Abstract
In this work, a new carboxylate-modified pine cone is synthesized via esterification of pine cone with isopropylidene malonate. The chemical modification procedure is optimized, and then, the adsorbent prepared is characterized by different techniques such as field emission scanning electron microscopy, energy-dispersive X-ray diffraction, and FT-IR spectroscopy in order to approve the presence of carboxylic groups on the surface of pine cone. The performance of the bio-sorbent is investigated for the removal of lead ions from the aqueous solution. The factors affecting the performance of the prepared adsorbent are analyzed and optimized in an experimental setup. Under the optimum conditions, studying the experimental data illustrates that the Langmuir isotherm can suitably describe the equilibrium data, and at a temperature of 298 K, pH 4.8, and 0.40 g L−1 of the adsorbent, the maximum adsorption capacity of 400.0 mg g−1 is obtained; this value is much higher than some of the newly reported ones. The high lead ion adsorption can be attributed to the abundant functional groups present in the adsorbent. Carboxylate-modified pine cone can be successfully regenerated for 3 times using EDTA-2Na as the solvent elution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The pollution of industrial effluents by heavy metal ions has been one of the serious environmental problems in the recent decades [1]. Among the heavy metal ions, lead is a grievous one due to its acute toxicity [2, 3]. The Environmental Protection Agency standard for lead ions in wastewater is 0.5 mg L−1 [4], and consequently, eliminating lead ions from the ecosystem is an essential requirement.
Different techniques like coagulation, membrane filtration, and adsorption have been reported to remediate the effluents containing lead ions [4,5,6,7,8,9]. Due to its practicability and low consumption of reagents, the adsorption using a low-cost bio-sorbent has earned a great attention in the recent years with respect to the other mentioned methods [9]. The low-cost bio-sorbents such as agricultural wastes have functional groups in their structure that have a strong tendency to adsorb pollutants [10]. However, the application of a crude adsorbent has important drawbacks such as a low adsorption capacity [9, 11]. By treating the agricultural wastes using the chemical reagents such as acids or amines, one can load an extra functional group on the surface of the adsorbent and, therefore, improve its efficiency [1, 12, 13].
In the present research work, pine cone (PC), as an abundant agricultural waste, was treated to refine the effluent containing lead ions. Like other lignocellulosic materials, PC has a large amount of hydroxyl groups that can be employed as reactive sites to synthesize an adsorbent with specific properties [10]. These functional groups have lone pairs of electrons that can be attacked by the electrophiles such as the carbonyl group in organic reactions [9]. Therefore, in this context, we intended to modify PC using isopropylidene malonate (as an esterification agent) and study the adsorption efficiency of carboxylate-modified pine cone (CMPC) for eliminating lead ions from aqueous media. The influence of various experimental factors such as the pH, adsorbent dosage, initial lead ion concentration, and contact time on the bio-sorption of lead ions was studied. Two adsorption kinetic models were employed to analyze the experimental data. In addition, the adsorption isotherms and the possible mechanism for the adsorption of lead ions onto the prepared adsorbent were described. Finally, the experiments were performed on some real samples in order to determine the practicality of this novel adsorbent.
Experimental
Materials and standard solutions
PC was gathered from the campus of the Shahrood University of Technology, Shahrood, Iran. The chemicals including lead nitrate, sodium hydroxide, toluene, 2,2-isopropylidene malonate, sodium bicarbonate, hydrochloric acid, sodium chloride, ethylene diamine tetra acetic acid disodium (EDTA-2Na), and ethanol were supplied from the Merck Company (Darmstadt, Germany) and used without any purification. Doubly distilled water was used throughout the work.
A stock solution of 1200 mg L−1 of lead ions was prepared by dissolving 0.4844 g of lead nitrate in distilled water and diluting it in a 250-mL volumetric flask. The synthetic wastewater of lead ions was prepared by diluting appropriate volumes of the stock solution.
