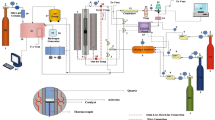
Abstract
The present work introduces the optimization of a synthetic procedure for oleate-coated iron-oxide nanoparticles by the thermal decomposition of Fe oleate dried at 30 and 70 °C in high-boiling organic solvents. The attention is focused on the temperature of the thermal decomposition, the nature of organic solvent, heating rate and the mode of the heating. In particular, heating on Wood alloy with simultaneous bubbling of argon through the reaction mixture versus the heating on mantel with magnetic stirring is highlighted as a route to improve the monodispersity of the nanoparticles. The effect of heating mode and rate on the nanoparticles size is estimated. The obtained tendencies point to the heating mode and rate as additional factors affecting the kinetic separation between nucleation and nanoparticle growth processes.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent decades, superparamagnetic iron-oxide nanoparticles with sub-50 nm diameters have attracted considerable attention of researches owing to their potential application in various fields. In particular, they are widely employed in targeted drug delivery [1, 2], catalysis [3], magnetic hyperthermia [4], magnetic data storage [5] and as contrast agents in MRI [6–10]. Thus, diverse synthetic routes to prepare iron-oxide nanoparticles have been developed. The synthesis through the so-called co-precipitation method, in which NH4OH solutions are added to the aqueous solution of FeCl2 and FeCl3 mixed in a certain molar ratio [11–13], is performed in mild conditions and in aqueous media, which makes it ecologically friendly. Although this general approach is employed for the synthesis of commercially used contrast agents, it results in the formation of quite polydisperse nanoparticles with quite large DLS sizes [12, 14]. The electrochemical synthesis is a facile route to obtain iron-oxide nanocrystals in mild conditions, although they have quite uncertain shape [15]. The use of K3[Fe(CN)6] as precursors [16] or sonochemical [17] techniques for the generation of iron-oxide particles also does not significantly improve the size distribution.
Having analysed the literature, we believe that the most reliable way to synthesize monodisperse spherical iron-oxide nanoparticles is by the thermal decomposition of iron salts such as oleate, stearate, chloride and acetylacetonate [18–21]. It must be added that iron oleate is the most popular precursor for the iron-oxide synthesis [22–24]. In particular, Mao et al. [25] prepared monodisperse oleate-coated iron-oxide nanoparticles of different sizes (3.5–19.9 nm) by simple varying the refluxing time in 1-octadecene at 320 °C. Bronstein et al. [26] has employed the thermal decomposition of Fe oleate to prepare uniform-sized iron-oxide nanoparticles of different diameters and shapes. The simple variations of synthetic conditions (temperature, concentration) and the post-synthetic treatment of Fe oleate (washing with acetone or ethanol, drying at different temperatures) provide the possibility to control over the particle size, size distribution and shape [26]. In general, a great number of papers are devoted to the synthesis of iron-oxide nanoparticles but none of them describe the experimental synthetic setup in detail, although the proper setup is crucial for the preparation of high-quality nanoparticles.
The literature data introduce the application of various heat carriers for the synthesis of iron-oxide nanoparticles. In particular, microwave-assisted [27] and laser-assisted heating [28] should be mentioned as fine examples for the synthesis of monodisperse iron-oxide nanoparticles. Nevertheless, both procedures require equipment, which is not always readily accessible to the wide circles of researches.
In this article, we emphasize the heating mode as the key reason for the fabrication of monodisperse spherical iron-oxide nanoparticles. The developed approach is based on the use of a Wood alloy without a magnetic stirring. Moreover, the present report introduces some simple and efficient practical advice on the experimental setup, described in detail in the “Experimental” section, for the synthesis, which to the best of our knowledge has not been published before.
Experimental section
Materials
Eicosane (99 %), octadecane (99 %), chloroform (99.9 %), acetone (99.8 %) and oleic acid (97 %) were purchased from Acros Organics. Hexane (97 %) was purchased from Sigma-Aldrich. Sodium oleate was purchased from J.T. Baker Reagent Chemicals. All the chemicals were used as received without further purification.
