Abstract
Tetrabutylammonium prolinate ionic liquid (TBAPIL) was prepared, and mesoporous silica nanoparticles (NPs) were synthesized. Both of these were linked through propyltriethoxysilane to prepare a reusable catalyst TBAPIL@Si(CH2)3@silica NPs (TBAPILS). The formation of TBAPIL was checked through Fourier-transform infrared spectroscopy (FT-IR) and nuclear magnetic resonance (NMR) analysis. X-ray diffraction analysis confirmed the structure of silica NPs and linking of TBPAIL on it. Transmission electron microscopy proved the flourishing development of silica NPs. Scanning electron microscopy graphs exposed the altering in morphology of silica NPs and TBAPILS. FT-IR analysis also confirmed the formation of TBAPILS catalyst. Moreover, the effectiveness of the TBAPILS was also checked for the synthesis of various derivatives of tetrahydrobenzoxanthenes-11-ones. The formation and structure of obtained compounds were confirmed by FT-IR, elemental analysis, 1HNMR and 13C NMR spectral analysis. The catalyst TBAPILS was found to be used successfully up to five cycles without significant loss of activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nano-catalysis is a very exciting field which is originated from nanoscience. Nano-catalysts are gaining importance increasingly [1]. The main aim of the nano-catalysts is to control the chemical reactions through changing their size, morphology and composition of reaction center [2]. The overall approach opens various areas for designing of nano-catalysts having a distinct, selective and specific chemical activity. The nano-shape, size and a large surface area by volume ratio provide unique characteristics to nano-catalysts due to the structural and electronic changes which differentiate them from the original materials [3]. Nanoparticles (NPs) can replace conventional materials and serve as heterogeneous catalytic systems or as a material of support for different catalytic systems [4, 5]. Catalyst is a substance which speeds up the chemical reactions, enhances the product yield and shrinks the energy of activation, but a severe trouble with reactions in catalytic conditions is the parting of catalytic system from the ongoing reaction mixture so that it may be used again [6]. To defeat this trouble, a heterogeneous catalytic system may be used in synthetic organic chemistry. Currently, heterogeneous catalytic systems may be prepared by a variety of substances in a straightforward way [7]. Heterogeneous catalytic systems may capably be formed by modifying the support with NPs and combining these with catalytic system [8, 9]. Recently a range of methods have been reported for the synthesis of silica NPs with controlled size and chemical stability with a variety of applications in drug delivery [10]. Moreover, these silica-based catalysts have also been used to catalyze a range of chemical reactions [11, 12].
Ionic liquids are salts having melting point lower than the boiling point of water. Ionic liquids have several alternative names such as designer solvents, molten salts and ionic fluids [13]. Ionic liquids are generally colorless liquids with moderately low viscosity. Ionic liquids are made up of two parts positive and negative ions, therefore there is wide scope to prepare them in large numbers and to fine-tune their properties according to the requirement. These advantages are unavailable for single constituent molecular solvents. In addition to all these properties, ionic liquids can be readily formed by using commercially accessible reagents [14]. Ionic liquids also have an important role in catalysis and synthesis [15]. Various methods for the preparation along with their applications in catalysis have been reported for tetrabutylammonium prolinate and other ionic liquids [16,17,18]. But most of these ionic liquids are associated with disadvantages like harsh conditions and non-reusability.
Tetrahydrobenzoxanthen-11-one and its derivatives are a chief class of bioactive compounds having activities like anti-inflammatory [19], free radical scavenging [20], mGlu1 receptor enhancing [21], antiplasmodial [22], antitumor [23], inhibitors of recombinant human calpain I [24], molecular probes in chemical biology [25], antibacterial [26, 27], antiproliferative [28], antineoplastic [29], drug development [30, 31], antileukemic [32] and insecticidal [33]. Due to special spectroscopic properties, these derivatives have also been used as pH-indicators [34], fluorescent materials in visualization of molecules [35] and as photodynamic therapy agents [36]. Various methods have been developed for the synthesis of tetrahydrobenzoxanthen-11-one using PMA/SiO2 [37], ZnO [38], InCl3/ionic liquid [39], p-dodecylbenzenesulfonic acid [40], iron oxide NPs [41], trichloroacetic acid [42], silica sulfuric acid [43], strontium triflate [44], PEG [45], bismuth nitrate [46], phenylboronic acid [47], molecular iodine [48], p-TSA [49] and other heterogeneous catalysts [50, 51]. Most of them have the limitations such as low yield, formation of mixture of products, long reaction time and harsh reaction conditions, so it is imperative to develop new and efficient methods with reduced reaction time and mild reaction conditions for the synthesis of derivatives of biologically active tetrahydrobenzoxanthen-11-one compounds. To the best of our knowledge, we are first to report the synthesis of tetrahydrobenzoxanthen-11-one in solvent-less condition by using TBAPILS nano-catalyst.
Experimental
Materials and methods
All reagents and chemicals were of L.R. grade and procured from Hi-media and Molychem and directly used with no additional purification. The glasswares used during the study were made of Borosil. IR spectra were taken on BRUCKER FT-IR spectrophotometer of BRUCKER. BRUCKER AVANCE II 400 MHz instrument was used for 1HNMR analysis with CDCl3 solvent. Decibel digital melting point equipment was used for recording of melting points. The progress of reaction and compounds purity was checked by TLC on silica gel plates with hexane and ethyl acetate solvents and visualized through vapors of iodine and UV light. JSM-1011 transmission electron microscope was used for TEM analysis and JEOL (JSM-6610 LV) with a prime ray power of 5 kV apparatus was used to record SEM analysis. XRD analysis of powdered samples was recorded at room temperature (RT) over Rigaku-Geigerflex X-Ray diffractometer by using Cu-Ka radiation (k = 0.154 nm) in the series of 108–708 at 30 kV and 15 mA with step size 0.05 and step time of 19.2 s.
Synthesis of mesoporous silica nanoparticles (NPs)
In a round-bottom flask, 100 mL methanol was placed. To it, 60 mL solution of ammonia (32%) and 1.98 mL water were mixed. The mixture was stirred up to 5–6 min and after that 10.40 g tetraethyl orthosilicate (TEOS) was added drop-wise. The solution was again stirred for 72 h at an ambient temperature [52, 53]. Then, it was centrifuged for 30 min, after which solvent was evaporated by rotavapor, washed with ethanol and particles obtained were put in oven at 250 °C for 1 h.