Apparatus
A Shimadzu flame atomic absorption spectrophotometer, model AA-670, equipped with air-acetylene flame and lead hollow cathode lamp (wavelength 283.3 nm) was applied for quantification of the concentration of lead ions in the solutions.
The pH values for the solutions were measured using a Metrohm 744 pH meter (Herisau, Switzerland) equipped with a combined glass electrode. Stirring of the solution was made using a Heidolph magnetic stirrer (MRHei-standard). The X-ray diffraction (XRD) patterns were recorded using an XRD diffractometer (model pw1730). The functional groups and surface morphology of CMPC were studied using an FT-IR spectrophotometer (WQF-520) and a field emission scanning electron microscope (Zeiss sigma 300 HV-Germany), respectively.
Preparation of adsorbent
Initially, the collected PC was grounded using a ball mill. The powdered sample was filtered through a sieve to obtain particles of identical size. Then an alkaline treatment was applied to release the hydroxyl functional groups and remove the natural fat and wax present in it [14, 15]. In this regard, 10 g of the prepared powder was placed in 250 L of a 0.1 M NaOH solution. The slurry was stirred for 24 h, and then, the filtered mixture was neutralized with distilled water. The dried alkaline-treated adsorbent and isopropylidene malonate with different ratios (1:1, 1:2, 1:3, 1:4, and 1:5) were refluxed in toluene (30 mL) at 110 °C for 6 h. After cooling the mixture, it was washed with ethanol, NaHCO3 solution (0.1 M), and distilled water for several times. Finally, the modified pine cone was dried at 100 °C. The optimum ratio for the dried alkaline-treated adsorbent and isopropylidene malonate was found to be 1:2. The relevant chemical reaction is demonstrated in Fig. 1 [16, 17].
Batch metal adsorption studies
The batch adsorption experiments were carried out by placing a specific quantity of CMPC in a 50 mL solution containing a definite concentration of lead ions. The initial solution pH was adjusted by a dilute HCl or NaOH solution. The resulting solution was stirred at a rate of 250 rpm in order to investigate the effects of the factors influencing the adsorption procedure. Next, at the predetermined times, 3 mL of the sample solution was centrifuged at 3000 rpm for 1 min. The remaining concentration of lead ions was quantified using flame atomic absorption spectrometry at its maximum wavelength (λ = 283.3 nm) and a calibration graph was constructed by standard solutions of lead ions. After determination of the lead ion concentration, the uptaked amounts of lead ions per weight of the adsorbent at time t (qt,i) and the removal percentage of lead ions (R%) were calculated using Eqs. (1) and (2), respectively:
where C0 is the initial lead concentration (mg L−1), Ct is the remaining lead concentration at time t (mg L−1), W is the weight of CMPC used (g), and V is the volume of the solution (L).
Results and discussion
Characterization of PC and CMPC
The structural and crystallographic makeup of PC and CMPC were specified using the X-ray diffractograms (Fig. 2). For the raw PC, the important characteristic peaks for cellulose (I) were identified at 2θ = 15.7°, 21.3°, and 34.4° [18, 19]. These peaks indicate that the raw PC mainly has organized crystalline cellulose. After treatment, a little shift in the cellulosic peaks and a substantial decrease in their intensities were detected, which were due to the decrease in the crystalline cellulose content. Similar results have been reported by other researchers [17, 19,20,21].
