Abstract
A new surfactant-based nanocomposite cation exchanger, sodium dodecyl sulfate–Th(IV) tungstate (SDS–TT) was prepared by the sol–gel method. The SDS–TT was characterized by FTIR, XRD, TGA, SEM, EDS and TEM. The distribution studies for various metal ions on SDS–TT were performed in different acidic mediums. On the basis of distribution coefficient values, SDS–TT was found to be selective for Cu2+ metal ion. SDS–TT was successfully used for the quantitative separation of Cu2+ from the synthetic mixture, pharmaceutical formulation and brass sample. The regeneration studies were performed which showed a decrease in the recovery of Cu2+ from 88 to 70 % after seven consecutive cycles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The improvement in the characteristics of ion exchange materials has always been an objective for the researchers by changing the inorganic, organic and polymeric ion exchangers to obtain the material with desirable ion exchange properties, reproducibility and stability [1, 2]. In the past few decades, a number of ion exchanger materials have been synthesized. The inorganic ion exchangers are thermally stable, while organic ion exchangers have good mechanical and chemical strength and can be applied for the treatment of large volume of waste effluents. By combining these two different materials having entirely different properties, composite ion exchanger materials are produced with unique properties which are distinct from the constituent’s material. In addition to other advantages, composite ion exchangers are more stable at high temperature and radiation fields [3]. The composite ion exchangers exhibit enhanced characteristics by bridging the property gap between two divergent types of materials [4, 5]. Thus, the composite ion exchanger materials have been utilized for diverse applications such as quantitative estimations by ion selective electrodes, catalysis, antimicrobial activity, bimolecular separations, chromatography, hydrometallurgy and environmental science [4–8]. However, there are still some major limitations. So, the efforts are continuously going on to improve the mechanical, chemical and thermal stability and their selectivity for heavy metal ions [9–12]. In this favors, the advanced composite ion exchangers with nanodomain have also been given consideration to summarize the desired properties of both inorganic and organic counter parts into single molecule. These nanocomposite ion exchangers have been developed by adding organic polymer into the matrix of inorganic precipitates by sol–gel method and have been used in environmental applications [4, 9–13]. Water pollution by heavy metals is an important environmental concern which is related negatively with the health and economy. Various kinds of health hazards are arising due to the contamination of heavy metals from the waste effluents of fast growing industries in the surrounding soils as well as ground and surface waters, i.e., in the food chain and drinking water. All heavy metals are highly toxic, non-biodegradable and readily accumulate to toxic levels [14]. Therefore, it is a main goal to remove these toxic heavy metals from industrial wastewater which commonly includes Hg, As, Cu, Zn, Cd, Cr, and Pb [15, 16]. Several methods have been applied for the removal of toxic metals from aqueous solution such as reverse osmosis, ultra-filtration, chemical precipitation, electrodialysis, oxidation, ion exchange and adsorption on several low-cost adsorbents [4–8, 17–21].
In separation science and technology, adsorption and ion exchange are more attractive, cost-effective, simple and widely used techniques for the treatment of wastewater from industrial and domestic sources [4–8]. The adsorption of metal ions on the ion exchanger surface in the presence of surfactant is affected by the charge density of the interface which helps and increases the selective adsorption of heavy metals onto the porous surface of ion exchangers [22–26]. Surfactants are the amphiphilic substance having the hydrophobic tail made up of hydrocarbon chain and hydrophilic charged or polar head group [27]. The capability of the charged head group of surfactant molecules to bind with the ions of opposite charge makes the ion exchanger quite effective and selective in nature [22–26]. The head groups of surfactant molecule bind metal ions reversibly through complexation, electron attraction and/or charge neutralization [22]. The higher moieties for heavy metal ions may be due to higher binding constant for them on surfactant molecules in the matrix of inorganic precipitate [28].
