Abstract
An efficient, simple, ecofriendly and cost-effective method has been developed for the synthesis of benzimidazole/benzothiazole derivatives by a two-component reaction, involving 1,2-diamino benzene/2-amino thiophenol and substituted aromatic aldehydes using recyclable nano-Fe2O3 catalyst (10 mol%) in water afforded with excellent yields (75–85%). The most important feature of this protocol is short reaction times, high yields, aqueous reaction medium, efficient recycling and high stability of the catalyst.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanochemistry is an emerging research field in modern science. The nanoscale catalysts can provide higher surface areas and lower coordinating sites, which are responsible for the higher catalytic activity [1]. In recent years, magnetic nanoparticles (MNPs) have gained attention as a useful group of heterogeneous catalysts and in view of their recovery. Magnetic nanoparticles provide a convenient method for the separation of magnetized species from the reaction mixture with an external magnet because it is simpler and more efficient than conventional separation i.e filtration or centrifugation [2, 3]. Among them, iron oxide nanoparticles (Fe2O3 NPS) have gained attention toward various organic transformations [4, 5].
Benzimidazole/benzothiazole moieties are important scaffolds in pharmaceutical applications, and these moieties appear in many drugs encompassing a broad range of activities (Fig. 1). Benzimidazole/benzothiazole derivatives were associated with a wide variety of medicinal, biological activities such as antifungal, antiviral, antibacterial, anticancer, anti-inflammatory, antiulcer, antihypertensive, antihistaminic, anticonvulsant, and antiparkinsonian activities [6–11]. They are also widely used in organic synthesis as intermediates. In particular, benzimidazoles are useful in controlling the diseases such as hypertension [12], ischemia–reperfusion injury [13], as well as obesity [14].
Several methodologies have been reported for the synthesis of benzimidazole/benzothiazoles. Mirkhani et al. [15] have developed the synthesis of 2-imidazolines and bis-imidazolines by using starting materials ethylenediamine and nitriles in the presence of sulfur under ultrasonic irradiation. The synthesis of benzimidazoles was reported by Lin and co-workers [16] taking starting materials phenylenediamines and aldehydes using molecular iodine. Hornberger et al. [17] described one-pot synthesis of disubstituted benzimidazoles from 2-nitroanilines with palladium charcoal as a catalyst in the presence of trimethyl orthoformate and catalytic pyridinium p-toluenesulfonate (PPTS) at room temperature. Das et al. [12] reported the synthesis of benzimidazoles from 1,2-phenylenediamine, with aldehydes by using (bromodimethyl)sulfonium bromide at room temperature. Recently Ramesh et al. [18] reported the aqueous phase synthesis of benzimidazoles/benzothiazoles using recyclable β-cyclodextrin.
However, the above-mentioned methods have been associated with different draw backs such as the use of hazardous organic solvents, strongly acidic conditions, expensive, moisture-sensitive catalysts, or tedious workup conditions as well as low yields. In continuation of our earlier efforts of novel environmentally benign methodologies using magnetically recyclable iron oxide nanoparticles [19, 20]. Herein, we report an efficient one-pot protocol for the synthesis of benzimidazole/benzothiazole derivatives by a two-component reaction, involving 1,2-diamino benzene/2-amino thiophenol and substituted aromatic aldehydes for the first time promoted by recyclable iron oxide nanoparticles in aqueous medium (Scheme 1).
Experimental section
Instruments and reagents
All chemicals, reagents, and the catalyst were obtained from Aldrich (Sigma-Aldrich, Saint Louis, MO, USA) or Spectrochem Pvt. Ltd. (Mumbai, India) and were used without further purification. The reactions were monitored by thin-layer chromatography (TLC) using Merck silica gel glass plate (60 F254, 0.25 mm), and TLC plates were visualized by UV light or I2 stain. Column chromatography was carried out with Merck 60–120 sized mesh silica gel using ethyl acetate and hexane as eluent. All products were characterized by their NMR and MS spectra. The 1H NMR (300 MHz) spectra and 13C NMR (75 MHz) spectra were recorded on Bruker Avance 300 nuclear magnetic resonance spectrometer taking the compounds in CDCl3 using TMS as an internal standard. The chemical shifts (δ) were reported in parts per million (ppm) downfield from TMS and coupling constants nJ values were expressed in Hz. Melting point was measured on Electrothermal 9100 Melting point apparatus. Surface morphology was recorded on Hitachi S-3000N scanning electron microscope. X-ray diffraction (XRD) pattern was obtained on a Bruker D8 Advance X-ray powder diffractometer.