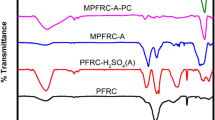
The FT-IR spectra (Fig. 3) show the presence of abundant functional groups in the raw PC and CMPC. Before modification with carboxylic acid, the adsorbent exhibits bands at 1460 cm−1 and 2925 cm−1, representative of the bending and stretching vibrations of the methyl and methylene groups [4, 20]; bands at 1730 cm−1 and 1624 cm−1, indicative of the stretching vibration of the C = O bond in non-ionic and ionic carboxyl groups, respectively; a band for the stretching vibration of O−H at 3428 cm−1; and a band for the phenyl ring skeletal vibrations of lignin at 1517 cm−1 [10]. For CMPC in the acidic and basic forms (Figs. 3b and c), the new bands appearing at 1155 and 1748 cm−1 are related to the stretching vibrations of C–O and C=O in the ester, respectively. It should be noted that the band for C=O at 1745 cm−1 is the result of overlapping of the absorption at 1712 cm−1 for carboxylic acid with that at 1750 cm−1 for C=O bond in the ester [17, 19, 22, 23]. In the basic form of CMFC, the asymmetric and symmetric stretching vibrations of carboxylate groups at 1608 cm–1 and 1380 cm–1 augmented. This specifies that a fraction of carboxylic groups was changed to the carboxylate ions.
After adsorption of lead ions onto the prepared adsorbent, the characteristic FT-IR absorbance peaks shifted significantly. For instance, the peaks at 3428 cm−1, 1624 cm−1, and 1381 cm−1 shifted to 3410 cm−1, 1572 cm−1, and 1375 cm−1, respectively. This can be attributed to the connection of lead ions to the functional groups, especially to the oxygen-containing groups [4] because lead ions have been recognized as intermediate ions and, therefore, an affinity with two types of intermediates and hard ligands containing oxygen atoms [24].
The field emission scanning electron microscopy (FESEM) images presented in Fig. 4 display a clear change in the morphology of the adsorbent surface. As it can be seen in this figure, after modification, the adsorbent has a rougher surface. This property is beneficial for metal adsorption [24]. By adsorption of lead ions, the surface of the bio-sorbent became smoother.
Moreover, a comparison between the energy-dispersive X-ray diffraction (EDX) images for CMPC before and after bio-sorption of lead ions demonstrates that the loading of lead ions on the surface of bio-sorbent takes place successfully (Fig. 5).
Effect of initial pH
The solution pH is the most important factor affecting the removal efficiency of metal ions from aqueous environments [20, 25]. Thus in this research work, the effect of the initial pH value on the adsorption of lead ions on the prepared adsorbent was investigated by adding 0.020 g of CMPC to 50 mL of 100 mg L−1 of lead ions. The initial pH value was changed from 3 to 5.5 using a dilute solution of HCl or NaOH. It should be noted that higher pH values were not investigated because lead ions would precipitate in the form of hydroxides at pH values higher than 5.6. The data obtained (Fig. 6) clarify that at a highly acidic pH value, due to the competition between the H+ ions and the lead ions for adsorption on the surface of the bio-sorbent, the adsorption efficiency of lead ions decreases. However, by increasing the pH value, the concentration of H+ ions reduces. Consequently, the adsorption efficiency of lead ions increases and reaches maximum values at the pH interval of 4.80–5.5. Since the pH values for the electroplating wastewater, pigment wastewater, and printing wastewater were commonly to some extent acidic [1], pH = 4.8 (the same pH as the initial lead ion solution) was chosen as the optimal one and used in the next steps.
Effect of adsorbent dosage
The adsorbent dosage is another important factor involved in the adsorption process because it specifies the capacity of the proposed adsorbent for a definite quantity of the adsorbate [26]. In order to determine the optimum value for this parameter, different dosages of CMPC (0.010–0.030 g) were contacted with 50 mL of the lead ion solution (120 ppm) at pH = 4.8, and the removal percentages of the lead ions were calculated. The derived data (Fig. 7) revealed that the removal efficiency improved with the increase in the adsorbent dosage. This behavior is due to the increase in the surface area and binding sites on the bio-sorbent, which causes an easier penetration of the metal ions into the active sites. As it can be seen in Fig. 7, the maximum removal efficiency of lead ions was detected with an adsorbent dosage of 0.020 g, and therefore, this value was selected as an optimum one.