Synthesis of tetrabutylammonium prolinate ionic liquid (TBAPIL)
Tetrabutylammonium hydroxide [(TBA)(OH)] aqueous solution was prepared by modified literature method [54] from Tetrabutylammonium bromide (20 mmol) using anion exchange resin AMBERLITE IRA400 OH. The obtained [(TBA)(OH)] aqueous solution (10 mmol) was then added drop-wise with a slightly excess aqueous proline solution (10 mmol). This combination was stirred at RT for 12 h, then water was removed in vacuum and obtained residue was dissolved in CH3CN (40 mL) and the solution was filtered to remove unreacted proline. Filtrate was dried over anhydrous Na2SO4 and solvent was removed in vacuum to give the desired TBAPIL as low viscous colorless oil [17].
Preparation of TBAP@Si(CH2)3@nano-silica (TBAPILS) catalyst
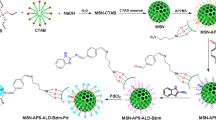
The supporting of the TBAPIL on propyl silane-grafted silica nanoparticles is shown in Scheme 1. Silica NPs (1 g) and 3-chloropropyltriethoxysilane (3 mmol) were added to 20 mL of dry toluene and then refluxed for 12 h. The resultant grafted SiO2@Si(CH2)3Cl was filtered, washed thrice with dry toluene and with dry diethyl ether and dried at 75 °C for 6 h in vacuum oven. Then, TBAPIL (10 mmol) was added to the round-bottom flask containing SiO2@Si(CH2)3Cl (1 mmol), K2CO3 (5 mmol) in 50 mL of dry toluene. The mixture was refluxed for 24 h. The resultant solid was then filtered, washed and dried to give TBAPILS. After the usual workup and washings, the material was dried at 75 °C for 5 h in a vacuum oven.
Synthesis of tetrahydrobenzoxanthen-11-one derivatives using TBAPILS catalyst
β-naphthol (10 mmol)/α-naphthol(10 mmol), cyclic 1,3 dicarbonyl compound (10 mmol) and benzaldehyde derivative (10 mmol) were mixed with TBAPILS (5 mol%). The mixture of was stirred vigorously at 80 °C (Scheme 2) and progress of reaction was observed using TLC (ethyl acetate: n-hexane:: 3:7 v/v). After completion of reaction, cold water was added to the reaction mixture to ease the precipitation of product. The solid obtained was dissolved in ethyl alcohol and filtered. The catalyst obtained as solid was washed three times with alcohol to remove any traces of product and then dried in vacuum oven at 70 °C to be used for next cycle. Solvent was distilled off from filtrate to get the solid product. The crude product was then recrystallized from dichloromethane to give pure product.
Results and discussion
TBAPILS catalyst
TEM analysis of Mesoporous Silica NPs
The size of silica NPs was characterized from TEM. The results are shown in Fig. 3a, b. The size of silica NPs was found to be 40–50 nm.
1H NMR of TBAPIL
1H NMR (CDCl3, 400 MHz): δppm 3.28 (t, 2H), 3.26 (t, 1H), 2.33 (t, 2H), 1.93(s, 1H), 1.62 (m, 2H), 1.58 (m, 2H), 1.56 (m, 2H), 1.37 (m, 2H), 0.91(t, 3H) (Fig. 1).
FT-IR analysis
The functional groups of the prepared TBAPILS catalyst and its precursors were characterized by FT-IR technique, as shown in Fig. 2. In mesoporous nano-silica, two characteristic bands by the side of 963 cm−1 and 783 cm−1 are for Si–O–Si symmetric and asymmetric stretching, and at 3562 cm−1 due to surface Si–OH stretching. Two bands at 459 and 1633 cm−1 are due to Si–O–Si and Si–OH bending, respectively (Fig. 2a). The characteristic bands of chloropropylsilane-coated silica appearing at 1057, 1237 and 2965 cm−1 are due to the Si–O–Si stretching, C–H bending and stretching, respectively (Fig. 2b). The characteristic bands at 1359, 1625, 3524, 2938 cm−1 are due to –C–OH, –C = O, –NH and –C–H stretching. The bands by the side of 1455 and 735 cm−1are due to the –C–H and –C = O bending (Fig. 2c). The presence of groups in TBAPILS catalyst such as Si–O–Si, –C = O, –C–H is confirmed by bands at 461, 791, 1121, 1241, 1681, 2986 and 3600 cm−1 (Figs. 2d, 3).
SEM analysis silica NPs and TBAPILS catalyst
The surface morphology of silica NPs and TBAPILS catalyst was characterized by SEM. Silica nanoparticles demonstrate a porous morphology (Fig. 4a). TBAPILS grafted on silica nanoparticles fill the pores in the surface thereby making a different topological arrangement which gives rise to changed morphology (Fig. 4b). Perusal of Fig. 4a, b clearly indicates the difference in surface morphology of silica NPs and TBAPILS (Fig. 4a, b).
XRD analysis
The XRD pattern of silica NPs clearly conforms to the amorphous nature of it with the broad peak (2θ) at 23.70. The XRD results are in well agreement with the reported facts JCPDS card number (00-049-1711) [55]. The change in intensity in TBAPILS (Fig. 5b) in comparison with silica NPs (Fig. 5a) clearly indicates the linking of TBAPIL on surface of silica NPs. These XRD results are supported by the SEM results whereby changed morphology is observed after grafting on the silica NPs.
Mechanism
A plausible mechanism has been proposed for the preparation of tetrahydrobenzoxanthen-11-one derivatives using TBAPILS catalyst (Scheme 3). The cation is attached to the oxygen of the carbonyl group of aldehyde which increases the electrophilicity of carbonyl group and anion activates the nucleophile b-naphthol, consequent upon this nucleophilic attack of β-naphthol at carbonyl carbon is facilitated and ortho-quinone methide intermediates (1a) is formed. After removal of a water molecule, 1b is formed. With the removal of a water molecule, the catalyst TBAPILS which is attached to oxygen of carbonyl group of aldehyde is detached and now it interacts with active methylene group containing compound (A) via oxygen and makes it more reactive while anion interacts with enolic –OH of bicarbonyl. Due to combined effect of these activations, Michael addition is facilitated. Ring closure step, next to Michael addition step, is also facilitated by the catalyst. Here also, electrophilicity of carbonyl carbon of bicarbonyl is increased due to its interaction with cation and interaction of anion with –OH group of naphthol moiety assists in removal of hydrogen thus facilitating ring closure. After ring closure, dehydration takes place and final product (1e) is obtained (Scheme 3). The mechanism proposed is the modification to the mechanism reported in the literature [47, 51] in which interaction of anion and combined effect of cation and anion are not discussed. In our view, it is simultaneous interaction of cation as well as anion of the catalyst with substrates in each step that makes the catalyst more active. Comparison of the activity of catalyst with those of tetrabutylammonium bromide and proline supports this view (Table 3).
