Abstract
MPTMS-functionalized silica immobilized with biphenyl-2,2′-dioic acid, an efficient heterogeneous catalyst, was synthesized and characterized by various spectroscopic techniques such as FTIR, TGA, TEM and SEM. The catalytic activity of the synthesized catalyst has been demonstrated for the one-pot Knoevenagel/Michael condensation reaction of aromatic amines and 5,5-dimethyl-1,3-cyclohexanedione with various aldehydes. This method results in the synthesis of high-yielding acridine-1,8-dione derivatives and also provides simple and facile recyclability of the catalyst. The synthesized derivatives were characterized by IR, 1H-NMR, 13C-NMR and mass spectroscopy. Molecular structures of three acridine-1,8-dione derivatives were determined by single-crystal X-ray diffraction which determines that 3,3,6,6-tetramethyl-9,10-diphenyl-1,8-dioxodecahydroacridine crystallizes in the monoclinic with space group P2 1 /c, 3,3,6,6-tetramethyl-9-(4-chlorophenyl)-10-(4-methylphenyl)-1,8-dioxodecahydroacridine crystallizes in the monoclinic with space group P2 1 /n and 3,3,6,6-tetramethyl-9-(4-chlorophenyl)-10-(4-methoxyphenyl)-1,8-dioxodecahydroacridine crystallizes in the monoclinic with space group P2 1 /n.
Graphical Abstract
The one-pot tandem synthesis of acridine-1,8-dione derivatives was carried out successfully with good yields under the catalytic influence of MPTMS-modified silica immobilized with biphenyl-2,2′-dioic acid which has proved to be an efficient heterogeneous catalyst with good recyclable property.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Following the ideology of green chemistry, the development of heterogenized mesoporous material-supported catalysts has engrossed immense attention due to the combining advantage of both homogeneous and heterogeneous catalytic systems [1]. Nowadays, different types of mesoporous materials are known and extensively employed as catalysts for diverse organic transformations. Among various mesoporous materials, silica especially immobilized siliceous surface with various organic functional groups has received great attention [2]. In the literature, several silica-functionalized acidic and basic derivatives, viz silica/sulfuric acid/NaNO2 [3, 4], sulfuric acid([3-(3-silicapropyl)sulfanyl]propyl)ester [5, 6], solid silica-based sulfonic acid [7], SBA-15-supported sulfonic acid [8], silica-bonded S-sulfonic acid (SBSSA) [9, 10], silica-bonded N-propyl sulfamic acid (SBNPSA) [11], silica-bonded propyl-diethylene-triamine-N-sulfamic acid (SPDTSA) [12], silica-bonded propylpiperazine-N-sulfamic acid (SBPPSA) [13], silica-bonded N-propylpiperazine sodium n-propionate (SBPPSP) [14, 15], silica-bound N-propyl triethylenetetramine sulfamic acid (SBPTETSA) [16], Pd nanoparticles on silica-bonded N-propylpiperazine sodium N-propionate (SBPPSP) [17], were reported and shown good activity toward various organic transformations. For the introduction of desired organic functional groups into the mesoporous silica materials for their surface modifications, different techniques have been intensely developed [18]. The silylization process is one of the techniques in which silica surface interacts with the silane reagent and forms covalent bond with the surface [19, 20] by the partial conversion of surface silanols to new organofunctional surface groups [21] that in turn act as precursors for further immobilization of organic molecules. Because of these modifications, the immobilized organofunctionalized mesoporous silica materials exhibit properties like concurrent tandem catalysis. Based on the above facts, in this communication, we introduce a facile methodology for the preparation of highly organofunctionalized mesoporous silica material, i.e., MPTMS-functionalized silica immobilized with biphenyl-2,2′-dioic acid as an efficient heterogenous catalyst which is employed in the one-pot tandem reaction for synthesis of acridine-1,8-dione derivatives.
