1 correspond to stretching vibration of C-H bonds of ethylenediamine. The absorption band observed at 494 cm−1 corresponds to Zn–O stretching. From the obtained spectral data, it can be realized that the ethylenediamine has been grafted on Zn-MOF surface. The FT-IR spectrum of Zn-MOF/COF hybrid displayed a series of new characteristic stretching vibrations at 1547, 1465 and 1338 arising from the C=N, C=C and C–N bonds, respectively, were observed showing condensation and tautomerization.
Figure 3 represents the XRD patterns of Zn-MOF, COF and Zn-MOF/COF that exhibited crystal structure of Zn-MOF is a well-defined cubic structure. The XRD pattern of Zn-MOF reveals the positions of the main peaks appearing at 2θ = 7.0, 10.0, 13.8, 20.8, 19.6, 15.7, 22.8 and 25.1 in the spectra corresponded to the previously reported XRD pattern of Zn-MOF. The characterization of this compound by XRD shows distinct peaks belong to [111], [200], [220] and [311] and indicate its cubic lattice structure [46]. The XRD pattern of Zn-MOF/COF (Fig. 3) shows the two broad peaks at 2θ of 8 and 20. The observed broadening in two peaks of the Zn-MOF/COF is probably related to the overlap of XRD peaks of Zn-MOF and COF.
Figure 4 shows FE-SEM images of Zn-MOF and Zn-MOF/COF. The morphology of Zn-MOF is characterized by well-defined cubic crystals of 2.5 μm in width. The FE-SEM investigation of Zn-MOF/COF confirms that smooth pure Zn-MOF structure significantly changed due to the loading of ordered, regular structure and spherical of COF on the surface of Zn-MOF. The FE‐SEM images of the resulting Zn-MOF/COF hybrid show that the COF in the range of 80 nm is dispersed on the surface of Zn-MOF. In addition, the COF may cause the deformation of the Zn-MOF structure. However, the surface of the Zn-MOF/COF hybrid tends to be rougher after the loading of COF on Zn-MOF crystals. Therefore, the FE‐SEM images indicated that COF had a crucial effect on the structure of Zn-MOF support.
Figure 5 displays the weight loss of Zn-MOF/COF with increasing the temperature. A weight loss was identified between 100 and 120 °C, which could be attributed to the removal of moisture and solvent molecules. From 120 to 400 °C, no noticeable mass loss can be found, which demonstrated the superior thermodynamic stability of Zn-MOF/COF till 400 °C. Further increasing, the temperature to 600 °C leads to a weight loss of around 450 °C due to the degradation of COF structure and a weight loss of around 550 °C due to the degradation of the MOF structure. Then no mass loss can be found when the temperature was further increased to 900 °C.
Catalytic activity test
The catalytic application of Zn-MOF/COF was examined in the Knoevenagel reaction under diverse conditions (Table 1). For the optimization of the reaction conditions, the reaction between malononitrile with benzaldehyde in the presence of Zn-MOF/COF as a catalyst was designated as a test reaction. The reaction was performed at 5, 10 and 15 mg of Zn-MOF/COF loading. With increasing catalyst loading from 5 to 15 mg, the yield was enhanced, and the best result in an appropriate time was obtained using 15 mg of catalyst (Table 1, entry 4). The effect of different solvents such as H2O, EtOH, CH2Cl2 and CH3CN and solvent-free conditions was also investigated, and the results revealed that H2O and EtOH provide moderate yields (Table 1, entries 5 and 6). Based on the obtained results, the yields in H2O and EtOH solvents are lower than solvent-free conditions. It might be attributed to the solvation of the active functional groups by these solvents, also it might be owing to hydrogen bonding between active protonic sites of Zn-MOF/COF and these solvents which decline the catalytic efficiency. Hence, the use of 15 mg of the catalyst under solvent-free conditions at 25 °C was designated as optimum conditions. After the optimization of the conditions, generality and the scope of this system were examined using several aromatic aldehydes bearing both electron-donating and electron-withdrawing groups. Based on the obtained results all substrates gave corresponding products in relatively high yield in very short reaction time as revealed in Table 2. The benzaldehyde derivatives containing electron-withdrawing groups such as –NO2, and –Cl were transformed to the corresponding products with a high yield (Table 2, entries 2–4). Whereas, the benzaldehyde derivatives possessing electron-donating moieties including –OCH3, –CH3 and -OH provided the lower yields (Table 2, entries 5–7). This result proves that the benzaldehyde derivatives having electron-withdrawing groups are slightly reactive compared to those with electron-donating moieties.