Effects of initial concentration of lead ions and contact time
In order to investigate the effect of the initial concentration of lead ions, 0.40 g L−1 of CMPC was mixed with 50 mL of the solutions containing various concentrations of lead ions in the range of 80–210 mg L−1; the relevant findings are shown in Fig. 8. According to these results, the removal efficiency (Ri) and the initial Pb ion concentration have an inverse relation. In other words, by increasing the initial pollutant concentration, the removal percentage (Ri) decreased. These results can be explained based on the ratio of the available active sites of the bio-sorbent to the lead ions. This ratio is high at low lead ion concentrations, but at upper initial lead ion concentrations, due to the occupancy of the binding sites, the value for this parameter is less, and therefore, Ri is diminished.
The equilibrium time is known as one of the most essential factors involved for designing the economical wastewater purification systems [27]. Consequently, in order to determine the time required for the equilibrium, the removal efficiency of lead ions (in different concentration ranges) against the contact time was investigated (Fig. 9). The data obtained explain that in the first 2 min, the adsorption rate of lead ions is rapid, which is related to the abundance of the active sites on the surface of the adsorbent and a high concentration gradient of the pollutant. However, at higher times, owing to the decrease in the concentration gradient of the pollutant and active sites and the repulsion force between the lead ions adsorbed onto the bio-sorbent and the lead ions existing in the aqueous solution, the adsorption rate gradually slows down [28], and lastly, at the equilibrium times (in 20–25 min), the rate of the adsorption process is almost constant for all the studied lead ion concentrations. The rapid adsorption of the metal ion pollutant is probably created from the fast coordination between the lead ions and the active donor atom sites present on the sorbent. This result is very important in the economy clean-up of the toxic metal ions from the effluents [24].
Kinetic investigation
In order to specify the mechanism and the adsorption rate, which is essential for designing the sorption industrial columns, the adsorption kinetics should be studied [24, 29]. In this regard, the concentration changes of lead ions as a function of contact time were calculated, while the adsorbent dosage and the solution pH value were fixed at optimum values (pH = 4.8, adsorbent dosage = 0.04 g). Then two commonly used rate models (pseudo-first-order and pseudo-second-order [Eq. (3) and (4)] were employed for the simulation of the kinetic data [9]:
where the variables qt and qe are the uptaked amounts of lead ions at time t and equilibrium time (mg/g), respectively, k1 (min−1) is the rate constant of the pseudo-first-order model, and k2 (g min mg−1) is the rate constant of the pseudo-first-order model. In the pseudo-first-order kinetics model, the constants k1 and qe are derived from the slope and intercept of the straight-line graph of ln(qe–qt) versus t, whereas for the second-order kinetics model, the constants k2 and qe are derived from the intercept and slope of the straight-line graph of (t/qt) versus t, respectively. The value for qe, extracted from the related graph, is detected as the predicted qe (qe,pred). According to the data obtained (Fig. 10 and Table 1), the values for this parameter, predicted by the second-order kinetics model, are much closer to the experimental ones in comparison with the values predicted using the first-order kinetics model for all the concentrations of the pollutant studied. The R2 value is another reason for a better agreement of the experimental data with the pseudo-second-order kinetic model [17, 27]. The adaptability with this type of kinetic model implies that the sorption of lead ions onto the presented adsorbent is chemisorption, approving that the functional groups are responsible for the adsorption of metal ions [24].
Investigation of adsorption isotherms
The applicability of a new adsorbent was specified by different physicochemical constants like the adsorptive capacity. In order to obtain such parameters, it is necessary to study the adsorption isotherms [30]. In the present research, the experiments were conducted by two common theoretical isotherms [Freundlich and Langmuir (Table 2)]; the theoretical background of these models can be found elsewhere [9]. The evaluation of the prediction accuracy and the quality of fitness of the experimental data were made by calculation of the correlation coefficient (R2). The model that had the largest R2 value was elected as the best model [31]. As it can be understood from Fig. 11 and Table 3, the Langmuir isotherm is more suitable. This isotherm offers a monolayer adsorption on the proposed adsorbent [9].