Acridine-1,8-dione/1,8-dioxodecahydroacridine derivatives are an important class of heterocyclic compounds containing a 1,4-dihydropyridine (DHP) parent nucleus [22] or are polyfunctionalized 1,4-DHP derivatives. These compounds possess a wide range of pharmaceuticals activities [23] including antimicrobial [24,25,26], antibacterial [27], antileishmanial activities [28], fungicidal [29, 30], antimalarial [31, 32], antitumor [33] and antimulti-drug resistant [34]. Multi-drug resistance (MDR) is a serious obstacle in the management of breast cancer. Therefore, acridine diones having MDR modifier potency used to overcome MDR problems [35]. These derivatives were also used in the treatment of cardiovascular diseases such as angina pectoris and hypertension, DNA-binding [36] and DNA photo-damaging ability [37]. They also exhibit properties such as positive inotropic effects which promote the entry of calcium to the intracellular space [38] and also act as potassium channel blockers [39]. In addition, acridine diones exhibit important properties such as high fluorescence efficiency allowing them to be used as laser dyes [40, 41]. It is also reported that these polyfunctionalized DHPs are useful in Alzheimer’ disease due to their platelet antiaggregatory activity [42]. They have similarities in structure to the biologically important compounds NADH and NADPH [43]. These findings gave activations to many scientists to prepare some new acridine derivatives by different methodologies.
Many protocols have already been reported for the synthesis of acridine-1,8-dione derivatives that are Bronsted acidic imidazolium salts containing perfluoroalkyl tails [44], [CMIM][HSO4] [22], indium(III) triflate [23], Amberlyst-15 [45], p-dodecylbenzenesulfonic acid [46], triethylbenzylammonium chloride [47], CuSO4.5H2O [48], [B(C6F5)3] [49], Nano TiO2 [50, 51], refluxing water [38], Cu-doped ZnO [52], SiO2-Pr-SO3H [53], water-mediated oxalic acid [42], TPA NPs/PAA [54], etc.
Most of the methods described above suffer from one or more limitations such as longer reaction time, use of stoichiometric amount of expensive catalysts and hazardous solvents. Further, these methods exemplify limited substrate scope. Thus, an effective, convenient and fast one-pot tandem condensation–cyclization method is required for the regioselective synthesis of acridone-1,8-derivatives. Therefore, we would like to report an effective method for their synthesis using the reaction of aldehydes, 5,5-dimethyl-1,3-cyclohexanedione and aromatic amines. This multi-component reaction was accomplished in the presence of MPTMS-functionalized silica immobilized with biphenyl-2,2′-dioic acid as an efficient heterogenous catalyst as shown in Scheme 1.
Experimental section
Materials and instrumentations
All the chemicals and solvents were purchased from Sigma-Aldrich and Merck and were used without further purification. Silica gel was purchased from ACROSS Organics. All melting points of the products were taken on Perfit melting point apparatus and compared with those reported in the literature. TGA analysis of the catalyst was carried on Perkin-Elmer Simultaneous Thermal Analyzer STA 6000. SEM images of catalyst were recorded from JEOL Model JSM-6390LV microscope, and TEM images were recorded from PHILIPS CM200 microscope (STIC Cochin University, Kerala). FTIR spectra of the catalyst and synthesized products were recorded on SHIMADZU prestige spectrophotometer, and 1H-NMR and 13C-NMR spectra of the products were recorded in DMSO-d 6 on Bruker Avance III 400 MHz spectrometer using TMS as an internal standard (Department of Chemistry, University of Jammu, Jammu). The mass spectra were recorded on Esquire 3000 Bruker Daltonics spectrometer (ESI) (IIIM, Jammu). The good quality crystals of acridine-1,8-dione compounds were selected, and their single-crystal X-ray data were recorded on a CCD area-detector diffractometer (X’calibur system—Oxford diffraction make, UK) (Department of Physics and Electronics, University of Jammu, Jammu).
Preparation of heterogeneous catalyst: organofunctionalized silica immobilized with biphenyl-2,2′-dioic acid
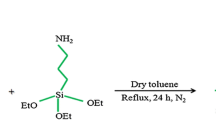
The preparation of organofunctionalized silica immobilized with biphenyl-2,2′-dioic acid catalyst was achieved initially by surface modification of activated silica with 3-mercaptopropyl trimethoxysilane (MPTMS). The procedure for surface modification of activated silica by 3-mercaptopropyl trimethoxysilane (MPTMS) as silane coupling reagent was already reported in the literature [55], and this step results in the conversion of surface silanols to new organofunctional surface groups that act as precursors for further immobilization of organic molecules.