Optimization of reaction conditions
A model reaction of β-naphthol, benzaldehyde and cyclic 1,3 dicarbonyl was carried out using TBAPILS under solvent less condition to optimize temperature. No yield was obtained at RT even up to 10 h stirring. After this, the reaction was tried at different temperatures from 40 to 100 °C with a difference of 10 °C. As a result of rising temperature, the yield increased and the reaction time decreased. After 80 °C, no rise in yield was observed. Time also remained constant. So, 80 °C was taken as the optimum temperature for the reaction.
Scope of substrate
Derivatives of substituted tetrahydrobenzoxanthen-11-one were produced by the reaction between substituted α/β-naphthol, benzaldehyde derivative and cyclic 1, 3 dicarbonyl. The substituted group, % yield, M.P. and reaction time are displayed in Table 1. To inspect the scope of substrate, a range of benzaldehyde derivative was used. All the products were obtained in good to excellent yield. The reaction was completed within 15 to 180 min. The reaction was viable for electron-withdrawing as well as for electron-donating groups substituted on the benzene ring of benzaldehyde. The reaction could be carried out well for both α-naphthol and β-naphthol which clearly establishes broad substrate scope of the reaction catalyzed by TBAPIS (Table 1). Turn over number (TON) and turn over frequency (TOF) values of catalyst for all the products have been calculated and are presented in Table 1. TON values were obtained in the range 144–192. The values clearly indicate that catalyst is quite stable. The TOF values calculated per minute were obtained in the range 1–11 indicating variable efficiency of the catalyst for different reactants.
Loading of TBAPILS catalyst
To optimize the amount of catalyst, model reaction of β-naphthol (10 mmol), benzaldehyde derivative (10 mmol) and cyclic 1,3 dicarbonyl (10 mmol) was tried in the presence of various amounts of catalyst such as 1, 2, 3, 4, 5, 6 and 7 mol%, there is an increase in yield and decrease in time with increase in the amount of catalyst up to 5 mol% (Fig. 6). No further significant increase in yield was observed in by further increasing the catalyst amount (Table 2). The results showed that 5 mol% of the catalyst and 80 °C temperature was the best combination to afford product A1 in excellent yield.
Structure and catalytic ability relationship of TBAPILS catalyst
Perusal of structure of the catalyst TBAPILS reveals that it is a bifunctional ionic liquid organocatalyst. It contains cation (tetrabutylammonium) and an effective anion which also contains base (–N). These features make it excellent and task specific for this reaction. These features are lacking in tetrabutylammonium bromide and proline. Though tetrabutylammonium bromide contains same cation, it does not have an effective anion or a base. Proline contains both acidic as well as basic group but it lacks effective cation. This is supported by experimental observations (Table 3). The catalyst prepared by us has activity much higher than tetrabutylammonium bromide and proline. The interaction of the catalyst with substrates at different stages of reaction is explained to understand its structure activity relationship. Cation of the catalyst is effective in catalyzing the reaction of benzaldehyde and b-naphthol by interacting with the carbonyl group of benzaldehyde via oxygen while its base/anion interacts with the nucleophile (b-naphthol) via hydrogen of –OH group of naphthol. Then, in the reaction between the intermediate formed thus and dicarbonyl compound, cation interacts in the same way with the carbonyl group of the intermediate and anion/ base interacts with the dicarbonyl facilitating Michael addition. Finally, in the cyclization step, carbonyl group of bicarbonyl moiety and OH group of b-naphthol moiety interact with cation and base/ anion, respectively. Thus, in every step, catalyst functions as acid as well as a base. Jimenez et al. [56] have explained these interactions of bifunctional organocatalyst containing thiourea moiety working as Lewis acid and amine working as base on the basis of electron density topological analysis using quantum chemical technology.
Hot filtration test
To confirm the heterogeneity of TBAPILS catalyst and in order to prove that tetrabutylammonium ionic liquid was not leaching out from the TBAPILS catalyst, the hot filtration test was performed with the model reaction of β-naphthol, benzaldehyde derivative and cyclic 1,3 dicarbonyl under optimized reaction conditions. After 30 min which is half of the reaction time, the catalyst was filtered off and 45% yield was obtained and filtrate was allowed to react further but no significant amount of product was obtained. These results show that immobilized ionic liquid is not leached out from the coated silica NPs and the TBAPILS catalyst is heterogeneous in nature.
Recycling studies of TBAPILS catalyst
After completion of reaction, the catalyst was filtered off the reaction mixture, washed, dried and used for the next cycle in the synthesis of A1. The process was repeated constantly up to seven cycles and observed not a major reduction in yield until fifth cycle. In sixth and seven cycles, the yield decreased significantly (Fig. 7). By these results, we can say that catalyst successfully may be used up to five cycles without significant loss of activity.
Comparison of the efficiency of TBAPILS catalyst with other reported catalysts
To prove the superiority of TBAPILS catalyst (Table 4), we compared our results with some of those catalysts reported in the literature for compound A1. The results clearly indicate the superiority of the catalyst developed by us. Moreover, solvent less condition makes the reaction economic.
1HNMR, 13CNMR and elemental analysis of tetrahydrobenzoxanthen-11-one derivatives
A1-9,9-dimethyl-12-phenyl-9,10-dihydro-8H-benzo[a]xanthen-11(12H)-one
Anal. Calcd. For C25H22O2: C, 84.74; H,6.21; N, Nil; O,9.03 Found: C, 82.58; H, 5.96; N, Nil; O,10.28% 1H NMR (CDCl3, 400 MHz): δppm 8.10 (d,1H,–Ar), 7.74–7.80 (m,2H, –Ar), 7.43–7.31 (m, 5H, –Ar),7.21–7.14(m, 2H, –Ar), 7.06 (m, 1H, –Ar), 5.71 (s,1H, –CH), 2.57 (s, 2H, –CH2), 2.26(m, 2H, –CH2), 1.11 (s, 3H, –CH3), 0.96 (m, 3H, –CH3); 13CNMR (CDCl3,100 MHz,δppm): 197.10, 164.06, 147.81, 144.82, 131.57, 128.93, 128.52, 127.10, 126.33, 124.99, 123.76, 117.78, 117.13, 50.94, 41.48, 34.78, 32.36, 29.40, 27.24.
A2-9,9-dimethyl-12-(3-nitrophenyl)-9,10-dihydro-8H-benzo[a]xanthen-11(12H)-one
1H NMR (CDCl3, 400 MHz): δppm 8.10 (m, 1H, –Ar), 7.92–7.94 (dd, 1H, –Ar), 7.84 (d, 1H, –Ar),7.82–7.79(m, 3H, –Ar), 7.45–7.35 (m, 4H, –Ar), 5.81 (s, 1H, –CH), 2.61 (s, 2H, –CH2), 2.26 (m, 2H, –CH2), 1.13 (s, 3H, –CH3), 0.98 (m, 3H, –CH3).