Syntheses
Synthesis of Fe oleate(III)
The Fe oleate was prepared according to literature procedure [26].
Synthesis of 1A
2 g of Fe oleate (dried at 70 °C) together with 9.4 ml of eicosane and 0.60 ml of oleic acid was mixed in a three-neck round-bottom reaction flask. The mixture was heated under vigorous stirring up to 60 °C to melt the eicosane. Then, the experimental setup was transferred on a mantel installed on a magnetic stirrer and the temperature was raised to 325 °C (average heating rate 3.35 °C/min). The mixture was kept refluxing for 30 min with vigorous stirring (800 rpm) under argon. No additional isolation of the mantel with glass fibre or by any other means was applied in the experimental setup. Next, the mixture was cooled down to 35 °C and 36 ml of hexane/acetone mixture (volume ratio 1:4) was added to precipitate the crystals. The iron-oxide nanoparticles were isolated by centrifugation and washed with hexane/acetone mixture 7 times. Then, the obtained black powder of iron-oxide particles was annealed in vacuum at 80 °C to get rid of the hexane and acetone. Yield: 33.5 % (58 mg).
Synthesis of 1B
1.73 g of Fe oleate (dried at 70 °C) together with 8.17 ml of eicosane and 0.52 ml of oleic acid was mixed in a three-neck round-bottom reaction flask. The mixture was heated under vigorous stirring up to 60 °C to melt the eicosane. Then, the experimental setup was transferred on a mantel installed on a magnetic stirrer and the temperature was raised to 330 °C (average heating rate 3 °C/min). The mixture was kept refluxing for 30 min with vigorous stirring (800 rpm) under argon. Next, the mixture was cooled down to 35 °C and 31.3 ml of hexane/acetone mixture (volume ratio 1:4) was added to precipitate the crystals. The iron-oxide nanoparticles were isolated by centrifugation and washed with hexane/acetone mixture 7 times. Then, the obtained black powder of iron-oxide particles was annealed in vacuum at 80 °C to get rid of the hexane and acetone. Yield: 66.8 % (100.6 mg).
Synthesis of 1C
1.96 g of Fe oleate (dried at 30 °C) together with 7.28 ml of eicosane and 0.68 ml of oleic acid was mixed in a three-neck round-bottom reaction flask. The mixture was heated under vigorous stirring up to 60 °C to melt the eicosane. Then, the experimental setup was transferred on a mantel installed on a magnetic stirrer and the temperature was raised to 330 °C (average heating rate 3.28 °C/min). The mixture was kept refluxing for 30 min with vigorous stirring (800 rpm) under argon. Next, the mixture was cooled down to 35 °C and 35.5 ml of hexane/acetone mixture (volume ratio 1:4) was added to precipitate the crystals. The iron-oxide nanoparticles were isolated by centrifugation and washed with hexane/acetone mixture 7 times. Then, the obtained black powder of iron-oxide particles was annealed in vacuum at 80 °C to get rid of the hexane and acetone. Yield: 46.2 % (78.6 mg).
Synthesis of 1D
2.34 g of Fe oleate (dried at 70 °C) together with 8.62 ml of eicosane and 0.80 ml of oleic acid was mixed in a two-neck round-bottom reaction flask. The mixture was heated under vigorous stirring up to 35 °C to melt the octadecane. Wood alloy is a solid at room temperature and has the melting point about 68.5 °C so that by the time all alloy had been melted, the temperature reached 160 °C. Then, the reaction flask was carefully immersed into fully melted Wood alloy and the temperature was raised to 330 °C and kept refluxing for 1 h (average heating rate 3.35 °C/min) without isolation glass fibre. It must be added that no magnetic stirring was used in the synthesis. Instead, we applied bubbling of argon through a homemade capillary both for the sake of stirring and inert atmosphere. One must always watch whether the bubbles of argon go freely through the capillary. If argon does not freely pass through the capillary or capillary is broken during the synthesis, one must turn off the heat and stop the experiment immediately. Next, the mixture was cooled down to 35 °C and 42 ml of hexane/acetone mixture (volume ratio 1:4) was added to precipitate the crystals. The iron-oxide nanoparticles were isolated by centrifugation and washed with hexane/acetone mixture 7 times. Then, the obtained black powder of iron-oxide particles was annealed in vacuum at 80 °C to get rid of the hexane and acetone. Yield: 71.5 % (143.8 mg).