General experimental procedure for the synthesis of benzimidazoles/benzothiazoles using nano-Fe2O3
The aldehyde (1.0 mmol) and 1,2-diamino benzene/2-amino thiophenol (1.0 mmol) were added to a suspension of nano-Fe2O3 (10 mol%) in water 3 mL. The reaction mixture was heated to reflux and monitored by TLC until total conversion of the starting materials. After completion of the reaction, the catalyst was separated with the aid of a magnet. The separated catalyst was washed several times with methanol, dried under vacuum. The aqueous layer was extracted with ethyl acetate (3 × 10 mL).The combined organic layers were washed with water, saturated brine solution, and dried over anhydrous Na2SO4. The combined organic layers were evaporated under reduced pressure, and the resulting crude product was purified by column chromatography using ethyl acetate and hexane (1:9) as an eluent. The identity and purity of the products were confirmed by 1H, 13C NMR, and mass spectra.
2-Phenylbenzo[d]thiazole (Table 2, Entry 1)
1H NMR (300 MHz, CDCl3) δ = 7.42–7.36 (m, 2H, H-Ar) 7.52–7.47 (m, 3H, H-Ar), 7.90 (d, 1H, J = 7.9 Hz, H-Ar), 8.11–8.07 (m, 3H, H-Ar),; 13C NMR (75 MHz, CDCl3) δ = 121.5, 123.1, 125.1, 126.2, 127.5, 128.9, 130.9; MS(ESI): m/z = 212 [M + H]+.
2-(4-Fluorophenyl)benzo[d]thiazole (Table 2, Entry 2)
1H NMR (300 MHz, CDCl3) δ = 7.18 (t, 2H, J = 7.0 Hz, H-Ar), 7.49 (t, 1H, J = 7.1 Hz, H-Ar), 7.89 (d, 1H, J = 7.6 Hz, H-Ar), 8.09–8.05 (m, 4H, H-Ar); 13C NMR (75 MHz, CDCl3) δ = 115.9, 116.1, 121.5, 123.0, 125.1, 126.3, 129.3, 129.4, 134.9, 153.9, 166.6; MS(ESI): m/z = 230 [M + H]+.
2-(4-Bromophenyl)benzo[d]thiazole (Table 2, Entry 3)
1H NMR (300 MHz, CDCl3) δ = 7.43–7.38 (m, 1H, H-Ar), 7.53–7.48 (m, 1H, H-Ar), 7.65–7.61 (m, 2H, H-Ar), 7.91 (d, 1H, J = 7.9 Hz, H-Ar), 7.99–7.94 (m, 2H, H-Ar), 8.08 (d, 1H, J = 8.1 Hz, H-Ar),; 13C NMR (75 MHz, CDCl3) δ = 121.6, 123.2, 125.4, 126.4, 128.8, 132.1, 135.0, 154.0, 166.6; MS(ESI): m/z = 290 [M + 2]+.
2-(2-Bromophenyl)benzo[d]thiazole (Table 2, Entry 4)
1H NMR (300 MHz,CDCl3) δ = 7.56–7.30 (m, 4H, H-Ar), 7.73 (d, 1H, J = 7.9 Hz, H-Ar), 8.01–7.94 (m, 2H, H-Ar), 8.15 (d, 1H, J = 7.9 Hz, H-Ar); 13C NMR (75 MHz, CDCl3) δ = 121.3, 123.4, 125.4, 126.2, 127.4, 131.1, 132.0, 133.9, 152.5, 165.6; MS(ESI): m/z = 290 [M + 2]+.
2-(2-Methoxyphenyl)benzo[d]thiazole (Table 2, Entry 5)
1H NMR (300 MHz, CDCl3) δ = 4.06 (s, 3H, -OCH3), 7.38–7.35 (m, 2H, H-Ar), 7.49–7.44 (m, 2H, H-Ar), 7.50 (d, 1H, J = 1.2 Hz, H-Ar), 8.09 (d, 1H, J = 8.2 Hz, H-Ar), 8.52 (d, 1H, J = 1.6 Hz, H-Ar), 8.53 (d, 1H, J = 1.8 Hz); 13C NMR (75 MHz, CDCl3) δ = 111.5, 121.1, 122.7, 124.5, 125.8, 129.4, 131.7, 152.0, 157.1; MS(ESI): m/z = 242 [M + H]+.