A3-9,9-dimethyl-12-(4-chlorophenyl)-9,10-dihydro-8H-benzo[a]xanthen-11(12H)-one
1H NMR (CDCl3, 400 MHz): δppm 7.91 (d,1H, –Ar), 7.80–7.76 (m,2H, –Ar), 7.44–7.38 (m, 2H, –Ar),7.33–7.26 (m, 2H, –Ar), 7.17–7.11 (m, 2H, –Ar), 6.99 (s,1H, –Ar), 5.68 (s,1H, –CH), 2.52 (s, 2H, –CH2), 2.29 (m, 2H, –CH2), 1.10 (s, 3H, –CH3), 0.96 (s, 3H, –CH3).
A4-9,9-dimethyl-12-(N,N-dimethylphenyl)-9,10-dihydro-8H-benzo[a]xanthen-11(12H)-one
1H NMR (CDCl3, 400 MHz): δppm 7.92 (d,1H, –Ar), 7.80–7.70 (m,2H, –Ar), 7.43–7.30 (m, 5H, –Ar),7.17–6.99 (m, 2H, –Ar), 5.67 (s,1H, –CH), 2.97 (s, 6H, –CH3), 2.57 (s, 2H, –CH2), 2.27 (s, 2H, –CH2), 1.14 (s, 3H, –CH3), 0.97 (s, 3H, –CH3).
A5-9,9-dimethyl-12-(3-hydroxyphenyl)-9,10-dihydro-8H-benzo[a]xanthen-11(12H)-one
1H NMR (CDCl3, 400 MHz): δppm 8.19 (d,1H, –Ar), 7.96–7.90 (m, 2H, –Ar), 7.72–7.78 (m, 1H, –Ar),7.47–7.36 (m, 1H, –Ar), 7.29–7.24 (m, 5H, –Ar), 5.96 (s, 1H, –OH), 5.66 (s, 1H, –CH), 2.59 (s, 2H, –CH2), 2.26 (m, 2H, –CH2), 1.10 (s, 3H, –CH3), 0.96 (s, 3H, –CH3); 13CNMR(CDCl3,100 MHz,δppm): 194.26, 161.98, 155.51, 145.67, 144.54, 129.47, 127.27, 126.81, 125.30, 123.22, 121.66, 117.44, 115.84, 115.50, 113.71, 111.85, 48.80, 38.23, 32.52, 30.35, 27.39, 25.04.
A6-9,9-dimethyl-12-(3,4-dimethoxyphenyl)-9,10-dihydro-8H-benzo[a]xanthen-11(12H)-one
1H NMR (CDCl3, 400 MHz): δppm 8.00 (d, 1H, –Ar), 7.79–7.70 (m, 2H, –Ar), 7.43–7.37 (m, 2H, –Ar),7.30 (d, 1H, –Ar), 6.96 (d, 2H, –Ar), 6.76–6.74 (dd, 1H, –Ar), 6.41 (s, 1H, –Ar), 5.66 (s, 1H, –CH), 3.79 (s, 3H,–OCH3), 3.74 (s, 3H, –OCH3), 2.56 (s, 2H, –CH2), 2.29 (m, 2H, –CH2), 1.11 (s, 3H, –CH3), 0.97 (s, 3H, –CH3).
A8-9,9-dimethyl-12-(4-hydroxy,3-methoxyphenyl)-9,10-dihydro-8H-benzo[a]xanthen-11(12H)-one
1H NMR (CDCl3, 400 MHz): δppm 8.00 (d, 1H, –Ar), 7.79–7.74 (m, 2H, –Ar), 7.62 (dd, 1H, –Ar),7.43–7.37 (m, 3H, –Ar), 6.69–6.60 (m, 2H, –Ar), 5.65 (s, 1H, –CH), 5.51 (s, 1H, –OH),3.79 (s, 3H, –OCH3), 2.55 (s, 2H, –CH2), 2.05 (s, 2H, –CH2), 1.11 (s, 3H, –CH3), 0.97 (s, 3H, –CH3).
A9-9,9-dimethyl-12-(2-hydroxy,3-methoxyphenyl)-9,10-dihydro-8H-benzo[a]xanthen-11(12H)-one
1H NMR (CDCl3, 400 MHz): δppm 7.83–7.73 (m, 3H, –Ar), 7.41–7.26 (m, 3H, –Ar),6.62–6.55 (m, 2H, –Ar), 6.32–6.30 (m, 1H, –Ar), 5.82 (s, 1H, –CH),5.29 (s, 1H, –OH), 3.85 (s, 3H, –OCH3), 2.62 (s, 2H, –CH2), 2.36 (m, 2H, –CH2), 1.13 (s, 3H, –CH3), 0.99 (s, 3H, –CH3).
A10-9,9-dimethyl-12- (4-fluorophenyl)-9,10-dihydro-8H-benzo[a]xanthen-11(12H)-one
1H NMR (CDCl3, 400 MHz): δppm 7.93–7.91 (d, 1H, J = 8 Hz, –Ar), 7.79–7.76 (m, 2H, –Ar),7.44–7.38 (m, 2H, –Ar),7.31–7.26 (m, 2H, –Ar), 6.89–6.82 (m, 2H, –Ar), 5.69 (s, 1H, –CH), 3.85(s, 3H, –OCH3), 2.56 (s, 2H, –CH2), 2.22–2.33(m, 2H, –CH2), 1.12(s, 3H, –CH3), 0.96(s, 3H, –CH3).
A11-12-phenyl-9,10-dihydro-8H-benzo[a]xanthen-11(12H)-one
1H NMR (CDCl3, 400 MHz): δppm 7.97–7.95 (d, 1H, J = 7.96 Hz, –Ar), 7.79–7.75 (m, 2H, –Ar),7.42–7.32 (m, 5H, –Ar),7.19–7.15 (m, 2H, –Ar),7.08–7.06 (m, 1H, –Ar),5.74 (s, 1H, –CH), 2.73–2.71 (m, 2H, –CH2), 2.37–2.44 (s, 2H, –CH2), 2.01–2.04 (m, 2H, –CH2); 13C NMR(CDCl3,100 MHz,δppm): 197.26, 165.76, 147.78, 145.08, 131.51, 128.89, 128.42, 127.04, 126.32, 124.93, 123.71, 117.72, 117.01, 115.55, 37.08, 34.67, 27.75, 20.26.