Synthesis of 2A
0.902 g of Fe oleate (dried at 30 °C) together with 3.25 ml of octadecane and 0.315 ml of oleic acid was mixed in a two-neck round-bottom reaction flask. Then, the reaction flask was carefully immersed into fully melted Wood alloy and the temperature was raised to 319 °C and kept refluxing for 1 h with argon bubbling (average heating rate 3.08 °C/min) without isolation glass fibre. Next, the mixture was cooled down to 35 °C and 16 ml of hexane/acetone mixture (volume ratio 1:4) was added to precipitate the crystals. The iron-oxide nanoparticles were isolated by centrifugation and washed with hexane/acetone mixture 7 times. Then, the obtained black powder of iron-oxide particles was annealed in vacuum at 80 °C to get rid of the hexane and acetone. Yield: 88.3 % (68 mg).
Synthesis of 2B
0.81 g of Fe oleate (dried at 30 °C) together with 3 ml of octadecane and 0.28 ml of oleic acid was mixed in a two-neck round-bottom reaction flask. The mixture was heated under vigorous stirring up to 35 °C to melt the octadecane. Then, the reaction flask was carefully immersed into Wood alloy and the temperature was raised to 319 °C and kept refluxing for 1 h (average heating rate 3.35 °C/min) with argon bubbling without isolation glass fibre. Next, the mixture was cooled down to 45 °C and 14.5 ml of hexane/acetone mixture (volume ratio 1:4) was added to precipitate the crystals. The iron-oxide nanoparticles were isolated by centrifugation and washed with hexane/acetone mixture 7 times. Then, the obtained black powder of iron-oxide particles was annealed in vacuum at 80 °C to get rid of the hexane and acetone. Yield: 90.2 % (80.3 mg).
Synthesis of 2C
1.7 g of Fe oleate (dried at 70 °C) together with 6.1 ml of octadecane and 0.6 ml of oleic acid was mixed in a two-neck round-bottom reaction flask. The mixture was heated under vigorous stirring up to 35 °C to melt the octadecane. Then, the reaction flask was carefully immersed into Wood alloy and the temperature was raised to 319 °C and kept refluxing for 1 h (average heating rate 3.08 °C/min) with argon bubbling without isolation glass fibre. The mixture was kept refluxing for 1 h with vigorous stirring (800 rpm). Next, the mixture was cooled down to 35 °C and 30.5 ml of hexane/acetone mixture (volume ratio 1:4) was added to precipitate the crystals. The iron-oxide nanoparticles were isolated by centrifugation and washed with hexane/acetone mixture 7 times. Then, the obtained black powder of iron-oxide particles was annealed in vacuum at 80 °C to get rid of the hexane and acetone. Yield: 53.5 % (78.3 mg).
Characterization
The X-ray diffraction patterns have been obtained on a Rigaku Miniflex apparatus with a Cu Kα source.
High-resolution transmission electron microscopy (HRTEM) studies were conducted at the FEI Tecnai F30 TEM operating at 300 kV of acceleration voltage.
Microscopic images for 1D were obtained by means of FE-SEM measurements. Samples were mounted on a 25-mm aluminium specimen stub and fixed by conductive silver paint. Then, the measurements were performed on a Hitachi SU8000 field-emission scanning electron microscope at 10 kV acceleration voltage.
For 1C sample, TEM image was obtained with Hitachi HT7700, Japan. The images were acquired at an accelerating voltage of 100 kV. Sample was ultrasonicated in absolute ethanol for 10 min and then dispersed on 200 mesh copper grids with continuous formvar support films.