2-(3,4,5-Trimethoxyphenyl)benzo[d]thiazole (Table 2, Entry 6)
1H NMR (300 MHz, CDCl3) δ = 3.99 (s, 9H, -OCH3) 7.13 (s, 1H, H-Ar), 7.33 (s, 1H, H-Ar), 7.51–7.48 (m, 2H, H-Ar), 7.89 (d, 1H, J = 7.6 Hz, H-Ar), 8.06 (d, 1H, J = 8.2 Hz, H-Ar); 13C NMR (75 MHz, CDCl3) δ = 56.2, 60.9, 104.6, 121.4, 122.9, 125.0, 126.3, 134.8, 153.4, 153.8; MS(ESI): m/z = 302 [M + H]+.
2-Phenyl-1H-benzo[d]imidazole (Table 2, Entry 7)
1H NMR (300 MHz, CDCl3) δ = 5.47 (s, 1H, –NH), 7.10 (d, 1H, J = 6.8 Hz, H-Ar), 7.29–7.27 (m, 2H, H-Ar), 7.49–7.44 (m, 2H, H-Ar), 7.68–7.64 (m, 3H, H-Ar), 8.07 (d, 1H, J = 6.2 Hz, H-Ar); 13C NMR (75 MHz, CDCl3) δ = 121.7, 126.3, 128.2, 129.2, 129.9, 151.5; MS(ESI): m/z = 195 [M + H]+.
2-(4-Chlorophenyl)-1H-benzo[d]imidazole (Table 2, Entry 8)
1H NMR (300 MHz, CDCl3) δ = 7.57 (d, 1H, J = 7.6 Hz, H-Ar), 7.41 (d, 2H, J = 7.6 Hz, H-Ar), 7.32–7.20 (m, 3H, H-Ar), 7.12 (d, 1H, J = 7.6 Hz, H-Ar), 7.01 (d, 1H, J = 7.6 Hz, H-Ar); 13C NMR (75 MHz, CDCl3) δ = 121.5, 123.1, 125.1, 126.2, 127.5, 128.9, 130.9, 154.4; MS(ESI): m/z = 229 [M + H]+.
2-(4-Nitrophenyl)-1H-benzo[d]imidazole (Table 2, Entry 9)
1H NMR (300 MHz, CDCl3) δ = 7.32–7.25 (m, 3H, H-Ar), 7.44–7.40 (m, 3H, H-Ar), 7.66–7.64 (m, 2H, H-Ar); 13C NMR (75 MHz, CDCl3) δ = 110.3, 114.6, 119.7, 122.6, 127.1, 128.1, 130.6, 136.0, 123.0, 153.9,158.5, 160.2; MS(ESI): m/z = 240 [M + H]+.
2-(p-Tolyl)-1H-benzo[d]imidazole (Table 2, Entry 10)
1H NMR (300 MHz, CDCl3) δ = 2.44 (s, 3H, –CH3), 3.13 (s, 1H, –NH), 7.32 (d, 4H, J = 8.1 Hz, H-Ar), 8.12 (d, 4H, J = 8.3 Hz, H-Ar); 13C NMR (75 MHz, CDCl3) δ = 21.0, 110.4, 120.5, 123.2, 125.7, 128.8, 129.4, 129.7, 132.7, 137.7, 140.3, 161.4; MS(ESI): m/z = 209 [M + H]+.
2-(4-Methoxyphenyl)-1H-benzo[d]imidazole (Table 2, Entry 11)
1H NMR (300 MHz, CDCl3) δ = 3.73 (s, 3H, -OCH3), 6.79 (d, 1H, J = 8.6 Hz, H-Ar), 6.98–6.93 (m, 3H, H-Ar), 7.65–7.46 (m, 3H, H-Ar), 8.11 (d, 1H, J = 8.6 Hz, H-Ar); 13C NMR (75 MHz, CDCl3) δ = 58.4,115.1, 119.0, 123.4, 132.9, 154.8, 164.1, 166.6; MS(ESI): m/z = 225 [M + H]+.
2-(2-Chlorophenyl)-1H-benzo[d]imidazole (Table 2, Entry 12)
1H NMR (300 MHz, CDCl3) δ = 4.92 (s, 1H, NH), 6.70 (d, 1H, J = 8.3 Hz, H-Ar), 7.25 (s, 1H, H-Ar), 7.47 (s, 2H, H-Ar), 7.93 (d, 3H, J = 5.8 Hz, H-Ar), 8.62 (s, 1H, H-Ar); 13C NMR (75 MHz, CDCl3) δ = 121.5, 123.1, 125.1, 126.2, 127.5, 128.9, 130.9, 158.2; MS(ESI): m/z = 229 [M + H]+.