A12-12-(3-nitrophenyl)-9,10-dihydro-8H-benzo[a]xanthen-11(12H)-one
1H NMR (CDCl3, 400 MHz): δppm 8.07 (m,1H, –Ar), 7.93–7.91 (m, 1H, –Ar),7.81–7.78 (m, 4H, –Ar),7.40–7.34 (m, 2H, –Ar),5.82 (s, 1H, –CH), 2.75–2.71(m, 2H, –CH2), 2.39–2.43 (s, 2H, –CH2), 2.05–2.06(m, 2H, –CH2).
A13-12-(4-chlorophenyl)-9,10-dihydro-8H-benzo[a]xanthen-11(12H)-one
1H NMR (CDCl3, 400 MHz): δppm 7.86 (d, 1H, J = 6.4 Hz, –Ar), 7.80–7.76 (m, 2H, –Ar),7.44–7.38 (m, 2H, –Ar),7.31 (d, 1H, J = 6.4 Hz, –Ar), 7.26–7.24 (s, 2H, –Ar),7.15–7.11 (m, 2H, –Ar), 5.71(s, 1H, –CH), 2.73–2.67(m, 2H, –CH2), 2.45–2.38 (s, 2H, –CH2), 2.08–1.97 (m, 2H, –CH2); 13CNMR(CDCl3,100 MHz,δppm): 197.26, 165.91, 147.72, 143.56, 132.20, 131.51, 131.18, 129.92, 129.15, 128.52, 127.14, 125.05, 123.48, 117.01, 115.07, 109.46, 36.99, 34.19, 31.32, 27.72, 20.25.
A14 10,10-dimethyl-7-phenyl-10,11-dihydro-7H-benzo[c]xanthen-8(9H)-one
1H NMR (CDCl3, 400 MHz): δppm 8.34 (d,1H, –Ar), 8.12 (d, 1H, J = 8.72 Hz, –Ar),7.88 (d, 2H, –Ar),7.78 (d,2H, –Ar), 7.61–7.43 (m, 3H, –Ar),7.29–7.12 (m, 4H, –Ar), 5.14 (s,1H, –CH), 2.64–2.76 (m, 2H, –CH2), 2.30 (d, 2H, –CH2), 1.54 (m, 3H, –CH3), 1.08(s, 3H, –CH3).
A15- 10,10-dimethyl-7-(3-nitrophenyl)-10,11-dihydro-7H-benzo[c]xanthen-8(9H)-one
1H NMR (CDCl3, 400 MHz): δppm 8.29 (d, 1H, J = 8 Hz, –Ar), 8.00–7.95 (m, 2H, –Ar),7.78–7.50 (m, 4H, –Ar),7.44–7.36 (m, 3H, –Ar), 5.24 (s, 1H, –CH), 2.67–2.80 (m, 2H, –CH2), 2.04 (s, 2H, –CH2), 1.26 (m, 3H, –CH3), 0.98 (s, 3H, –CH3).
A16- 10,10-dimethyl-7-(4-Chlorophenyl)-10,11-dihydro-7H-benzo[c]xanthen-8(9H)-one
1H NMR (CDCl3, 400 MHz): δppm 8.27 (m,2H, –Ar), 7.80–7.71 (m, 2H, –Ar),7.47–7.39 (m, 3H, –Ar),7.26–7.18 (m,3H, –Ar), 5.48 (s,1H, –CH), 2.75–2.61 (m, 2H, –CH2), 2.04 (s, 2H, –CH2), 1.20 (m, 3H, –CH3), 1.08(s, 3H, –CH3).
A17-10,11-dihydro-7-phenyl-7H-benzo[c]xanthen-8(9H)-one
1H NMR (CDCl3, 400 MHz): δppm 7.28 (d, 1H, –Ar), 7.27–7.26 (m, 2H, –Ar), 7.25–7.16 (d, 3H, J = 8 Hz, –Ar), 7.11–7.09 (m, 5H, –Ar), 5.47 (s, 1H, –CH), 2.80 (m, 2H, –CH2), 2.60 (m, 2H, –CH2), 2.02 (m, 2H, –CH2).
A18-10,11-dihydro-7-(3-nitrophenyl-7H-benzo[c]xanthen-8(9H)-one
1H NMR (CDCl3, 400 MHz): δppm 8.16 (d, 1H, J = 8.2 Hz, –Ar), 8.03–7.97 (m, 4H, –Ar), 7.85–7.76 (m, 2H, –Ar), 7.49 (d, 1H, J = 8 Hz, –Ar), 7.42–7.38 (m, 3H, –Ar), 4.88 (s, 1H, –CH), 2.68 (m, 2H, –CH2), 2.36 (m, 2H, –CH2), 2.06 (m, 2H, –CH2).
A19-10,11-dihydro-7-(4-chlorophenyl-7H-benzo[c]xanthen-8(9H)-one.
1H NMR (CDCl3, 400 MHz): δppm 8.30 (d, 1H, J = 8.2 Hz, –Ar), 8.83–7.77 (m, 2H, –Ar), 7.57–7.38 (m, 4H, –Ar), 7.49 (d, 1H, J = 8 Hz, –Ar), 7.26–7.10 (m, 3H, –Ar), 4.76 (s, 1H, –CH), 2.62 (m, 2H, –CH2), 2.35–2.32 (m, 2H, –CH2), 2.03–2.00 (m, 2H, –CH2).
Conclusion
Tetrabutylammonium prolinate ionic liquid (TBAPIL) was grafted on silica NPs through propyltriethoxysilane linkage to afford supported catalyst TBAP@Si(CH2)3@nano-silica (TBAPILS). SEM results revealed the successful grafting of TBAPIL on silica NPs by indicating morphological changes. The TEM micrographs confirmed formation of silica NPs of the size of 40–50 nm and well matched with the values obtained for silica NPs from XRD measurements using JCPDS card number. The preparation of the catalyst was also confirmed by FT-IR analysis. The prepared catalyst TBAPILS was successfully and efficiently used for the synthesis of tetrahydrobenzoxanthene-11-one derivatives in solvent less condition in short reaction time and with easy workup. The catalyst could be used up to five cycles without significant loss of activity. The key advantages of the process are cost effectiveness, high efficiency and easy workup.