Results and discussion
The Fe oleate prepared according to the published procedure [26] was dried at two temperatures of 30 and 70 °C (hereinafter called Fe1 and Fe2, respectively). The elemental analysis data are presented in Table 1. The obtained results perfectly agree with the results reported by Bronstein et al. [26]. The significant difference in the percentage of hydrogen is due to the complete removal of the crystal hydrate water from the product upon drying at 70 °C [26].
The further thermal decomposition of the foregoing Fe oleate in eicosane and octadecane is the way to prepare iron-oxide nanoparticles. The employed reaction conditions and resulting nanoparticles’ sizes and shapes are summarized in Table 2.
It is known that drying of Fe2 (dried at 70 °C) facilitates formation of its stable modification with total decomposition at about 380 °C [26]. Thus, the temperature of thermal decomposition of Fe2 has an impact on the synthesis of monodisperse iron-oxide nanoparticles. Indeed, the mantel-facilitated high-temperature decomposition of Fe2 in eicosane performed in diverse temperature regimes, which are 325 and 330 °C, results in different size and polydispersity of the nanoparticles. According to TEM analysis, the decomposition at 325 °C resulted in fabrication of two types of nanoparticles, which are spherical with mean diameter of 31 nm and larger (~50 nm) polydisperse nanoparticles of different shapes. It must be added that, after thermal decomposition of Fe2, we observed a red colour of the undecomposed iron oleate, which shows that temperature 323–325 °C is not enough for complete decomposition of the foregoing precursor previously dried at 70 °C. This agrees well with the results of Bronstein et al. [26] who showed that complete decomposition of iron oleate dried at 70 °C completely occurs only at higher temperatures, while lower temperature conditions lead to incomplete decomposition and quite polydisperse nanoparticles. The total yield is 33.5 % (Fig. 1 (panel 1A); Table 2 (sample 1A)), while the higher temperature (330 °C) gives rise to the formation of smaller and more monodisperse particles (~22 nm) (Fig. 1 (panel 1B); Table 2 (sample 1B)) with a 66.8 % yield.
According to literature data, the decomposition of Fe1 results in worse monodispersity. This tendency is highlighted by Bronstein et al. [26], who reveals a small separation between nucleation and growth processes of the iron oleate dried at 30 °C as the reason for the enhanced polydispersity [26]. The present results also indicate an increased polydispersity of the nanoparticles obtained by the decomposition of Fe1 in eicosane at 330 °C (Table 2, samples 1B and 1C). The presence of smaller particles along with the larger (23 nm) ones (Fig. 1, panel 1C) illustrates the above-mentioned polydispersity.
The solvent was changed from eicosane to octadecane with the aim to facilitate in keeping the temperature on the level of 318 °C, since this is the boiling point of octadecane. It is worth noting that the use of octadecane instead of eicosane in the thermal decomposition of Fe1 improves both the monodispersity and the shape of nanoparticles. The data and image presented in Table 2 and Fig. 2, 2A indicate that the decomposition of Fe1 at 318 °C results in the spherical, monodisperse 22 nm sized particles.
The obtained results prompt further optimization of synthetic conditions. The impact of kinetic separation between nucleation and growth processes [26] points to the heating mode and heating rate as the factors affecting the size and polydispersity of iron-oxide nanoparticles. A conventional heating setup for iron-oxide nanoparticle preparation involves the use of a mantel wrapped around a reaction flask with iron oleate. It is clear that homogeneous heating in this case is restricted due to the presence of air between the mantel and the reaction flask. In contrast, no air is present between the hot liquid Wood alloy and the reaction flask, which provides more homogenous conditions than those in the case of mantel-facilitated heating. This heating setup requires another mode of stirring, since the magnetic stirring is inconvenient in these conditions. Argon bubbling provides enough stirring for this synthetic procedure. Thus, the decomposition of Fe2 and Fe1 in eicosane and octadecane correspondingly was performed by heating on a Wood alloy. This also results in monodisperse spherical nanoparticles, although they are significantly larger (35 nm, Fig. 1, 1D; Table 2 (sample 1D)) than those heated by mantel (22 nm).