2-(4-Ethoxyphenyl)-1H-benzo[d]imidazole (Table 2, Entry 13)
1H NMR (300 MHz, DMSO-d6) δ = 7.59–7.55 (m, 2H, H-Ar), 7.25–7.14 (m, 3H, H-Ar), 6.77 (d, 1H, J = 8.3 Hz, H-Ar), 6.92–6.88 (m, 2H, H-Ar), 4.08–3.94 (q, 2H, –CH2–), 1.43 (t, 3H, J = 7.5 Hz, –CH3); 13C NMR (75 MHz, DMSO-d6): δ = 63.3, 110.3, 114.6, 119.7, 122.6, 127.1, 128.1, 130.6, 136.0, 143.0, 153.9, 258.5, 160.2; MS (ESI): m/z = 239 [M + H]+.
2-(1H-Indol-3-yl)benzo[d]thiazole (Table 2, Entry 14)
1H NMR (300 MHz, CDCl3) δ = 7.48–7.27 (m, 5H, H-Ar), 7.87 (d, 1H, J = 7.9 Hz, H-Ar), 7.93 (d, 1H, J = 2.8 Hz, H-Ar), 8.02 (d, 1H, J = 8.1 Hz, H-Ar), 8.44 (d, 1H, J = 7.1 Hz, H-Ar), 8.94 (s, 1H. –NH); 13C NMR (75 MHz, CDCl3) δ = 111.7. 112.2, 120.9, 121.2, 121.7, 121.9, 123.3, 124.1, 124.8, 126.0, 126.4, 133.7, 153.6, 163.2; MS (ESI): m/z = 251 [M + H]+.
Results and discussion
Chemistry
A preliminary survey of reaction conditions was conducted with 2-amino thiophenol and benzaldehyde as a model reaction (Table 2, entry 1). In the first case, the impact of catalyst was tested (Table 1 ), and in the absence of catalyst, no formation of product was obtained after stirring for 24 h (entry 1). The effect of solvents was also studied, and it was observed that the reaction was effective in polar solvents, such as H2O, CH3OH, CH3CN, DMF, and DMSO, whereas trace amount of product was observed in toluene and THF (Table 1, entries 2–8). As indicated in Table 1, the reaction could be progressed very efficiently in H2O (entry 2). This solvent was chosen as a green solvent for the synthesis of benzimidazole/benzothiazole.
To further improve the yield, the same reaction was carried out in the presence of 5, 10, and 20 mol% of nano-Fe2O3 (Table 1, entries 2, 9, 10). From the obtained results shown in Table 1, it was clear that the yields depend on the amount of catalyst and the optimum amount of catalyst was 10 mol% (entry 2). The reaction (10 mol% nano-Fe2O3) in water stirring at room temperature yileded only trace amount of product even after prolonged reaction time (Table 1, entry 11). Thus, the optimized reaction conditions turned out to be using 10 mol% nano-Fe2O3 in water at 80 °C temperature for 2 h. With the optimized conditions defined, the scope of the iron oxide catalyzed synthesis of benzimidazole/benzothiazole derivatives was further expanded with a variety of 1,2-diamino benzene/2-amino thiophenol and substituted aromatic aldehydes. The results are summarized in Table 2. The aldehydes bearing either electron-withdrawing or electron-donating groups reacted satisfactorily to furnish the corresponding 2-substituted benzimidazole/benzothiazole.
Recyclability of nano-Fe2O3 catalyst
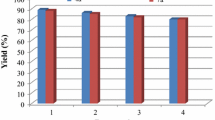
Important features of the nano-Fe2O3 catalysts are reusability, recyclability, and selectivity, which make them green catalysts, especially considering from an environmental point of view. In this regard, the life time as well as reusability of the catalyst is verified. The recyclability of nano-Fe2O3 was studied using 2-amino thiophenol and benzaldehyde as a model reaction. After completion of reaction, the reaction mixture was cooled to room temperature and the catalyst was recovered magnetically and washed several times with methanol, dried at 60 °C for 30 min. The recovered catalyst was reused directly in the next cycle with fresh reactants, under the same conditions, and it was reused for four times without any change in activity (Fig. 2). The powder XRD patterns of nano-Fe2O3 catalyst before and after the reaction revealed no substantial variation was observed in the powder XRD patterns of fresh and used catalyst, which was revealing the retention of the original structure of nano-Fe2O3 in the used catalyst (Fig. 3). Further, The SEM image of the catalyst taken after the fourth cycle of the reaction did not show any significant change in the morphology and the size of the catalyst, which indicates the retention of the catalytic activity after recycling (Fig. 4).