In the present research works, we have synthesized a surfactant-based nanocomposite cation exchanger sodium dodecyl sulfate–Th(IV) tungstate (SDS–TT) via a simple sol–gel method. This nanocomposite ion exchanger was subjected for different physiochemical analyses and the results demonstrated that the addition of SDS into the matrix of TT improved the ion exchange capacity, thermal stability and selectivity toward metal ions. Specifically, this nanocomposite ion exchange material was found to be highly selective for Cu2+ metal ion, and hence, it has been exploited for the remediation of toxic Cu2+ ion from aqueous medium. Copper is greatly used in the industries such as mining, plating, smelting, electroplating industries, brass manufacture and petroleum refining. These industries generate much wastewater and sludge having Cu2+ ions with various concentrations which have harmful effects on the water environment [29]. According to the World Health Organization, the maximum permissible limit of Cu(II) in drinking water is 1.5 mg L−1 [30]. Hence, the level of Cu2+ ions must be reduced to the level that satisfies environmental regulations for various bodies of water. SDS–TT was successfully used for the adsorption of Cu2+ ion from the synthetic mixture of metal ions, pharmaceutical formulation and brass alloy sample.
Experimental
Reagents and instruments
The main reagents used for the synthesis of SDS–TT were sodium dodecyl sulfate, thorium nitrate and sodium tungstate. These reagents were purchased from Sigma Aldrich, Germany. All other reagents and chemicals were of analytical reagent grade.
The main instruments used during the study were single electrode pH meter (Orion 2 star, Thermo Scientific, USA), automatic thermal analyzer (V2.2A Du Pont 9900), FTIR spectrophotometer (Nicolet 6700, Thermo Scientific, USA), atomic absorption spectrometer (AAS, Perkin Elmer, USA), a PW 1148/89 based X-ray diffractometer (Phillips, Holland), high-performance scanning electron microscope with a high resolution of 3.0 nm (JSM-6380 LA, Japan), transmission electron microscope (H-7500, Hitachi, Japan) and a water bath incubator shaker (SW22/9550322, Julabo, Germany).
Preparation of SDS–TT nanocomposite cation exchanger
To get a stable product with good ion exchange properties, several samples of SDS–TT nanocomposite cation exchanger were synthesized at different reactants volume ratios by sol–gel mixing method [4]. Initially, 0.005 M sodium dodecyl sulfate solution was added into 0.1 M thorium nitrate solution with constant stirring for 30 min. Subsequently, 0.1 M sodium tungstate solutions was added drop wise into the aforementioned mixture of sodium dodecyl sulfate and thorium nitrate at pH 1.8 and mixed thoroughly with constant stirring for 2 h at 25 ± 2 °C (Scheme 1). The white precipitate so formed was allowed to stand for 24 h and then washed with Milli-Q water to remove the excess reagents. The product was dried at 30 ± 2 °C in an oven. The dried product was then kept in Milli-Q water for cracking and converted into H+ form by placing in 0.01 M HNO3 solution and washed with Milli-Q water to remove excess acid and finally dried at 35 ± 2 °C. The sample S-7 was selected for detailed studies due to its better ion exchange capacity among all samples (Table 1).
Column ion exchange capacity and thermal stability of SDS–TT
1.0 g of SDS–TT (H+ form) was packed in a column (internal diameter-0.6 cm) fitted with glass wool at the bottom. 250 mL of 0.1 M solution of numerous metal nitrates was applied to elute the H+ ions completely from the column. The effluent was titrated against a standard solution of 0.1 M NaOH solution to check the total amount of H+ ions released which was equivalent to the metal ions retained by the material [22].
To see the effect of heating temperature on the ion exchange capacity of this material, 1.0 g of SDS–TT in H+ form was heated at different temperatures in a muffle furnace for 1 h and Na+ ion exchange capacity was found after cooling them at room temperature by standard column process as described above.
Effect of eluent concentration and elution behavior
To get the optimal concentration of the eluent for the complete elution of H+ ions, 250 mL of NaNO3 solution of different concentrations was passed through the columns which had 1.0 g of the SDS–TT nanocomposite cation exchanger (H+ form). The effluents were titrated against a standard solution of 0.1 M NaOH to find the eluted H+ ions.