As it can be seen from Table 2 (samples 1D, 2B, 2C) and Figs. 1 (panel 1D) and 2 (panels 2B, 2C), the decomposition process of Fe1 in octadecane upon the heating by means of a Wood alloy is considerably different from results obtained by the conventional heating on a mantel with magnetic stirring. In particular, it generates highly monodisperse spherical nanoparticles with the size of 7 and 12 nm (Fig. 2, 2B, 2C) in the case of decomposition of Fe1. The opposite trend, namely the increase in size, is observed for Fe2 in eicosane from 22 (sample 1B) to 35 nm (sample 1D). It is worth noting that the oleic acid concentrations are not the same for samples 1B and 1D, although no significant influence of these concentration changes on the nanoparticles size is known. Thus, the variation of the heating mode results in the change of the size.
It is worth noting that a heating rate of 3.35 °C/min is commonly applied [18, 26]. The work [19] highlights the duration of the stirring at constant high temperature as a more important factor than the heating rate, which affects the particle size. Nevertheless, the decrease of heating rate from 3.35 to 3.08 °C/min (Table 2, samples 2B, 2C) results in high-quality 12-nm spherical nanoparticles (Fig. 2, panel 2C) instead of the 7 nm sized at the rate of 3.35 °C/min. This fact is a very rare example of the significant effect of the heating rate on the size of the obtained particles, the reasons for that, to date, are unexplored. Moreover, the use of octadecane helps keeping the temperature regime at the constant level of 318 °C, which is the boiling point of octadecane. This in turn provides additional favouring the monodisperse nanoparticles formation. In contrast, the boiling temperature of eicosane is about 342 °C and, therefore, temperature regime can fluctuate when performing synthesis at 330 °C. Thus, the use of octadecane along with Wood alloy makes the perfect conditions for monodisperse nanoparticles.
It is worth noting that although Bronstein et al. [26] reported the formation of polydisperse samples from iron oleate dried at 30 °C, the present report introduces a route to produce monodisperse nanoparticles from this precursor. This route is based on the use of a special heat-transfer agent for the iron oleate decomposition, that is, Wood alloy (the detailed description is given in the “Experimental” section). Taking into consideration the temperature-dependent behaviour of the particles formation, it is not surprising that the production of highly monodisperse samples is favoured by the use of more homogeneous heating mode than the conventional mantel-facilitated heating.
Conclusions
We publish for the first time the synthesis of iron-oxide nanoparticles using Wood alloy as a heat carrier. Our heating setup has one big advantage: the heating process is very homogeneous what allows the fabrication of uniform-sized nanoparticles. Argon bubbling is enough for the efficient stirring in the introduced procedure. The use of this heating mode results in the improved monodispersity of the iron-oxide nanoparticles, although their size also tends to change. The obtained data indicate opposite trends of the size change for Fe oleates dried at 30 and 70 °C. The reasons for the different trends are not clear at the moment. Nevertheless, the obtained results open new route to tune both size and polydispersity of iron-oxide nanoparticles, which would be useful for the further improvement of the synthetic procedure for the optimized and reliable experimental synthesis of high-quality iron-oxide nanoparticles.