Finally, immobilization of MPTMS-functionalized silica (SiMPTMS) with biphenyl-2,2′-dioic acid
To a suspension of MPTMS-functionalized silica (SiMPTMS) (5 g) in DMF (50 ml) was added biphenyl-2,2′-dioic acid (0.5 g, 1 mol%, 2.06 mmol) and the resulting mixture was refluxed at 120 °C in an oil bath for 24 h. After completion of 24 h, the mixture was allowed to cool down at room temperature and filtered off using Buchner funnel. The residue was given continuous distilled water washing for 3–4 times. After washing, the residue was dried in an oven for 24 h, and finally, free flowing powder was obtained.
The complete preparation procedure is shown in Scheme 2.
One-pot regioselective synthesis of acridine-1,8-dione derivatives
A mixture of aldehyde (1 mmol), 5,5-dimethyl-1,3-cyclohexanedione (2 mmol) and aniline/toluidine/p-anisidine (1 mmol) was taken in a round-bottom flask (50 ml) containing solution of ethanol (2 ml) and water (0.4 ml). To this mixture, MPTMS-functionalized silica immobilized with biphenyl-2,2′-dioic acid (0.05 g) as a heterogeneous catalyst was added and the reaction mixture was allowed to refluxed at 80 °C in an oil bath for the appropriate time. On completion of the reaction (monitored by TLC), the reaction mixture was cooled to room temperature and diluted with EtOAc (20 mL). After dilution, the reaction mixture was filtered to separate the catalyst followed by washing of filtrate with distilled water and dried over anhydrous Na2SO4. Finally, the solvent was allowed to evaporate over water bath and crude product was obtained. The crude product was further recrystallized from ethanol at room temperature to isolate pure product.
Result and discussions
Catalyst characterization
FTIR
The proposed configuration of MPTMS-modified silica immobilized with biphenyl-2,2′-dioic acid catalyst was confirmed and characterized by FTIR technique and also compared the obtained spectrum with FTIR spectrum of MPTMS-modified silica as shown in Fig. 1. The main absorption frequencies are mentioned in Table 1. As proposed by the preparation method of the catalyst, immobilization of MPTMS-modified silica with biphenyl-2,2′-dioic acid takes place which leads to the formation of thioester linkage between –SH of MPTMS-modified silica and –OH of biphenyl-2,2′-dioic acid. The presence of thioester linkage (–S–C=O) in the immobilized catalyst was confirmed from the presence of absorption frequency at 1690.68 cm−1. This linkage formation was further supported by the fact that MPTMS-modified silica shows the characteristic absorption frequency of thiol (-SH) group at 2574.97 cm−1 which was completely absent in the immobilized catalyst. The presence of absorption peaks at 1443.48 and 1370.48 cm−1 in the FTIR spectrum of immobilized catalyst concludes that only one of the two –COOH groups present in the biphenyl-2,2′-dioic acid undergoes the formation of thioester linkage with –SH of MPTMS-modified silica. This conclusion was also supported by the phenomenon of steric hindrance which opposes the involvement of both –COOH groups of acid in thioester linkage formation and finally leads to the stability of catalyst.
Thermal gravimetric analysis
The thermal stability of MPS-modified silica immobilized with biphenyl-2,2′-dioic acid was determined by thermal gravimetric analysis (Fig. 2). The TGA curve obtained for the synthesized catalyst showed the first weight loss of 2.397% up to 111.70 °C which may be ascribed to the removal of surface-adsorbed solvent and gases. The second weight loss of 1.524% up to 239.04 °C was probably due to the loss of structural water within modified silica support. The third weight loss of 3.505% up to 431.88 °C may be considered due to the decomposition of organic functionalities like benzene rings of biphenyl-2,2′-dioic acid and also CO and CO2 groups from the catalyst. The fourth weight loss of 4.695% and more up to 800 °C for the catalyst may probably attributed to thermal decomposition of MPS-modified silica chains. This thermal analysis of the synthesized catalyst indicates that it is stable up to 250 °C, and hence, it is safe to carry out the organic transformations at 80 °C. The calculation of weight loss is shown in Table 2.