References
K. Philippot, P. Serp, Concepts in nanocatalysis. Nanomater. Catal. First Ed. (2012). https://doi.org/10.1002/9783527656875.ch1
S. Chaturvedi, P.N. Dave, N.K. Shah, Applications of nano-catalyst in new era. J. Saudi Chem. Soc. 16, 307–325 (2012). https://doi.org/10.1016/j.jscs.2011.01.015
S.B.Singh, Tandon, P.K., Catalysis : A brief review on Nano-Catalyst Catalysis : A Brief Review on Nano-Catalyst. (2016)
H. Filian, A. Kohzadian, M. Mohammadi, A. Ghorbani-Choghamarani, A. Karami, Pd(0)-guanidine@MCM-41: a very effective catalyst for rapid production of bis (pyrazolyl)methanes. Appl. Organomet. Chem. 34, 42–44 (2020). https://doi.org/10.1002/aoc.5579
F. Feizpour, M. Jafarpour, A. Rezaeifard, Band gap modification of TiO2 nanoparticles by ascorbic acid-stabilized pd nanoparticles for photocatalytic suzuki-miyaura and ullmann coupling reactions. Catal. Letters. 149, 1595–1610 (2019). https://doi.org/10.1007/s10562-019-02749-z
A. Agrwal, V. Kasana, [Fesipmim]Cl as highly efficient and reusable catalyst for solventless synthesis of dihydropyridine derivatives through Hantzsch reaction. J. Chem. Sci. (2020). https://doi.org/10.1007/s12039-020-01770-9
M. Nikoorazm, M. Khanmoradi, M. Mohammadi, Guanine-La complex supported onto SBA-15: a novel efficient heterogeneous mesoporous nanocatalyst for one-pot, multi-component Tandem Knoevenagel condensation–Michael addition–cyclization reactions. Appl. Organomet. Chem. 34, 1–18 (2020). https://doi.org/10.1002/aoc.5504
M. Nikoorazm, M. Mohammadi, M. Khanmoradi, Zirconium@guanine@MCM-41 nanoparticles: an efficient heterogeneous mesoporous nanocatalyst for one-pot, multi-component tandem knoevenagel condensation–michael addition–cyclization reactions. Appl. Organomet. Chem. (2020). https://doi.org/10.1002/aoc.5704
A. Ghorbani-Choghamarani, M. Mohammadi, R.H.E. Hudson, T. Tamoradi, Boehmite@tryptophan-Pd nanoparticles: a new catalyst for C–C bond formation. Appl. Organomet. Chem. 33, 1–11 (2019). https://doi.org/10.1002/aoc.4977
I.I. Slowing, B.G. Trewyn, S. Giri, V.S.Y. Lin, Mesoporous silica nanoparticles for drug delivery and biosensing applications. Adv. Funct. Mater. 17, 1225–1236 (2007). https://doi.org/10.1002/adfm.200601191
B. Karimi, D. Zareyee, A high loading sulfonic acid-functionalized ordered nanoporous silica as an efficient and recyclable catalyst for chemoselective deprotection of tert-butyldimethylsilyl ethers. Tetrahedron Lett. 46, 4661–4665 (2005). https://doi.org/10.1016/j.tetlet.2005.04.100
A.R. Karimi, Z. Alimohammadi, M. Mostafa Amini, Wells-Dawson heteropolyacid supported on silica: a highly efficient catalyst for synthesis of 2,4,5-trisubstituted and 1,2,4,5-tetrasubstituted imidazoles. Mol Divers. 14, 635–641 (2010). https://doi.org/10.1007/s11030-009-9197-x
R. Ratti, Ionic liquids: synthesis and applications in catalysis. Adv. Chem. 2014, 1–16 (2014). https://doi.org/10.1155/2014/729842
T. Welton, Ionic liquids in catalysis. Coord. Chem. Rev. 248, 2459–2477 (2004). https://doi.org/10.1016/j.ccr.2004.04.015
J.P. Hallett, T. Welton, Room-temperature ionic liquids: Solvents for synthesis and catalysis. 2. Chem. Rev. 111, 3508–3576 (2011). https://doi.org/10.1021/cr1003248
N. Ferlin, M. Courty, A.N. Van Nhien, S. Gatard, M. Pour, B. Quilty, M. Ghavre, A. Haiß, K. Kümmerer, N. Gathergood, S. Bouquillon, Tetrabutylammonium prolinate-based ionic liquids: aA combined asymmetric catalysis, antimicrobial toxicity and biodegradation assessment. RSC Adv. 3, 26241–26251 (2013). https://doi.org/10.1039/c3ra43785j
U.C. Rajesh, D. Divya, D.S. Rawat, Functionalized superparamagnetic Fe3O4as an efficient quasi-homogeneous catalyst for multi-component reactions. RSC Adv. 40, 41323–41330 (2014). https://doi.org/10.1039/c4ra06803c
R. Fareghi-Alamdari, M.N. Niri, H. Hazarkhani, A novel hydrogen-bonded silica-supported acidic ionic liquid: An efficient, recyclable and selective heterogeneous catalyst for the synthesis of diesters. J. Chem. Sci. 130, 1–13 (2018). https://doi.org/10.1007/s12039-018-1454-z
H.N. Hafez, M.I. Hegab, I.S. Ahmed-Farag, A.B.A. El-Gazzar, A facile regioselective synthesis of novel spiro-thioxanthene and spiro-xanthene-9′,2-[1,3,4]thiadiazole derivatives as potential analgesic and anti-inflammatory agents. Bioorg. Med. Chem. Lett. 18, 4538–4543 (2008). https://doi.org/10.1016/j.bmcl.2008.07.042
N. Hashim, M. Rahmani, M.A. Sukari, A.M. Ali, N.B. Alitheen, R. Go, H.B.M. Ismail, Two new xanthones from Artocarpus obtusus. J. Asian Nat. Prod. Res. 12, 106–112 (2010). https://doi.org/10.1080/10286020903450411
E. Vieira, J. Huwyler, S. Jolidon, F. Knoflach, V. Mutel, J. Wichmann, 9H-Xanthene-9-carboxylic acid [1,2,4]oxadiazol-3-yl- and (2H-tetrazol-5-yl)-amides as potent, orally available mGlu1 receptor enhancers. Bioorg. Med. Chem. Lett. (2005). https://doi.org/10.1016/j.bmcl.2005.05.135
F. Zelefack, D. Guilet, N. Fabre, C. Bayet, S. Chevalley, S. Ngouela, B.N. Lenta, A. Valentin, E. Tsamo, M.G. Dijoux-Franca, Cytotoxic and antiplasmodial xanthones from Pentadesma butyracea. J. Nat. Prod. 72, 954–957 (2009). https://doi.org/10.1021/np8005953
S.J. Tao, S.H. Guan, W. Wang, Z.Q. Lu, G.T. Chen, N. Sha, Q.X. Yue, X. Liu, D.A. Guo, Cytotoxic polyprenylated xanthones from the resin of Garcinia hanburyi. J. Nat. Prod. 72, 117–124 (2009). https://doi.org/10.1021/np800460b