Adsorption thermodynamic study
In an attempt to observe the effect of the temperature on the adsorption capacity and to determine the thermodynamic parameters, a number of experiments were performed with a concentration of 120 mg/L of lead ions at different temperatures using 0.020 g of the adsorbent at pH = 4.8. It was realized that with increase in the temperature, the amount of adsorbed metal ion increased. This effect can be attributed to the increasing mobility of lead ions with rising temperature. Also this result means that the adsorption process is endothermic. The thermodynamic parameters such as entropy (ΔS°), enthalpy (ΔH°), and Gibb's free energy (ΔG°) for the sorption of the considered ions on the prepared adsorbent were determined according to Eqs. (5)–(7):
In the abovementioned equations, Kd is the distribution coefficient for adsorption, R is the universal gas constant (0.008314 kJ/mol K),\( \Delta S^{^\circ }\) is the standard entropy (kJ/mol K), \(\Delta H^{^\circ }\) is the standard enthalpy (skJ/mol), \(\Delta G^{^\circ }\) is the standard free energy (kJ/mol), cad is the uptaked amount of ions, and T is the temperature of the solution (K) [32]. The ΔH° and ΔS° parameters were computed using the slope and intercept of the graph of ln Kd versus 1/T, respectively. The values for these parameters are tabulated in Table 4. The positive values for ΔS° point out that there is an increase in the disorder or randomness at the solid/solution interface during the adsorption of ions onto the prepared adsorbent. The positive values for ΔH° reflect the endothermic nature of the adsorption process, and the reduction in the ΔG° values represents the feasibility of the adsorption while the temperature is raised. Similar results have been reported for the endothermic nature of the adsorption process in the removal of Cu(II) and Pb(II) ions onto potassium hydroxide treated pine cone powder [33].
Comparison between adsorption capacities of proposed adsorbent and some recently reported adsorbents
Table 5 displays a comparison between qmax of the proposed adsorbent and some other adsorbents reported for the adsorption of lead ions. With respect to the other adsorbents, CMPC shows a comparable or better adsorption performance. This result is due to the type and amount of the functional groups existing at the surface of the modified adsorbent [34].
Regeneration
From an economic viewpoint, a good absorbent should not only have the right absorption capacity but also should have a suitable regeneration [31]. Thus the regeneration experiments were performed using the HCl, HNO3, and EDTA-2Na solutions. In this procedure, 1.00 g of the adsorbent spent was added to 15 mL of 0.1 mol L−1 of the mentioned solvents, separately, and the resultant suspension was stirred for 1 h. Then the adsorbent was washed with distilled water for its neutralization. After that, 0.020 g of dried CMPC was applied for the removal of 120 mg L−1 of lead ions. The experimental data demonstrated that the efficiency of the proposed adsorbent decreased to 30% after three cycles of regeneration with HNO3 or HCl. However, the adsorption efficiency was 53% after three cycles of regeneration with EDTA-2Na. Thus EDTA-2Na was selected as the desired eluent solvent. Breaking the ester bonds is the most important reason for reducing the efficiency of the adsorbent in acidic environments [9].
Applicability for real water samples
The applicability of the proposed adsorbent was investigated for the removal of lead ions from the samples of tap water (Shahrood and Jajarm, Iran) by the standard addition technique. The results obtained showed that the removal percentage was decreased up to 82% (Fig. 12). This is due to the competition between the different ions present in the solution for the occupancy of the active sites. These results show that the adsorbent has an outstanding potential for removing lead ions from the real water samples.
Conclusions
Pine cone (PC) is a type of low-cost and locally accessible agricultural waste. In this research work, we demonstrated that treating PC with carboxylic groups is an effective method for the production of an effective and suitable adsorbent for removing lead ions from aqueous solutions.
A summary of the most important results obtained in this work is as follows:
-
The removal of lead ions from aqueous solutions increased by increasing the pH value, and the maximum removal occurred at the pH value of 4.8.