TEM analysis of MPTMS-modified silica immobilized with biphenyl-2,2′-dioic acid
The TEM micrographs and the corresponding SAED (selected area electron diffraction) pattern were also used to further examine the particle size, morphology and crystallinity of the synthesized heterogeneous catalyst. The TEM micrographs indicated that the biphenyl-2,2′-dioic acid was uniformly distributed onto the surface of mercaptopropyl trimethoxysilane-modified silica and the magnified image showed that the catalyst was in shape of nanorods with size in the range of 3.56–5.99 nm. Further, the SAED pattern displays an arrangement of rings containing spots, proposing that the nanorods were highly crystalline in nature (see inset of Fig. 3a). The TEM micrographs and the SAED pattern are shown in Fig. 3.
TEM micrographs a 50 nm; b 100 nm and SAED pattern (inset of Fig. 3a)
SEM analysis of MPTMS-modified silica immobilized with biphenyl-2,2′-dioic acid
The nanostructure and morphology of the immobilized MPTMS-modified silica with biphenyl-2,2′-dioic acid were also studied using a scanning electron microscope (SEM) at different magnifications which also proved the presence of nanorod-like structures of the catalyst as indicated in Fig. 4.
Optimization of reaction conditions
To obtain optimized conditions for the designed protocol, p-chlorobenzaldehyde (1 mmol), 5,5-dimethyl-1,3-cyclohexanedione (2 mmol) and aniline/toluidine/p-anisidine (1 mmol) were selected as model substrates to form N-substituted acridine-1,8-dione derivative. Preliminarily, the catalytic potential of various solid acid catalysts has been assessed for the one-pot tandem condensation–cyclization reaction among the model substrates and it has been found that MPTMS-functionalized silica immobilized with biphenyl-2,2′-dioic acid, exhibiting concurrent tandem catalysis, was proved to be an efficient catalyst for the synthesis of desired acridine-1,8-dione derivatives in 70–95% yields. The model reactions were also performed using 0.05/0.1/0.2 mg amount of catalysts in the presence of different types of solvents, refluxing at different temperatures like 80/100 °C for obtaining high-yield products under proper reaction conditions. The complete test reaction conditions and final results are shown in Table 3.
To study the generality of the newly designed protocol, various types of aldehydes and also different aromatic amines were selected which provided good to excellent yields of products. The results are summarized in Table 4.
From the above table, the structure–activity relationship has been drawn which inferred that aromatic aldehydes substituted with electron-withdrawing groups and aromatic amines with electron-donating groups undergo faster reaction to generate high-yield acridine-1,8-dione derivatives as compared to aldehydes substituted with electron-donating groups as well as hetero-aromatic aldehydes and unsubstituted aromatic amines or substituted with electron-withdrawing groups. The catalytic effect of the synthesized catalyst was also studied by carrying out the reaction of model substrates in the absence of catalyst, and it was observed that without catalyst, reaction did not take place even after 120 min of refluxing at 80 °C.
Different types of acridine-1,8-dione derivatives have been regioselectively synthesized in good yields by the above-mentioned protocol as shown in Table 4. Among these synthesized derivatives, three derivatives (Entry 1, 16 and 26, Table 4) were grown as good crystals and their structures were confirmed by single-crystal X-ray analysis, IR, 1H-NMR, 13C-NMR and mass spectroscopy. These crystals were obtained by very slow evaporation of their ethanol solution at room temperature. The structures of the three derivatives were determined by single-crystal X-ray diffraction analysis. The molecular structures with atomic labelings of derivatives 1, 16 and 26 (Table 4) are shown below (Figs. 5, 6 and 7).