References
M. Halupka-Bryl, M. Bednarowicz, B. Dobozs, R. Krzyminiewski, T. Zalewski, B. Wereszczynska, G. Novaczyk, M. Jarek, Y. Nagasaki, Doxorubicin loaded PEG-b-poly(4-vinylbenzylphosphonate) coated magnetic iron oxide nanoparticles for targeted drug delivery. J. Magn. Magn. Mater. 384, 320–327 (2015)
M. Talelli, C.J.F. Rijcken, T. Lammers, P.R. Seevinck, G. Storm, C.F. van Nostrum, W.E. Hennink, Superparamagnetic iron oxide nanoparticles encapsulated in biodegradable thermosensitive polymeric micelles: toward a targeted nanomedicine suitable for image-guided drug delivery. Langmuir 25, 2060–2067 (2009)
M. Beygzadeh, M. Alizadeh, M.M. Khodaei, D. Kordestani, Biguanide/Pd(OAc)2 immobilized on magnetic nanoparticle as a recyclable catalyst for the heterogeneous Suzuki reaction in aqueous media. Catal. Commun. 32, 86–91 (2013)
R.R. Shah, T.P. Davis, A.L. Glover, D.E. Nikles, C.S. Brazel, Impact of magnetic field parameters and iron oxide nanoparticle properties on heat generation for use in magnetic hyperthermia. J. Magn. Magn. Mater. 387, 96–106 (2015)
G. Frolov, Film carriers for super-high-density magnetic storage. Tech. Phys. 12, 410–414 (2000)
M. Lewin, N. Carlesso, C.H. Tung, X.W. Tang, D. Cory, D.T. Scadden, R. Weissleder, Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat. Biotechnol. 18, 410–414 (2000)
E. Amstad, S. Zurcher, A. Mashaghi, J.Y. Wong, M. Textor, E. Reimhult, Surface functionalization of single superparamagnetic iron oxide nanoparticles for targeted magnetic resonance imaging. Small 5, 1334–1342 (2009)
D.G. You, G. Saravanakumar, S. Son, H.S. Han, R. Heo, K. Kim, I.C. Kwon, Y.G. Lee, J.H. Park, Dextran sulfate-coated superparamagnetic iron oxide nanoparticles as a contrast agent for atherosclerosis imaging. Carbohydr. Polym. 101, 1225–1233 (2014)
M. Corti, A. Lascialfari, M. Marinone, A. Masotti, E. Micotti, F. Orsini, G. Ortaggi, G. Poletti, C. Innocenti, C. Sangregorio, Magnetic and relaxometric properties of polyethylenimine-coated superparamagnetic MRI contrast agents. J. Magn. Magn. Mater. 320, e316–e319 (2008)
M. Branca, M. Marciello, D. Ciuculescu-Pradines, M. Respaud, del P.M. Morales, R. Serra, M.-J. Casanove, C. Amiens, Towards MRI T2 contrast agents of increased efficiency. J. Magn. Magn. Mater. 377, 348–353 (2015)
C.-L. Lin, C.-F. Lee, W.-Y. Chiu, Preparation and properties of poly(acrylic acid) oligomer stabilized superparamagnetic ferrofluid. Colloids Surf. A 370, 1–5 (2005)
D. Ramimoghadam, S. Bagheri, S.B.A. Hamid, In-situ precipitation of ultra-stable nano-magnetite slurry. J. Magn. Magn. Mater. 379, 74–79 (2015)
D. Ramimoghadam, S. Bagheri, A.T. Yousefi, S.B.A. Hamid, Statistical optimization of effective parameters on saturation magnetization of nanomagnetite particles. J. Magn. Magn. Mater. 393, 30–35 (2015)
R.Y. Hong, B. Feng, L.L. Chen, G.H. Liu, H.Z. Li, Y. Zheng, D.G. Wei, Synthesis, characterization and MRI application of dextran-coated Fe3O4 magnetic nanoparticles. Biochem. Eng. J. 42, 290–300 (2008)
D. Ramimoghadam, S. Bagheri, S.B.A. Hamid, Progress in electrochemical synthesis of magnetic iron oxide nanoparticles. J. Magn. Magn. Mater. 368, 207–229 (2014)
H. Wang, S. Liu, X. Yang, R. Yuan, Y. Chai, Mixed-phase iron oxide nanocomposites as anode materials for lithium-ion batteries. J. Power Sources 276, 170–175 (2015)