To study the elution performance, NaNO3 solution of optimal concentration was passed through the column containing 1.0 g of the SDS–TT nanocomposite cation exchanger (H+ form) for the complete elution of H+ ions. The collected effluent was titrated against a standard alkali solution.
Instrumental characterization
The Fourier transform infrared spectroscopy (FTIR) absorption spectra of TT inorganic ion exchanger and SDS–TT nanocomposite cation exchanger were recorded between 450 and 4000 cm−1. For X-ray powder diffraction (XRD) analysis, manganese-filtered CuKα radiation wavelength (l50.1542 nm) at 298 K was utilized. The instrument was equipped with graphite monochromator and operating at 40 kV and 30 mA. Thermogravimetric analysis (TGA) of SDS–TT was performed at a heating rate of 10 °C min−1 up to 1000 °C in the air atmosphere. To see the morphology, scanning electron micrographs (SEM) of TT and SDS–TT were performed using an electron microscope. TEM study was carried out to know the particle size of SDS–TT cation exchanger.
Distribution studies (K d)
The values of distribution coefficient (K d) for different metal ions, viz., Mg2+, Ca2+, Sr2+, Ba2+, Hg2+, Cd2+, Pb2+, Fe3+, Cu2+ and Zn2+ were evaluated by batch method in different acidic mediums. Various portions of (300 mg each) the SDS–TT exchanger in H+ form were taken in flasks and mixed with 30 mL of the above metal nitrate solution in the required medium and shaken for 5 h in the temperature controlled shaker at 25 ± 2 °C to achieve the equilibrium. The quantity of metal ion was determined by EDTA titration. The distribution coefficient values were evaluated using the equation
where I is the initial amount of the metal ion in the solution phase and F is final amount of metal ion in the solution phase after treatment with the exchanger.
Quantitative separations of Cu2+ ion from synthetic, pharmaceutical and alloy samples
For the selective separation of Cu2+, three sets of synthetic mixture of metal ions, viz., Hg2+, Cd2+, Fe3+, Pb2+, Cu2+ and Zn2+ were taken wherein the amount of Cu2+ was changed and the amount of rest metal ions was kept constant. The mixture of these metals was poured onto the top of columns and permitted to flow down at a rate of 0.5 mL min−1. It was recycled through the columns to ensure complete adsorption of the metal ions. The separation of metal ions was achieved by collecting the effluent i and titrated against the standard solution of 0.01 M EDTA.
The I-Vit antioxidant (Two tablets) was treated with 10 mL conc. HCl and heated to evaporate the excess acid. The aliquot was filtered and the filtrate was diluted to 100 mL using Milli-Q water. Different quantities of stock solutions were taken into the glass column which had 1.0 g SDS–TT nanocomposite cation exchanger. The solution was passed through the column with 0.5 mL min−1 flow rate. The effluent was recycled through the column to make the maximum adsorption of Cu2+ and it was eluted with a 0.01 M HNO3 solution and treated against 0.01 M EDTA solution.
A piece of brass alloy sample (0.5 g) was dissolved in 10 mL aqua regia solution and the mixture was evaporated to remove the excess acid. It was diluted to 100 mL with Milli-Q water and then utilized as a stock solution. Different quantities of stock solutions were poured into a glass column using the same method as described above.
Kinetics and isotherm studies
Kinetics studies were carried out by varying the concentration of Cu2+ (C o, 20 and 40 mg L−1). The samples were collected at different time gaps until equilibrium achieved. Isotherm studies were performed at different reaction temperatures (25, 30 and 40 °C) and initial concentration of Cu2+ solution (20–100 mg L−1).