-
The adsorption of lead ions could reach an equilibrium at 25 min. The rapid adsorption of this pollutant can be ascribed by the numerous functional groups present on carboxylate-modified pine cone (CMPC).
-
The adsorption process followed a pseudo-second-order kinetics, signifying that a chemical adsorption took place.
-
Compared to the Freundlich isotherm, the Langmuir isotherm model can explain the behavior of the adsorption of lead ions with a higher accuracy. Therefore, the adsorption of lead ions seems to be a multi-layer adsorption on a heterogeneous surface.
-
At the optimum experimental conditions, qmax for adsorption of lead ions on CMPC was found to be 400.00 s, which was comparable or better than the values reported in the literature.
-
The thermodynamic factors such as ΔG° and ΔH° displayed that the adsorption of lead ions was spontaneous and endothermic.
-
The proposed adsorbent could be reused for at least three cycles for eliminating lead ions using EDTA-2Na.
References
A. Tian, J. Xiaojun, L. Qingyu, Novel adsorbents based upon carboxylic acid-modified Phyllostachys pubescens powder: preparation, characterization and application for adsorbing lead (II) from aqueous solution. Sep. Sci. Technol. 55(7), 1249 (2020)
M. Kaur, S. Kumari, P. Sharma, Removal of Pb (II) from aqueous solution using nanoadsorbent of Oryza sativa husk: Isotherm, kinetic and thermodynamic studies. Biotechnol. Rep. 25, e00410 (2020)
A. Adewuyi, F.V. Pereira, Underutilized Luffa cylindrica sponge: A local bio-adsorbent for the removal of Pb (II) pollutant from water system. Beni-Suef Univ. J. Basic Appl. Sci. 6(2), 118 (2017)
H. Khoshsang, A. Ghaffarinejad, Rapid removal of lead (II) ions from aqueous solutions by saffron flower waste as a green biosorbent. J. Environ. Chem. Engin. 6(5), 6021 (2018)
R.M. Vieira, P.B. Vilela, V.A. Becegato, A.T. Paulino, Chitosan-based hydrogel and chitosan/acid-activated montmorillonite composite hydrogel for the adsorption and removal of Pb2+ and Ni2+ ions accommodated in aqueous solutions. J. Environ. Chem. Engin. 6, 2713 (2018)
N. Bowman, D. Patel, A.X.U.W. Sanchez, A. Alsaffar, S.M. Tiquia-Arashiro, Lead absorption mechanisms in bacteria as strategies for lead bioremediation. Appl. Microbiol. Biotechnol. 102, 2391 (2018)
N. Chanthapon, S. Sarkar, P. Kidkhunthod, S. Padungthon, Lead removal by a reusable gel cation exchange resin containing nano-scale zero valent iron. J. Chem. Engin. 331, 545 (2018)
M. Ashrafi, G. Bagherian, M.A. Chamjangali, N. Goudarzi, A.H. Amin, Simultaneous removal of Pb2+ and methylene blue from aqueous solution by a new carboxylic acid functionalized walnut shell: Optimization by multivariate method. Mater. Res. Expresss 5, 065510 (2018)
M. Ashrafi, H. Borzuie, G. Bagherian, M.A. Chamjangali, H. Nikoofard, Artificial neural network and multiple linear regression for modeling sorption of Pb2+ ions from aqueous solutions onto modified walnut shell. Sep. Sci. Technol. 55, 222 (2020)
H.S. Altundoğan, A. Topdemir, M. Çakmak, N. Bahar, Hardness removal from waters by using citric acid modified pine cone. J. Taiwan Inst. Chem. Eng. 58, 219–225 (2016)
T.A.H. Nguyen, H.H. Ngo, W.S. Guo, J. Zhang, S. Liang, Q.Y. Yue, T.V. Nguyen, Applicability of agricultural waste and by-products for adsorptive removal of heavy metals from wastewater. Bioresour. Technol. 148, 574 (2013)