Molecular structure (40% probability) of the [3,3,6,6-tetramethyl-9,10-diphenyl-1,8-dioxodecahydroacridine] (Entry 1, Table 4). H atoms are shown as small spheres of arbitrary radii
Molecular structure (40% probability) of the [3,3,6,6-tetramethyl-9-(4-chlorophenyl)-10-(4-methylphenyl)-1,8-dioxodecahydroacridine] (Entry 16, Table 4). H atoms are shown as small spheres of arbitrary radii
Molecular structure (40% probability) of the [3,3,6,6-tetramethyl-9-(4-chlorophenyl)-10-(4-methoxyphenyl)-1,8-dioxodecahydroacridine] (Entry 26, Table 4). H atoms are shown as small spheres of arbitrary radii
X-ray intensity data of these derivatives were collected on a CCD area-detector diffractometer (X’calibur system—Oxford diffraction make, UK) equipped with graphite-monochromated MoKα radiation (λ = 0.71073 Å) at room temperature. The crystal used for data collection was of dimensions 0.30 × 0.20 × 0.20 mm. The data were corrected for Lorentz and polarization factors. The structures were solved by direct methods using SHELXS97 [58]. All non-hydrogen atoms of the molecule were located in the best E-map. Full-matrix least-squares refinement was carried out using SHELXL97. Atomic scattering factors were taken from International Tables for X-ray Crystallography (1992, Vol. C, Tables 4.2.6.8 and 6.1.1.4). Molecular drawings were obtained using DIAMOND version 2.1 [59]. The crystallographic data and details of the data collection and structure solution and refinement are listed in Table 5.
Catalysts recyclability, heterogeneity and stability
In order to test the recyclability and stability of catalyst regarding green chemistry aspect, the catalyst was separated from the reaction mixture, washed with ethyl acetate and dried in an oven for 3 h. After drying, catalyst was ready to be reused again and again by following the same process of separation from the reaction mixture in case of the reaction among 4-chlorobenzaldehyde, 5,5-dimethyl-1,3-cyclohexanedione and aniline/toluidine/p-anisidine (Entry 5, 16 and 26, Table 4). After examining the six runs, catalyst did not show any significant drop in its catalytic activity or yield of the products. The results are shown in Fig. 8.
Recyclability graph of MPTMS-modified silica immobilized with biphenyl-2,2′-dioic acid catalyst in case of derivatives 5, 16 and 26 (Table 4)
For examining stability of the catalyst, the same reaction was performed with the dried catalyst which has been exposed to the atmosphere conditions for 5 days and showed similar results. This may conclude that the catalyst is quite stable even toward atmospheric conditions which did not deteriorate its activity.
The hot-filtration test has also been performed for testing the heterogeneity of the catalyst using 4-chlorobenzaldehyde, 5,5-dimethyl-1,3-cyclohexanedione and aniline as test substrates which finally concluded that there is no leaching of organic acid from the catalyst.
Mechanism
The role of MPTMS-modified silica immobilized with biphenyl-2,2′-dioic acid as an efficient and recyclable heterogeneous catalyst for the synthesis of acridine-1,8-dione derivatives can be understood by the mechanism proposed in Scheme 3. From the literature, it is clear that the synthesis of desired product involves Knoevenagel (path a) as well as Michael (path b) condensation reactions among aldehyde, dimedone and amine. The reaction starts immediately after the activation of reactants by the catalyst and leads to the formation of intermediates (1) and (2) which are further activated by the catalyst and engaged them to undergo cyclization process to form intermediate (3). Finally, loss of water takes place which leads to the synthesis of desired product.
Conclusion
The objective of this work is to introduce an effective and simple regioselective one-pot tandem Knoevenagel/Michael condensation–cyclization reaction for the synthesis of various types of acridine-1,8-dione derivatives under the catalytic influence of newly synthesized heterogeneous catalyst, MPTMS-modified silica immobilized with biphenyl-2,2′-dioic acid. The synthesized catalyst and products were characterized by various spectroscopy techniques. Molecular structures of three products have also been determined by single-crystal X-ray crystallography. Therefore, it has been concluded that the developed protocol was versatile in terms of the substrate scope in case of aldehydes and amines. A mechanistic insight also clearly revealed the influence of electronic and steric factors associated with aldehydes and amines and involvement of catalyst that finally led to the synthesis of products in less time but with good yields. Moreover, catalyst offers advantages like easy preparation and handling, recyclability and reusability which fulfills the triple bottom-line philosophy of green chemistry [60].
Supplementary information
The supplementary information includes spectral data of few synthesized products with their NMR and mass spectral figures. Crystallographic data (CIF) files of three derivatives are also provided as supporting information.