To verify whether the observed catalysis was due to the heterogeneous catalyst Zn-MOF/COF or to a leached zinc species in solution, the reaction of benzaldehyde with malononitrile was carried out until an approximately 50% of the reaction was completed. Then the Zn-MOF/COF catalyst was separated using simple filtration, and the solution was transferred to another reaction tube and stirred again at room temperature for 2 h. In this case, no significant increase in conversion was observed, indicating that leached zinc species from the catalyst (if any) are not responsible for the observed activity. It was confirmed by ICP analysis that no zinc species could be detected in the solution (below the detection limit). These results rule out any contribution to the observed catalysis from a homogeneous gold species, demonstrating that the observed catalysis was intrinsically heterogeneous.
Reusability of Zn-MOF/COF
To examine the reusability of Zn-MOF/COF, after the completion of the reaction, the catalyst was collected and separated and then reused under similar conditions as the first run. This experiment was repeated eight times, and it was found that Zn-MOF/COF is stable under the applied conditions and can be reused at least eight times without a considerable decrease in its catalytic activity (Fig. 6).
Comparison of the proposed catalyst with previously reported catalysts for the Knoevenagel condensation
The comparison between the performance of the Knoevenagel condensation based on the Zn-MOF/COF catalyst and some previously reported catalysts involving the Knoevenagel condensation is listed in Table 3. It was found that Zn-MOF/COF exhibited advantages in terms of cost-effectiveness and simplicity, low temperature and very short reaction time. Additionally, it consumed very short reaction time and mild conditions in the Knoevenagel condensation compared with the literature.
Conclusions
In this work, Zn-MOF/COF was constructed and characterized by FT-IR, XRD, SEM and STA techniques. The FT-IR and STA analyses successfully confirmed well-incorporation and immobilization of COF moieties onto Zn-MOF surface. The SEM images exhibited that the COF has grown well on the surface of MOF. This study examined the Knoevenagel condensation and synthesis of benzylidenemalononitrile in the presence of Zn-MOF/COF as a powerful heterogeneous catalyst. The Knoevenagel products were achieved in high yields under moderate conditions and short reaction time. The other features of the present study include straightforward preparation and cost-effectiveness of catalyst, accomplishing reaction at room temperature, solvent-free media and low loading of catalyst.