Desorption and regeneration studies
Desorption and regeneration studies were performed by batch process. 100 mL of 10 mg L−1 Cu2+ solution was treated with 0.5 g of SDS–TT in conical flask for 1 h. After 1 h, the SDS–TT was washed with Milli-Q water to remove the excess of Cu2+. Then, SDS–TT was treated with 100 mL of 0.01 M HNO3 solution in other flask for 1 h to desorb Cu2+. The solution was then filtered and the filtrate was treated against the standard solution of 0.01 M EDTA to check the desorbed Cu2+. The desorption studies of Cu2+ was also done in several other acids (0.01 M formic acid, 0.01 M hydrochloric acid, 0.1–0.01 M acetic acid, 0.01 M succinic acid and 0.01 M H2SO4) by following the aforementioned procedure.
For the regeneration study, 0.5 g of SDS–TT was saturated with 100 mL of 10 mg L−1 Cu2+ solution for 1 h. After 1 h, the SDS–TT was washed several times with Milli-Q water to remove excess of Cu2+. To regenerate the exhausted SDS–TT, it was treated with 100 mL of 0.01 M HNO3 solution. The same procedure was repeated for four consecutive cycles.
Results and discussion
Various samples of SDS–TT nanocomposite cation exchange material were prepared under different reaction conditions by sol–gel mixing method [5]. Table 1 shows that the volume ratio and the concentration of the reactants as well as the pH affected the ion exchange capacity of the SDS–TT cation exchanger. Once the surfactant (SDS) was added into the matrix of inorganic cation exchanger Th(IV) tungstate, the ion exchange capacity was enhanced because the surfactant minimized the interfacial tensions between the liquid and solid phases [22, 23]. Furthermore, when the ratio of sodium tungstate (anionic part) in the reaction mixture was increased, the ion exchange capacity was also enhanced because the replaceable hydrogen groups were attached to this group which is anionogenic group [4]. Whereas, an increase in the cationic part (thorium nitrate) did not increase the ion exchange capacity of SDS–TT nanocomposite cation exchanger. The optimum pH for the synthesis of the material was found to be 1.8. In all samples, S-7 had better Na+ ion exchange capacity and good reproducible behavior as SDS–TT was synthesized in various batches under identical conditions and there was no any significant change in the ion exchange capacities. So, sample S-7 was selected for detailed studies. The better ion exchange capacity of SDS–TT in comparison to its inorganic counterpart [Th(IV) tungstate] was owing to the addition of sodium dodecyl surfactant. Actually, sodium dodecyl sulfate enhanced the wettability of surface by minimizing the interfacial tensions between liquid and solid phases and, therefore, gave better interaction chance between the mobile phase and the ion exchange material [22]. The order of ion exchange capacities for alkali and alkaline earth metal ions was K+ > Na+ > Li+ and Ba2+ > Sr2+ > Ca2+ > Mg2+, respectively (Table 2). This sequence was in agreement with the hydrated ionic radii. The smaller hydrated radii ions easily entered the pores of SDS–TT nanocomposite cation exchanger, resulted in higher adsorption [31]. According to Kossel [32] and Pauling [33], the attraction between anions and cations in ionic crystals obeys Coulomb’s law. A small ion will be attracted either to a greater force or held more tightly than a larger ion. So, the ion exchange capacity should increase with decreasing hydrated radii and increase with electric potential. The effect of temperature on the ion exchange capacity of SDS–TT nanocomposite cation exchanger is presented in Table 3 which demonstrated that the ion exchange capacity of SDS–TT was decreased as the temperature increased (Table 3). The material was found thermally stable up to 500 °C as the sample retained 59 % of its initial ion exchange capacity. The loss in the ion exchange capacity might be due to the loss of external water molecule and condensation of material. The variations of ion exchange capacity with concentration suggested that the ion exchange capacity of SDS–TT depended upon the concentration of eluent (NaNO3). The highest capacity was observed with 1.0 M NaNO3 solution after that it became almost constant (Figure is not given). The elution behavior of the SDS–TT confirmed that all the exchangeable H+ ions were eluted out at 130 mL of the effluent (Figure is not given). From this observation, it has been found that the efficiency of the column was quite satisfactory.