References
S. Ray, M. Ray, A. Bhaumik, A. Dutta, C. Mukhopadhyay, Green Chem. 15, 1910 (2013)
P.K. Jal, S. Patel, B.K. Mishra, Talanta 62, 1005 (2004)
M.A. Zolfigol, Tetrahedron 57, 9509 (2001)
K. Niknam, B. Karami, M.A. Zolfigol, Catal. Commun. 8, 1427 (2007)
K. Niknam, D. Saberi, Appl. Catal. A 366, 220 (2009)
S. Tayebi, M. Baghernejad, D. Saberi, K. Niknam, Chin. J. Catal. 32, 1477 (2011)
B. Karimi, M. Khalkhali, J. Mol. Catal. A Chem. 232, 113 (2005)
B. Karimi, D. Zareyee, J. Mater. Chem. 19, 8665 (2009)
K. Niknam, D. Saberi, M.N. Sefat, Tetrahedron Lett. 51, 2959 (2010)
K. Niknam, F. Panahi, D. Saberi, M. Mohagheghnejad, J. Heterocycl. Chem. 47, 292 (2010)
F. Rashedian, D. Saberi, K. Niknam, J. Chin. Chem. Soc. 57, 998 (2010)
M.N. Sefat, A. Deris, K. Niknam, Chin. J. Chem. 29, 2361 (2011)
K. Niknam, A. Deris, F. Naeimi, F. Majleci, Tetrahedron Lett. 52, 4642 (2011)
K. Niknam, A. Jamali, Chin. J. Catal. 33, 1840 (2012)
K. Niknam, N. Borazjani, R. Rashidian, A. Jamali, Chin. J. Catal. 34, 2245 (2013)
K. Niknam, A. Jamali, M. Tajaddod, A. Deris, Chin. J. Catal. 33, 1312 (2012)
K. Niknam, A. Deris, F. Panahi, M.R.H. Nezhad, J. Iran. Chem. Soc. 10, 1291 (2013)
P. Sreekanth, S.W. Kim, T. Hyeon, B.M. Kim, Adv. Synth. Catal. 345, 936 (2003)
G.D. Brykina, L.S. Krysina, V.M. Ivanov, Zh. Anal. Khim. 43, 1547 (1988)
R.K. Iler, The chemistry of silica (Wiley, New York, 1979)
U. Pyell, G. Stork, Fresenius J. Anal. Chem. 343, 576 (1992)
D. Kour, D.R. Patil, M.B. Deshmukh, R. Kant, Eur. Chem. Bull. 3, 552 (2014)
Q.H. To, Y.R. Lee, S.H. Kim, Bull. Korean Chem. Soc. 33, 1170 (2012)
Y.M. Shchekotikhin, T.G. Nikolaeva, G.M. Shub, A.P. Krivenko, Pharmaceut. Chem. J. 35, 206 (2001)
N. Pyrko, Russ. J. Org. Chem. 44, 1215 (2008)
M. Shaikh, S.G. Konda, A.V. Mehare, G.G. Mandawad, S.S. Chobe, B.S. Dawan, Der Pharma Chemica 2, 25 (2010)
K. Palani, D. Thirumalai, P. Ambalavanan, M.N. Ponnuswamy, V.T.J. Ramakrishnan, Chem. Crystallogr. 35, 751 (2005)
D. Carole, D. Michel, C. Julien, D. Florence, N.J. Anna, D. Severine, T. Gerard, G. Pierre, Bioorg. Med. Chem. 13, 5560 (2005)
M. Wainwright, J. Antimicrob. Chemother. 47, 1 (2001)
C. Srivastava, Nizamuddin. Indian J. Heterocycl. Chem. 13, 261 (2004)
M. Kidwai, D. Bhatnagar, Tetrahedron Lett. 51, 2700 (2010)
O.A. Abd-Allah, A.A. Abdelhamid, S.K. Mohamed, Med. Chem. S. 2, 2161 (2015)
S. Tu, X. Zhang, F. Shi, T. Li, Q. Wang, X. Zhu, J. Zhang, J. Xu, J. Heterocycl. Chem. 42, 1155 (2005)
S. Gallo, S. Atifi, A. Mohamoud, C.S. Rouvier, K. Wolfart, J. Molnar, J. Barbe, Eur. J. Med. Chem. 38, 19 (2003)
S.A. Moallem, N. Dehghani, S. Mehri, M. Shahsavand, F.Hadizadeh Alibolandi, Res. Pharm. Sci. 10, 214 (2015)