References
D. Rani, P. Singla, J. Agarwal, Chitosan in water as an eco-friendly and efficient catalytic system for Knoevenagel condensation reaction. Carbohydr. Polym. 202, 355–364 (2018)
S.F. Amarante, M.A. Freire, D.T.S.L. Mendes, L.S. Freitas, A.L.D. Ramos, Evaluation of basic sites of ZIFs metal organic frameworks in the Knoevenagel condensation reaction. Appl. Catal. A Gen. 548, 47–51 (2017)
Q. Li, S. Jiang, S. Ji, M. Ammar, Q. Zhang, J. Yan, Synthesis of magnetically recyclable ZIF-8@SiO2@Fe3O4 catalysts and their catalytic performance for Knoevenagel reaction. J. Solid State Chem. 223, 65–72 (2015)
D. Markad, S. Khullar, S.K. Mandal, Engineering a nanoscale primary amide-functionalized 2D coordination polymer as an efficient and recyclable heterogeneous catalyst for the Knoevenagel condensation reaction. ACS Appl. Nano Mater. 1, 5226–5236 (2018)
J.P.H. Li, A.A. Adesina, E.M. Kennedy, M. Stockenhuber, A mechanistic study of the Knoevenagel condensation reaction: new insights into the influence of acid and base properties of mixed metal oxide catalysts on the catalytic activity. Phys. Chem. Chem. Phys. 19, 26630–26644 (2017)
M. Chaudhary, P. Mohanty, Nitrogen enriched polytriazine as metal-free heterogeneous catalyst for the Knoevenagel reaction in mild condition. New J. Chem. 42, 12924–12928 (2018)
R. Devi, P. Begum, P. Bharali, R.C. Deka, Comparative study of potassium salt-loaded mgal hydrotalcites for the Knoevenagel condensation reaction. ACS Omega 3, 7086–7095 (2018)
K. Jain, S. Chaudhuri, K. Pal, K. Das, The Knoevenagel condensation using quinine as an organocatalyst under solvent-free conditions. New J. Chem. 43, 1299–1304 (2019)
C. Guo, Y. Zhang, L. Zhang, Y. Zhang, J. Wang, 2-Methylimidazole-assisted synthesis of a two-dimensional MOF-5 catalyst with enhanced catalytic activity for the Knoevenagel condensation reaction. CrystEngComm 20, 5327–5331 (2018)
M. Joharian, A. Morsali, A. Azhdari Tehrani, L. Carlucci, D.M. Proserpio, Water-stable fluorinated metal-organic frameworks (F-MOFs) with hydrophobic properties as efficient and highly active heterogeneous catalysts in aqueous solution. Green Chem. 20, 5336–5345 (2018)
D.-W. Kang, X. Han, X.-J. Ma, Y.-Y. Liu, J.-F. Ma, Two cyclotriveratrylene metal-organic frameworks as effective catalysts for Knoevenagel condensation and CO2 cycloaddition with epoxides. Dalton Trans. 47, 16197–16204 (2018)
J.-S. Wang, F.-Z. Jin, H.-C. Ma, X.-B. Li, M.-Y. Liu, J.-L. Kan, G.-J. Chen, Y.-B. Dong, Au@Cu(II)-MOF: highly efficient bifunctional heterogeneous catalyst for successive oxidation-condensation reactions. Inorg. Chem. 55, 6685–6691 (2016)
X. Wu, B. Wang, Z. Yang, L. Chen, Novel covalent organic frameworks: preparation, characterization and application. J. Mater. Chem. A 7, 5650–5655 (2019)
X. Zhao, Y. Wang, D.-S. Li, X. Bu, P. Feng, Metal-organic frameworks for separation. Adv. Mater. 30, 1705189–1705223 (2018)
M.P. Suh, H.J. Park, T.K. Prasad, D.-W. Lim, Hydrogen storage in metal-organic frameworks. Chem. Rev. 112, 782–835 (2012)
P. Horcajada, R. Gref, T. Baati, P.K. Allan, G. Maurin, P. Couvreur, G. Ferey, R.E. Morris, C. Serre, Metal-organic frameworks in biomedicine. Chem. Rev. 112, 782–835 (2012)