B. Chen, Y. Liu, S. Chen, X. Zhao, X. Meng, X. Pan, Magnetically recoverable cross-linked polyethylenimine as a novel adsorbent for removal of anionic dyes with different structures from aqueous solution. J. Taiwan Inst. Chem. Eng. 67, 191 (2016)
G.Z. Kyzas, P.I. Siafaka, E.G. Pavlidou, K.J. Chrissafis, D.N. Bikiaris, Synthesis and adsorption application of succinyl-grafted chitosan for the simultaneous removal of zinc and cationic dye from binary hazardous mixtures. Chem. Engin. J. 259, 438 (2015)
B.S. Ndazi, S. Karlsson, J.V. Tesha, C.W. Nyahumwa, Chemical and physical modifications of rice husks for use as composite panels. Compos. Part A Appl. Sci. Manuf. 38, 925 (2007)
S. Hokkanen, E. Repo, M. Sillanpää, Removal of heavy metals from aqueous solutions by succinic anhydride modified mercerized nanocellulose. Chem. Eng. J. 223, 40 (2013)
B.C.S. Ferreira, F.S. Teodoro, A.B. Mageste, L.F. Gil, R.P. de Freitas, L.V.A. Gurgel, Application of a new carboxylate-functionalized sugarcane bagasse for adsorptive removal of crystal violet from aqueous solution: kinetic, equilibrium and thermodynamic studies. Ind. Crops. Prod. 65, 521 (2015)
M. Ashrafi, G. Bagherian, M.A. Chamjangali, N. Goudarzi, Application of artificial neural network and random forest methods for modeling simultaneous adsorption of safranin-O and methyl violet dyes onto modified pine cone powder. Desal. Water. Treat. 109, 90 (2018)
F.Z. Arrakhiz, M. El Achaby, K. Benmoussa, R. Bouhfid, E.M. Essassi, A. Qaiss, Evaluation of mechanical and thermal properties of Pine cone fibers reinforced compatibilized polypropylene. Mater. Des. 40, 528 (2012)
D.A. Gopakumar, D. Pasquini, M.A. Henrique, L.C. de Morais, Y. Grohens, S. Thomas, Meldrum’s acid modified cellulose nanofiber-based polyvinylidene fluoride microfiltration membrane for dye water treatment and nanoparticle removal. ACS Sustain. Chem. Eng. 5, 2026 (2017)
A.E. Ofomaja, A. Pholosi, E.B. Naidoo, Kinetics and competitive modeling of cesium biosorption onto iron(III) hexacyanoferrate modified pine cone powder. Int. Biodeterior. 92, 71 (2014)
H. Parab, M. Sudersanan, Engineering a lignocellulosic biosorbent–coir pith for removal of cesium from aqueous solutions: equilibrium and kinetic studies. Water Res. 44, 854 (2010)
A. Chadlia, K. Mohamed, L. Najah, Preparation and characterization of new succinic anhydride grafted Posidonia for the removal of organic and inorganic pollutants. J. Hazard. Mater. 172, 1579 (2009)
C.F. Liu, R.C. Sun, M.H. Qin, A.P. Zhang, J.L. Ren, J. Ye, Z.N. Cao, Succinoylation of sugarcane bagasse under ultrasound irradiation. Bioresour. Technol. 99, 1465 (2008)
S. Tighadouini, S. Radi, M. Ferbinteanu, Y. Garcia, Highly Selective Removal of Pb (II) by a Pyridylpyrazole-β-ketoenol Receptor Covalently Bonded onto the Silica Surface. ACS Omega. 4, 3954 (2019)
M.R. Awual, Mesoporous composite material for efficient lead (II) detection and removal from aqueous media. J. Environ. Chem. Engin. 7, 103124 (2019)
M. Arshadi, M. Mehravar, M.J. Amiri, A.R. Faraji, Synthesis and adsorption characteristics of a heterogenized manganese nanoadsorbent towards methyl orange. J. Colloid Interface Sci. 440, 189 (2015)