R. Vijayakumar, Yu. Koltypin, I. Felner, A. Gedanken, Sonochemical synthesis and characterization of pure nanometer-sized Fe3O4 particles. Mater. Sci. Eng., A 286(1), 101–105 (2000)
A. Stepanov, V. Burilov, M. Pinus, A. Mustafina, M. Rümmeli, R. Mendes, R. Amirov, S. Lukashenko, E. Zvereva, S. Katsuba, J. Elistratova, I. Nizameev, M. Kadirov, R. Zairov, Water transverse relaxation rates in aqueous dispersions of superparamagnetic iron oxide nanoclusters with diverse hydrophilic coating. Colloids Surf. A 443, 450–458 (2014)
D. Ramimoghadam, S. Bagheri, S.B.A. Hamid, Stable monodisperse nanomagnetic colloidal suspensions: an overview. Colloids Surf. B 133, 388–411 (2015)
Q. Yu, A. Fu, H. Li, H. Liu, R. Lv, J. Liu, P. Guo, X.S. Zhao, Synthesis and characterization of magnetically separable Ag nanoparticles decorated mesoporous Fe3O4@carbon with antibacterial and catalytic properties. Colloids Surf. A 457, 288–296 (2014)
J. Wang, B. Zhang, L. Wang, M. Wang, F. Gao, One-pot synthesis of water-soluble superparamagnetic iron oxide nanoparticles and their MRI contrast effects in the mouse brains. Mater. Sci. Eng., C 48, 416–423 (2015)
D. Kim, N. Lee, M. Park, B.H. Kim, K. An, T. Hyeon, Synthesis of uniform ferrimagnetic magnetite nanocubes. J. Am. Chem. Soc. 131, 454–455 (2008)
J. Park, K.J. An, Y.S. Hwang, J.-G. Park, H.J. Noh, Y.G. Kim, H.J. Park, N.M. Hwang, T. Hyeon, Ultra large-scale syntheses of monodisperse nanocrystals. Nat. Mater. 3, 891–895 (2004)
Z. Chen, Size and shape controllable synthesis of monodisperse iron oxide nanoparticles by thermal decomposition of iron oleate complex. Synth. React. Inorg. Met.-Org. Chem. 42, 1040–1046 (2012)
J. Huang, L. Wang, X. Zhong, Y. Li, L. Yang, H. Mao, Facile non-hydrothermal synthesis of oligosaccharide coated sub-5 nm magnetic iron oxide nanoparticles with dual MRI contrast enhancement effects. J. Mater. Chem. B. 2, 5344–5351 (2015)
L.M. Bronstein, X. Huang, J. Retrum, A. Schmucker, M. Pink, B.D. Stein, B. Dragnea, Influence of iron oleate complex structure on iron oxide nanoparticle formation. Chem. Mater. 19, 3624–3632 (2007)
W. Xiao, H. Gu, D. Li, D. Chen, X. Deng, Z. Jiao, J. Lin, Microwave-assisted synthesis of magnetite nanoparticles for MR blood pool contrast agents. J. Magn. Magn. Mater. 324, 488–494 (2012)
T. Gonzales-Carreno, M.P. Morales, M. Gracia, C.J. Serna, Preparation of uniform γ-Fe2O3 particles with nanometer size by spray pyrolysis. Materials Lett. 18, 151–155 (1993)
Acknowledgments
We are very thankful to Russian Fund for Basic Research (Project Number 13-03-12436_ofi_M2) for financial support. Mark H. Rümmeli thanks the IBS Korea (IBS-RO11-D1). Microscopic investigations for 1C sample were carried out in the laboratory “Transmission electron microscopy” of Kazan National Research Technological University. Electron microscopy characterization of 1D sample was performed in the Department of Structural Studies of Zelinsky Institute of Organic Chemistry, Moscow.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stepanov, A., Mustafina, A., Mendes, R.G. et al. Impact of heating mode in synthesis of monodisperse iron-oxide nanoparticles via oleate decomposition. J IRAN CHEM SOC 13, 299–305 (2016). https://doi.org/10.1007/s13738-015-0737-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-015-0737-2