X. Fang, B. Zong, S. Mao, Metal-organic framework-based sensors for environmental contaminant sensing. Nano-Micro Lett. 10, 64–82 (2018)
D. Yang, B.C. Gates, Catalysis by metal organic frameworks: perspective and suggestions for future research. ACS Catal. 9, 1779–1798 (2019)
M. Ranocchiari, J.A. van Bokhoven, Catalysis by metal-organic frameworks: fundamentals and opportunities. Phys. Chem. Chem. Phys. 13, 6388–6396 (2011)
N. Stock, S. Biswas, Synthesis of metal-organic frameworks (MOFs): routes to various MOF topologies, morphologies, and composites. Chem. Rev. 112, 933–969 (2012)
H.-C. Zhou, J.R. Long, O.M. Yaghi, Introduction to metal-organic frameworks. Chem. Rev. 112, 673–674 (2012)
W. Xiang, Y. Zhang, H. Lin, C.-J. Liu, Nanoparticle/metal-organic framework composites for catalytic applications: current status and perspective. Molecules 22, 2103–2126 (2017)
K. Koh, A.G. Wong-Foy, A.J. Matzger, MOF@MOF: microporous core-shell architectures. Chem. Commun. 41, 6162–6164 (2009)
C.L. Calvez, M. Zouboulaki, C. Petit, L. Peeva, N. Shirshova, One step synthesis of MOF-polymer composites. RSC Adv. 6, 17314–17317 (2016)
Y. Peng, M. Zhao, B. Chen, Z. Zhang, Y. Huang, F. Dai, Z. Lai, X. Cui, C. Tan, H. Zhang, Hybridization of MOFs and COFs: a new strategy for construction of MOF@COF core-shell hybrid materials. Adv. Mater. 30, 1705454–1705459 (2017)
P.J. Waller, F. Gandara, O.M. Yaghi, Chemistry of covalent organic frameworks. Acc. Chem. Res. 48, 3053–3063 (2015)
S.J. Lyle, P.J. Waller, O.M. Yaghi, Covalent organic frameworks: organic chemistry extended into two and three dimensions. Trends Chem. 1, 172–184 (2019)
M.S. Lohse, T. Bein, Covalent organic frameworks: structures, synthesis, and applications. Adv. Funct. Mater. 28, 1705553–1705623 (2018)
D.D. Medina, V. Werner, F. Auras, R. Tautz, M. Dogru, J. Schuster, S. Linke, M. Doblinger, J. Feldmann, P. Knochel, T. Bein, Oriented thin films of a benzodithiophene covalent organic framework. ACS Nano 8, 4042–4052 (2014)
S.Y. Ding, W. Wang, Covalent organic frameworks (COFs): from design to applications. Chem. Soc. Rev. 42, 548–568 (2013)
A. Sharma, A. Malani, N.V. Medhekar, R. Babarao, CO2 adsorption and separation in covalent organic frameworks with interlayer slipping. CrystEngComm 19, 6950–6963 (2017)
E. Klontzas, E. Tylianakis, G.E. Froudakis, Designing 3D COFs with enhanced hydrogen storage capacity. Nano Lett. 10, 452–454 (2010)
H. Ma, B. Liu, B. Li, L. Zhang, Y.-G. Li, H.-Q. Tan, H.-Y. Zang, G. Zhu, Cationic covalent organic frameworks: a simple platform of anionic exchange for porosity tuning and proton conduction. J. Am. Chem. Soc. 138, 5897–5903 (2016)
X. Yan, Y. Song, J. Liu, N. Zhou, C. Zhang, L. He, Z. Zhang, Z. Liu, Two-dimensional porphyrin-based covalent organic framework: a novel platform for sensitive epidermal growth factor receptor and living cancer cell detection. Biosens. Bioelectron. 126, 734–742 (2019)
A.K. Mandal, J. Mahmood, J.-B. Baek, Two-dimensional covalent organic frameworks for optoelectronics and energy storage. Chem. NanoMat. 3, 373–391 (2017)
J.I. Feldblyum, C.H. McCreery, S.C. Andrews, T. Kurosawa, E.J.G. Santos, V. Duong, L. Fang, A.L. Ayzner, Z. Bao, Few-layer, large-area, 2D covalent organic framework semiconductor thin films. Chem. Commun. 51, 13894–13897 (2015)