K. Venkatesan, S.S. Pujari, K.V. Srinivasan, Synth. Commun. 39, 228 (2009)
P. Yang, Q. Yang, X. Qian, L. Tong, X. Li, J. Photochem. Photobiol. B:Biol. 84, 221 (2006)
J.J. Xia, K.H. Zhang, Molecules 17, 5339 (2012)
M.G. Gunduz, A.E. Dogan, R. Simsek, K. Erol, C. Safak, Med. Chem. Res. 18, 317 (2009)
S. Balalaie, F. Chadegani, F. Darviche, H.R. Bijanzadeh, Chin. J. Chem. 27, 1953 (2009)
L.B. Li, S.J. Ji, Y. Liu, Chin. J. Chem. 26, 979 (2008)
J.N. Sangshetti, P.P. Dharmadhikari, R.S. Chouthe, B. Fatema, Arab. J. Chem. (2012). doi:10.1016/j.arabjc.2012.06.005
M. Kaya, Y. Yildirir, G.Y. Celik, Med. Chem. Res. 20(293), 293 (2011)
W. Shen, L.M. Wang, H. Tian, J. Tang, J.J. Yu, J. Fluorine Chem. 130, 522 (2009)
B. Das, P. Thirupathi, I. Mahender, V.S. Reddy, Y.K. Rao, J. Mol. Catal. A Chem. 247, 233 (2006)
T.S. Jin, J.S. Zhang, T.T. Guo, A.Q. Wang, T.S. Li, Synthesis 12, 2001 (2004)
X.S. Wang, M.M. Zhang, Z.S. Zeng, D.Q. Shi, S.J. Tu, X.Y. Wei, Z.M. Zong, ARKIVOC (ii), 117 (2006)
M.A. Pasha, R.R. Khan, R.K.B, Can. Chem. Trans. 4, 90 (2016)
S. Chandrasekhar, Y.S. Rao, L. Sreelakshmi, B. Mahipal, C.R. Reddy, Synthesis 11, 1737 (2008)
S. Rahmani, A. Amoozadeh, J. Nano Struct. 4, 91 (2014)
A. Khazaei, R.H. Zare, Z. Mohammadi, V. Khakyzadeh, J. Afsar, J. Chin. Chem. Soc. 63, 165 (2016)
H. Alinezhad, S.M. Tavakkoli, Sci. World J. 2013, 1 (2013)
G.M. Ziarani, A. Badiei, M.H.S. Mousavi, Arab. J. Chem. 7, 335 (2014)
M.N. Esfahani, Z. Rafiee, H. Kashi, J. Iran. Chem. Soc. (2016). doi. 10.1007/s13738-016-0860-8
P. Sharma, M. Gupta, Green Chem. 17, 1100 (2015)
Y.B. Shen, G.W. Wang, ARKIVOC (xvi), 1 (2008)
D.Q. Shi, S.N. Ni, F. Yang, J.W. Shi, G.L. Dou, X.Y. Li, X.S. Wang, J. Heterocycl. Chem. 45, 653 (2008)
G.M. Sheldrick, Acta Cryst. A64, 112 (2008)
K. Brandenburg, Diamond. Version 2.1. Crystal Impact GbR, Bonn, Germany, (1998)
D. Kumar, M. Sonawane, B. Pujala, V.K. Jain, S. Bhagat, A.K. Chakraborti, Green Chem. 15, 2872 (2013)
Acknowledgements
We are grateful to Head, Department of Physics and Electronics, University of Jammu, for recording single-crystal X-ray data of products and also thankful to Department of Chemistry, University of Jammu, for providing necessary facilities for accomplishing the work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vaid, R., Gupta, M. & Gupta, V.K. Immobilization of organofunctionalized silica (SiMPTMS) with biphenyl-2,2′-dioic acid and investigation of its catalytic property for one-pot tandem synthesis of acridine-1,8-dione derivatives. J IRAN CHEM SOC 14, 2199–2210 (2017). https://doi.org/10.1007/s13738-017-1156-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-017-1156-3