S. Chatterjee, M. Iqbal, J.C. Kauer, J.P. Mallamo, S. Senadhi, S. Mallya, D. Bozyczko-Coyne, R. Siman, Xanthene derived potent nonpeptidic inhibitors of recombinant human calpain I. Bioorg. Med. Chem. Lett. 6, 1619–1622 (1996). https://doi.org/10.1016/S0960-894X(96)00286-7
K. Kikuchi, K. Komatsu, T. Nagano, Zinc sensing for cellular application. Curr. Opin. Chem. Biol. (2004). https://doi.org/10.1016/j.cbpa.2004.02.007
A.M. El-Brashy, M. El-Sayed Metwally, F.A. El-Sepai, Spectrophotometric determination of some fluoroquinolone antibacterials by binary complex formation with xanthene dyes. Farmaco 59, 809–817 (2004). https://doi.org/10.1016/j.farmac.2004.07.001
A. Akbari, A. Hosseini-Nia, Biological evaluation and simple method for the synthesis of tetrahydrobenzo[a]xanthenes-11-one derivatives. J. Saudi Chem. Soc. 21, S7–S11 (2017). https://doi.org/10.1016/j.jscs.2013.09.009
A. Kumar, S. Sharma, R.A. Maurya, J. Sarkar, Diversity oriented synthesis of benzoxanthene and benzochromene libraries via one-pot, three-component reactions and their anti-proliferative activity. J. Comb. Chem. 12, 20–24 (2010). https://doi.org/10.1021/cc900143h
M.K. Schwaebe, T.J. Moran, J.P. Whitten, Total synthesis of psorospermin. Tetrahedron Lett. 46, 827–829 (2005). https://doi.org/10.1016/j.tetlet.2004.12.006
N. Singh, A.K. Shreshtha, M.S. Thakur, S. Patra, Xanthine scaffold: scope and potential in drug development. Heliyon. 4, e00829 (2018). https://doi.org/10.1016/j.heliyon.2018.e00829
P. Bedi, R. Gupta, T. Pramanik, Synthesis and biological properties of pharmaceutically important xanthones and benzoxanthone analogs: a brief review. Asian J. Pharm. Clin. Res. 11, 12–20 (2018). https://doi.org/10.22159/ajpcr.2018.v11i2.22426
S.L. Niu, Z.L. Li, F. Ji, G.Y. Liu, N. Zhao, X.Q. Liu, Y.K. Jing, H.M. Hua, Xanthones from the stem bark of Garcinia bracteata with growth inhibitory effects against HL-60 cells. Phytochemistry 77, 280–286 (2012). https://doi.org/10.1016/j.phytochem.2012.01.010
J.M. Khurana, D. Magoo, K. Aggarwal, N. Aggarwal, R. Kumar, C. Srivastava, Synthesis of novel 12-aryl-8,9,10,12-tetrahydrobenzo[a]xanthene-11-thiones and evaluation of their biocidal effects. Eur. J. Med. Chem. 58, 470–477 (2012). https://doi.org/10.1016/j.ejmech.2012.10.025
J. Liu, Z. Diwu, W.Y. Leung, Synthesis and photophysical properties of new fluorinated benzo[c]xanthene dyes as intracellular pH indicators. Bioorg. Med. Chem. Lett. 11, 2903–2905 (2001). https://doi.org/10.1016/S0960-894X(01)00595-9
R.J. Sarma, J.B. Baruah, One step synthesis of dibenzoxanthenes. Dye. Pigment. 64, 91–92 (2005). https://doi.org/10.1016/j.dyepig.2004.03.010
A. Banerjee, A.K. Mukherjee, Chemical aspects of santalin as a histological stain. Biotech. Histochem. 56, 83–85 (1981). https://doi.org/10.3109/10520298109067286
P. Srihari, S.S. Mandal, J.S.S. Reddy, R.S. Rao, J.S. Yadav, Synthesis of 1,8-dioxo-octahydroxanthenes utilizing PMA-SiO2 as an efficient reusable catalyst. Chin. Chem. Lett. 19, 771–774 (2008). https://doi.org/10.1016/j.cclet.2008.05.005
M.T. Maghsoodlou, S.M. Habibi-Khorassani, Z. Shahkarami, N. Maleki, M. Rostamizadeh, An efficient synthesis of 2,2′-arylmethylene bis(3-hydroxy-5,5-dimethyl-2-cyclohexene-1-one) and 1,8-dioxooctahydroxanthenes using ZnO and ZnO-acetyl chloride. Chin. Chem. Lett. 21, 686–689 (2010). https://doi.org/10.1016/j.cclet.2010.02.005
X. Fan, X. Hu, X. Zhang, J. Wang, InCl3·4H2O-promoted green preparation of xanthenedione derivatives in ionic liquids. Can. J. Chem. 83, 16–20 (2005). https://doi.org/10.1139/v04-155
T.-S. Jin, J.-S. Zhang, J.-C. Xiao, A.-Q. Wang, T.-S. Li, Clean synthesis of 1,8-dioxo-octahydroxanthene derivatives catalyzed by p -dodecylbenzenesulfonic acid in aqueous media. Synlett (2004). https://doi.org/10.1055/s-2004-820022
F. Rajabi, M. Abdollahi, E.S. Diarjani, M.G. Osmolowsky, O.M. Osmolovskaya, P. Gómez-López, A.R. Puente-Santiago, R. Luque, Solvent-free preparation of 1,8-dioxo-octahydroxanthenes employing iron oxide nanomaterials. Materials 12, 2386 (2019). https://doi.org/10.3390/ma12152386
Z. Karimi-Jaberi, S.Z. Abbasi, B. Pooladian, M. Jokar, Efficient, one-pot synthesis of tetrahydrobenzo[a]xanthen-11-ones and dibenzo[a, j]xanthenes using trichloroacetic acid as a solid heterogeneous catalyst under solvent-free conditions. E-Journal Chem. 8, 1895–1899 (2011)
M. Seyyedhamzeh, P. Mirzaei, A. Bazgir, Solvent-free synthesis of aryl-14H-dibenzo[a, j]xanthenes and 1,8-dioxo-octahydro-xanthenes using silica sulfuric acid as catalyst. Dye. Pigment. 76, 836–839 (2008). https://doi.org/10.1016/j.dyepig.2007.02.001
J. Li, W. Tang, L. Lu, W. Su, Strontium triflate catalyzed one-pot condensation of β-naphthol, aldehydes and cyclic 1,3-dicarbonyl compounds. Tetrahedron Lett. 49, 7117–7120 (2008). https://doi.org/10.1016/j.tetlet.2008.09.129
N.V. Shitole, S.B. Sapkal, B.B. Shingate, M.S. Shingare, A simple and green synthesis of tetrahydrobenzo[α]-xanthen-11-one using peg-400 as efficient and recyclable reaction media. Bull. Korean Chem. Soc. 32, 35–36 (2011). https://doi.org/10.5012/bkcs.2011.32.1.35
B.B.F. Mirjalili, A. Bamoniri, N. Salehi, Synthesis of tetrahydrobenzo[a]xanthenes-11-one derivatives in water promoted by Bi(NO3)3·5H2O. Chemija 23, 118–123 (2012)
S.V. Goswami, P.B. Thorat, S.S. Dhone., S.R. Bhusrae, Phenylboronic acid-catalyzed synthesis of 99-dimethyl-12-phenyl-910- dihydro-8H-benzo[a] xanthen-11(12H)-one derivatives. J. Chem. Pharm. Res. 3, 632–635 (2011).