Conclusion
In summary, we have developed a simple and efficient protocol for the synthesis of benzimidazole/benzothiazole derivatives by a two-component reaction, involving 1,2-diamino benzene/2-amino thiophenol and substituted aromatic aldehydes catalyzed by recyclable nano-Fe2O3 in water without using any toxic solvent or co-catalyst. This simple and novel methodology will be useful to green chemistry with some advantage that the reaction excludes nontoxic, moisture-sensitive or hazardous catalysts and elevated reaction temperatures, longer reaction times, and the catalyst nano-Fe2O3 is economically viable, readily available, easily handling. Moreover, this nanocatalyst can be recycled and reused for four times without loss of catalytic activity.
References
P. Serp, K. Philippot (eds.), Nanomaterials in Catalysis (Wiley-VCH, Weinheim, 2013)
S. Shylesh, W.R. Thiel, Angew. Chem. Int. Ed. 49, 3428 (2010)
M. Shokouhimehr, J.E. Lee, S.I. Han, T. Hyeon, Chem. Commun. 49, 4779 (2013)
F. Shi, M.K. Tse, M.M. Pohl, A. Brückner, S. Zhang, M. Beller, Angew. Chem. Int. Ed. 46, 8866 (2007)
N. Anand, K.H.P. Reddy, T. Satyanarayana, K.S.R. Rao, D.R. Burri, Catal. Sci. Technol. 2, 570 (2012)
V. Zaharia, A. Ignat, N. Palibroda, B. Ngameni, V. Kuete, C.N. Fokunang, M.L. Moungang, B.T. Ngadjui, Eur. J. Med. Chem. 45, 5080 (2010)
J.S. Kim, B. Gatto, C. Yu, A. Liu, L.F. Liu, E.J. LaVoie, J. Med. Chem. 39, 992 (1996)
A. Kamal, M.N.A. Khan, K.S. Reddy, K. Rohini, Bioorg. Med. Chem. 15, 1004 (2007)
T. Roth, M.L. Morningstar, P.L. Boyer, S.H. Hughes, R.W. Buckheit Jr., C.J. Michejda, J. Med. Chem. 40, 4199 (1997)
F. Azam, B.A. El-Gnidi, I.A. Alkskas, M.A. Ahmed, J. Enzyme Inhib. Med. Chem. 25, 818 (2010)
D.I. Shah, M. Sharma, Y. Bansal, G. Bansal, M. Singh, Eur. J. Med. Chem. 43, 1808 (2008)
Y. Ogino, N. Ohtake, Y. Nagae, K. Matsuda, M. Moriya, M. Suga, M. Ishikawa, Y. Kanesaka, Ito J. Mitobe, T. Kanno, T.A. Ishiara, H. Iwaasa, T. Ohe, A. Kanatani, T. Fukami, Bioorg. Med. Chem. Lett. 18, 5010 (2008)
P. Ghosh, A. Mandal, Catal. Commun. 12, 744 (2011)
V. Mirkhani, M. Moghadam, S. Tangestaninejad, H. Kargar, Tetrahedron Lett. 47, 2129 (2006)
S. Lin, L. Yang, Tetrahedron Lett. 46, 4315 (2005)
K.R. Hornberger, G.M. Adjabeng, H.D. Dickson, R.G. Davis-Ward, Tetrahedron Lett. 47, 5359 (2006)
B. Das, H. Holla, Y. Srinivas, Tetrahedron Lett. 48, 61 (2007)
K. Ramesh, C. Rakhi, S.M. Padma, L.C.D. Nelson, RSC Adv. 5, 41716 (2015)
M.S.R. Murty, M.R. Katiki, B.R. Rao, N.J. Babu, S.K. Buddana, R.S. Prakasham, Synth. Commun. 44, 2724 (2014)
M.S.R. Murty, M.R. Katiki, D. Kommula, Can. Chem. Trans. 4, 47 (2016)
Acknowledgements
The author DK is thankful to CSIR New Delhi, India, for the award of research fellowships. The authors thank CSIR New Delhi for financial support as a part of XII five year plan program under title ORIGIN (CSC-0108).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kommula, D., Madugula, S.R.M. Synthesis of benzimidazoles/benzothiazoles by using recyclable, magnetically separable nano-Fe2O3 in aqueous medium. J IRAN CHEM SOC 14, 1665–1671 (2017). https://doi.org/10.1007/s13738-017-1107-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-017-1107-z