The infrared spectra of Th(IV) tungstate and sodium dodecyl Th(IV) tungstate nanocomposite cation exchanger are shown in Fig. 1a, b, respectively. The inorganic cation exchanger Th(IV) tungstate showed the presence of extra water molecule in addition to –OH groups and metal oxides present in the material. A broad peak around 3500 cm−1 might be due to water molecule [8]. A sharp peak at 1660 cm−1 represented the free water molecule (water of crystallization) and strongly bonded OH group in the matrix [8]. The peaks at 790 and 550 cm−1 were owing to the presence of WO4 2− and metal oxides groups, respectively [34]. In addition to the above peaks, SDS–TT nanocomposite cation exchanger showed the peak at 1130 cm−1 which indicated the presence of sulfate group in the material, while the weak peaks at 2940 and 1430 cm−1 showed the presence of methyl and/or methylene groups, respectively [4]. These three peaks (1130, 1430 and 2940 cm−1) confirmed the presence of organic part (SDS) in the SDS–TT nanocomposite cation exchanger. The X-Ray diffraction pattern of SDS–TT (Fig. 1c) was recorded and the sharp peaks between 35° and 50° suggested a good crystalline nature of SDS–TT nanocomposite cation exchanger. To know the mechanism of ion exchange and adsorption phenomenon in a better way, the crystalline nature of any ion exchange material is a very good property [35]. As apparent from the thermogravimetric analysis (TGA) of SDS–TT nanocomposite cation exchanger (Fig. 1d), an initial weight loss of 3.9 % up to 190 °C was due to the loss of external water molecules as well as partial removal of surfactant molecules (SDS) from the SDS–TT nanocomposite cation exchanger [36]. The weight loss of 2.3 % observed from temperatures 200–390 °C was owing to the removal/decomposition of remaining surfactant molecules from the ion exchanger material. Further weight loss up to 450 °C might be assigned due to the decomposition of the complete organic part, present in the material [36]. Above 450 °C, the weight of SDS–TT nanocomposite cation exchange material became almost constant which demonstrated the complete conversion of the material to the oxide form [22]. The surface morphologies of TT and SDS–TT are shown in Fig. 2a, b, respectively, which exhibited the rough surface with different-sized particles of Th(IV) tungstate inorganic cation exchanger. It was observed that the surface morphology of SDS–TT nanocomposite cation exchanger was totally different from Th(IV) tungstate which was due to the binding of inorganic precipitate (Th(IV) tungstate) with the organic part (SDS). The EDS analysis (Fig. 2c) showed that Cu2+ element appeared after the adsorption of Cu2+ which confirmed the adherence of Cu2+ over SDS–TT surface. The TEM image of SDS–TT showed that the particle size was around 20 nm which revealed that SDS–TT was nanocomposite cation exchanger (Fig. 2d).
Analytical and environmental applications
To explore the applicability of SDS–TT nanocomposite cation exchange material for the metal ions separation, distribution studies of different metal ions were evaluated in different acidic systems (Table 4). It is apparent from Table 4 that SDS–TT was selective for Cu2+ metal ion because Cu2+ had the higher K d values in comparison to other metal ions in all solvent systems. It was also noted that all metal ions had the lowest K d values in nitric acid medium which may be due to the presence of high strength of H+ ions which reversed the process of adsorption and the process of regeneration predominated over the process of removal. The high K d values of Cu2+ in all solvent systems enabled its selective separation from the synthetic mixture (Hg2+, Cd2+, Fe3+, Cu2+ and Zn2+), pharmaceutical formulation (I-Vit) and brass alloy sample (Table 5). The results indicated the high efficiency of the column and the percentage recovery was more than 83 % which suggested that SDS–TT was suitable for the removal and recovery of Cu2+ in synthetic as well as real samples.
Adsorption kinetics and isotherms
The kinetics for the adsorption of Cu2+ onto SDS–TT was analyzed using pseudo-first-order and pseudo-second-order kinetic models.