X.J. Sun, J.F. Zhou, P.S. Zhao, Molecular iodine-catalyzed one-pot synthesis of tetrahydrobenzo[a]xanthene-11-one and diazabenzo[a]anthracene-9,11-dione derivatives under microwave irradiation. J. Heterocycl. Chem. 48, 1347–1350 (2011). https://doi.org/10.1002/jhet.742
J.M. Khurana, D. Magoo, p-TSA-catalyzed one-pot synthesis of 12-aryl-8,9,10,12-tetrahydrobenzo[a]xanthen-11-ones in ionic liquid and neat conditions. Tetrahedron Lett. 50, 4777–4780 (2009). https://doi.org/10.1016/j.tetlet.2009.06.029
B. Das, P. Thirupathi, I. Mahender, V.S. Reddy, Y.K. Rao, Amberlyst-15: An efficient reusable heterogeneous catalyst for the synthesis of 1,8-dioxo-octahydroxanthenes and 1,8-dioxo-decahydroacridines. J. Mol. Catal. A Chem. 247, 233–239 (2006). https://doi.org/10.1016/j.molcata.2005.11.048
B. Das, P. Thirupathi, K.R. Reddy, B. Ravikanth, L. Nagarapu, An efficient synthesis of 1,8-dioxo-octahydroxanthenes using heterogeneous catalysts. Catal. Commun. 8, 535–538 (2007). https://doi.org/10.1016/j.catcom.2006.02.023
M. Litschauer, M.A. Neouze, Nanoparticles connected through an ionic liquid-like network. J. Mater. Chem. 18, 640–646 (2008). https://doi.org/10.1039/b713442h
M. Mahkam, F. Hosseinzadeh, M. Galehassadi, Preparation of ionic liquid functionalized silica nanoparticles for oral drug delivery. J. Biomater. Nanobiotechnol. 03, 391–395 (2012). https://doi.org/10.4236/jbnb.2012.33038
K. Fukumoto, M. Yoshizawa, H. Ohno, Room temperature ionic liquids from 20 natural amino acids. J. Am. Chem. Soc. 127, 2398–2399 (2005). https://doi.org/10.1021/ja043451i
A. Kumari, B. Kaur, R. Srivastava, R.S. Sangwan, Isolation and immobilization of alkaline protease on mesoporous silica and mesoporous ZSM-5 zeolite materials for improved catalytic properties. Biochem. Biophys. Rep. 2, 108–114 (2015). https://doi.org/10.1016/j.bbrep.2015.05.009
E.I. Jiménez, W.E.V. Narváez, T. Rocha-Rinza, M. Hernández-Rodríguez, Design and application of a bifunctional organocatalyst guided by electron density topological analyses. Catal. Sci. Technol. 7, 4470–4477 (2017). https://doi.org/10.1039/c7cy00430c
G.C. Nandi, S. Samai, R. Kumar, M.S. Singh, An efficient one-pot synthesis of tetrahydrobenzo[a]xanthene-11-one and diazabenzo[a]anthracene-9,11-dione derivatives under solvent free condition. Tetrahedron 65, 7129–7134 (2009). https://doi.org/10.1016/j.tet.2009.06.024
V. Rama, K. Kanagaraj, K. Pitchumani, A multicomponent, solvent-free, one-pot synthesis of benzoxanthenones catalyzed by HY zeolite: Their anti-microbial and cell imaging studies. Tetrahedron Lett. 53, 1018–1024 (2012). https://doi.org/10.1016/j.tetlet.2011.10.143
Fatahpour, M., Hazeri, N., Maghsoodlou, M.T., Lashkari, M.: Lactic acid: a new application as an efficient catalyst for the green one-pot synthesis of 2-hydroxy-12-aryl-8,9, 10,12-tetrahydrobenzo[a]xanthene-11-one and 12-aryl-8,9,10,12-tetrahydrobenzo[a]xanthen-11-one Analogs. Iran. J. Sci. Technol. Trans. A Sci. 42, 533–538 (2018). https://doi.org/10.1007/s40995-016-0064-1.
B. Maleki, M. Gholizadeh, Z. Sepehr, 1,3,5-Trichloro-2,4,6-triazinetrion: a versatile heterocycle for the one-pot synthesis of 14-aryl-or alkyl -14H-dibenzo[a, j]xanthene, 1,8-dioxooctahydroxanthene and 12-aryl-8,9,10,12-tetrahydrobenzo[a]xanthene-11- one derivatives under solvent-free conditions. Bull. Korean Chem. Soc. 32, 1697–1702 (2011). https://doi.org/10.5012/bkcs.2011.32.5.1697
S. Sudha, M.A. Pasha, Ultrasound assisted synthesis of tetrahydrobenzo[c]xanthene-11-ones using CAN as catalyst. Ultrason Sonochem. 19, 994–998 (2012). https://doi.org/10.1016/j.ultsonch.2012.02.002
J. Li, L. Lu, W. Su, A new strategy for the synthesis of benzoxanthenes catalyzed by proline triflate in water. Tetrahedron Lett. 51, 2434–2437 (2010). https://doi.org/10.1016/j.tetlet.2010.02.149
H. Wang, X. Ren, Y. Zhang, Z. Zhang, Synthesis 12-aryl or 12-alkyl-8,9,10,12-tetrahydrobenzo[a]xanthen-11-one derivatives catalyzed by dodecatungstophosphoric acid. J. Braz. Chem. Soc. 20, 1939–1943 (2009)
Acknowledgements
We are thankful to Govind Ballabh Pant University of Agriculture and Technology, Pantnagar (Uttarakhand), India, for providing necessary research facility and KIET Group of Institutions for constant help and support during the research. We would also thank Manish Kumar, IIT Ropar, for providing NMR spectra and ISFAL, Moga, for providing IR spectra.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Agrwal, A., Kumar, V. & Kasana, V. Preparation and application of highly efficient and reusable TBAPIL@Si(CH2)3@nano-silica-based nano-catalyst for preparation of benzoxanthene derivatives. J IRAN CHEM SOC 18, 2583–2595 (2021). https://doi.org/10.1007/s13738-021-02211-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-021-02211-1