Q. Fang, J. Wang, S. Gu, R.B. Kaspar, Z. Zhuang, J. Zheng, H. Guo, S. Qiu, Y. Yan, 3D porous crystalline polyimide covalent organic frameworks for drug delivery. J. Am. Chem. Soc. 137, 8352–8355 (2015)
S.M.J. Rogge, A. Bavykina, J. Hajek, H. Garcia, A.I. Olivos-Suarez, A. Sepulveda-Escribano, A. Vimont, G. Clet, P. Bazin, F. Kapteijn, M. Daturi, E.V. Ramos-Fernandez, F.X. Llabres, I. Xamena, V. van Speybroeck, J. Gascon, Metal-organic and covalent organic frameworks as single-site catalysts. Chem. Soc. Rev. 46, 3134–3184 (2017)
M.G. Schwab, B. Fassbender, H.W. Spiess, A. Thomas, X. Feng, K. Mullen, Catalyst-free preparation of melamine-based microporous polymer networks through Schiff base chemistry. J. Am. Chem. Soc. 131, 7216–7217 (2009)
W. Zhang, L.-G. Qiu, Y.-P. Yuan, A.-J. Xie, Y.-H. Shen, J.-F. Zhu, Microwave-assisted synthesis of highly fluorescent nanoparticles of a melamine-based porous covalent organic framework for trace-level detection of nitroaromatic explosives. J. Hazard. Mater. 221–222, 147–154 (2012)
F.M. Zhang, J.L. Sheng, Z.D. Yang, X.J. Sun, H.L. Tang, M. Lu, H. Dong, F.C. Shen, J. Liu, Y.Q. Lan, Rational design of MOF/COF hybrid materials for photocatalytic H2 evolution in the presence of sacrificial electron donors. Angew. Chem. Int. Ed. 57, 12106–12110 (2018)
Y. Cheng, Y. Ying, L. Zhai, G. Liu, J. Dong, Y. Wang, M.P. Christopher, S. Long, Y. Wang, D. Zhao, Mixed matrix membranes containing MOF@COF hybrid fillers for efficient CO2/CH4 separation. J. Membr. Sci. 573, 97–106 (2019)
J. Fu, S. Das, G. Xing, T. Ben, V. Valtchev, S. Qiu, Fabrication of COF-MOF composite membranes and their highly selective separation of H2/CO2. J. Am. Chem. Soc. 138, 7673–7680 (2016)
Y. Yan, T. He, B. Zhao, K. Qi, H. Liu, B.Y. Xia, Metal/covalent-organic frameworks-based electrocatalysts for water splitting. J. Mater. Chem. A 6, 15905–15926 (2018)
H.F. Clausen, R.D. Poulsen, A.D. Bond, M.-A.S. Chevallier, B.B. Iversen, Solvothermal synthesis of new metal organic framework structures in the zinc-terephthalic acid-dimethyl formamide system. J. Solid State Chem. 178, 3342–3351 (2005)
H.-X. Jin, H.P. Xu, N. Wang, L.-Y. Yang, Y.-G. Wang, D. Yu, X.-K. Ouyang, Fabrication of carboxymethylcellulose/metal-organic framework beads for removal of Pb(II) from aqueous solution. Materials 12, 942–957 (2019)
A. Schejn, T. Mazet, V. Falk, L. Balan, L. Aranda, G. Medjahdi, R. Schneider, Fe3O4@ZIF-8: magnetically recoverable catalysts by loading Fe3O4 nanoparticles inside a zinc imidazolate framework. Dalton Trans. 44, 10136–10140 (2015)
U.P.N. Tran, K.K.A. Le, N.T.S. Phan, Expanding applications of metal-organic frameworks: zeolite imidazolate framework ZIF-8 as an efficient heterogeneous catalyst for the Knoevenagel reaction. ACS Catal. 1, 120–127 (2011)
Z.-W. Zhai, S.-H. Yang, Y.-R. Lv, C.-X. Du, L.-K. Li, S.-Q. Zang, Amino functionalized Zn/Cd-Metal-organic frameworks for selective CO2 adsorption and Knoevenagel condensation reaction. Dalton Trans. 48, 4007–4014 (2019)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rafiee, Z. Fabrication of efficient Zn-MOF/COF catalyst for the Knoevenagel condensation reaction. J IRAN CHEM SOC 18, 2657–2664 (2021). https://doi.org/10.1007/s13738-021-02221-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-021-02221-z