M. Ashrafi, M. Chamjangali, G. Bagherian, N. Goudarzi, S. Kavian, Evaluation of nanosilica, extracted from stem sweep, as a new adsorbent for simultaneous removal of crystal violet and methylene blue from aqueous solutions. Desal. Water Treat. 88, 207–220 (2017)
R. Lafi, A. Hafiane, Removal of methyl orange (MO) from aqueous solution using cationic surfactants modified coffee waste (MCWs). J. Taiwan Inst. Chem. Eng. 58, 424 (2016)
K.V. Kumar, A. Kumaran, Removal of methylene blue by mango seed kernel powder. Biochem. Engi. J. 27, 83 (2005)
M. Ghaedi, S. Heidarpour, S.N. Kokhdan, R. Sahraie, A. Daneshfar, B. Brazesh, Comparison of silver and palladium nanoparticles loaded on activated carbon for efficient removal of methylene blue: kinetic and isotherm study of removal process. Powder Technol. 228, 18 (2012)
M. Ashrafi, M.A. Chamjangali, G. Bagherian, N. Goudarzi, Application of linear and non-linear methods for modeling removal efficiency of textile dyes from aqueous solutions using magnetic Fe3O4 impregnated onto walnut shell. Spectrochim Acta A Mol. Biomol. Spectrosc. 171, 268 (2017)
B.H. Hameed, Spent tea leaves: a new non-conventional and low-cost adsorbent for removal of basic dye from aqueous solutions. J. Hazard. Mater. 161, 753 (2009)
A.E. Ofomaja, E.B. Naidoo, S.J. Modise, Biosorption of copper (II) and lead (II) onto potassium hydroxide treated pine cone powder. J. Environ. Manage 91(8), 1674–1685 (2010)
A. Çelekli, H. Bozkurt, F. Geyik, Artificial neural network and genetic algorithms for modeling of removal of an azo dye on walnut husk. Desal. Water Treat. 57, 15580 (2016)
L.V.A. Gurgel, O.K. Junior, R.P. de Freitas Gil, L.F. Gil, Adsorption of Cu(II), Cd (II), and Pb(II) from aqueous single metal solutions by cellulose and mercerized cellulose chemically modified with succinic anhydride. Bioresour. Technol. 99, 3077 (2008)
M.J. Akbarzadeh, S. Hashemian, N. Mokhtarian, Study of Pb (II) removal ZIF@ NiTiO3 nanocomposite from aqueous solutions. J. Environ. Chem. Engin. 8, 103703 (2020)
V.L. Medeiros, L.G. de Araujo, D.R. Ratero, A.S. Paula, E.F. Molina, C. Jaeger, J.G. Nery, Synthesis and physicochemical characterization of a novel adsorbent based on yttrium silicate: a potential material for removal of lead and cadmium from aqueous media. J. Environ. Chem. Eng. (2020). https://doi.org/10.1016/j.jece.2020.103922
S. Biswas, H. Siddiqi, B.C. Meikap, T.K. Sen, M. Khiadani, Preparation and characterization of raw and inorganic acid-activated pine cone biochar and its application in the removal of aqueous-phase Pb2+ metal Ions by adsorption. Water Air Soil Pollut. 231(1), 3 (2020)
P.T. Huong, B.K. Lee, J. Kim, C.H. Lee, M.N. Chong, Acid activation pine cone waste at differences temperature and selective removal of Pb2+ ions in water. Process Saf. Environ. Prot. 100, 80–90 (2016)
Acknowledgement
The authors are thankful to the Shahrood University of Technology Research Council for the support of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Bagherian, G., Nemati, E., Arab Chamjangali, M. et al. Removal of lead ions from aqueous solutions using functionalized pine cone powder. J IRAN CHEM SOC 18, 2369–2379 (2021). https://doi.org/10.1007/s13738-021-02196-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-021-02196-x