The pseudo-first-order equation is expressed as:
where, q t and q e are the amounts of Cu2+ adsorbed at time t, and at equilibrium, respectively, and k 1 is the rate constant for pseudo-first-order adsorption (min−1). The values of rate constant were determined from the slope of the plot log (q e − q t ) versus t.
The pseudo-second-order kinetic rate equation is given as [24]:
where k 2 is the rate constant of the pseudo-second-order sorption (g/mg min). The values of k 2 were determined from the intercepts of the plot t/q t versus time.
Both kinetic models are given in Fig. 5. The values of different constants and correlation coefficients are shown in Table 6. The fitted linear regression plot showed that the experimental data were well fitted to the pseudo-second-order model with better value of correlation coefficient (R 2 > 0.990).
In the present study, the isotherm results were evaluated using the Langmuir and Freundlich isotherms.
Langmuir isotherm model can be given as:
where, q e is the amount of Cu2+ adsorbed (mg g−1), C e is the equilibrium concentration of Cu2+ (mg L−1), Q m and b are the Langmuir constants related to maximum monolayer adsorption capacity and energy of adsorption, correspondingly.
The values of Q m and b were evaluated from the intercept and slope of linear plots of 1/q e versus 1/C e (Fig. 3), respectively, which are given in Table 7.
The linearized form of Freundlich isotherm is given as:
where, K f and n are the Freundlich adsorption constants which were determined from the intercept and slope of the linear plots of log q e versus log C e (Fig. 4), respectively. The values of correlation coefficients were higher for Freundlich isotherm which showed the better applicability of this model.
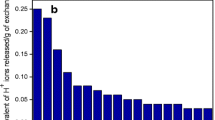
To make adsorption process economically feasible it is essential to testify the regeneration potential of ion exchangers. Adsorption/desorption and regeneration studies were carried out in batch mode using SDS–TT. The optimum recovery of Cu2+ was 89 % by 0.01 M HNO3 (Fig. 5). To check the reusability of exhausted SDS–TT nanocomposite cation exchanger, regeneration studies were conducted for seven consecutive cycles (Fig. 5). It was noticed that the adsorption was reduced from 90 to 80 %, while desorption reduced from 88 to 70 %, after seven consecutive cycles. The regeneration studies showed that SDS–TT was an excellent nanocomposite cation exchanger which could be utilized for the removal and recovery of Cu2+ without any considerable loss in the adsorptive performance.
Conclusion
In the present study, a new nanocomposite cation exchanger SDS–TT was synthesized by sol–gel method. The X-Ray diffraction pattern suggested a good crystalline nature of SDS–TT nanocomposite cation exchanger. The TEM image of SDS–TT showed that the particle size was around 20 nm. SDS–TT showed a high affinity for Cu2+ in comparison to other metal ions studied. On the basis of this behavior, SDS–TT was successfully used for the separation of Cu2+ quantitatively from the synthetic mixture of metal ions, pharmaceutical formulation and brass alloy sample. Freundlich isotherm and pseudo-second-order kinetic models were obtained as the most suitable models for the adsorption of Cu2+ onto SDS–TT nanocomposite cation exchanger. The adsorbed Cu2+ was recovered using 0.01 M HNO3 solution and the regenerated SDS–TT was effectively used up to seven consecutive cycles without any appreciable loss in the percent adsorption of Cu2+.
References
J. Keary, A.D. Mortimer, in Ion Exchange Developments and Applications, vol. 35, ed. by J.A. Greig (The Royal Society of Chemistry, Cambridge, UK, 1996), pp. 35–42
J. Lee, D.F. Hussey, G.L. Foutch, in Ion Exchange at the Millennium, vol. 61 ed. by J.A. Greig (Imperial College Press, London, 2000) pp. 61–68
A. Clearfield, Inorganic Ion Exchange Materials (CRC Press, Boca Raton, 1982)
Mu. Naushad, Chem. Eng. J. 235, 100 (2014)
Z.A. ALOthman, Inamuddin, Mu. Naushad, Chem. Eng. J. 171, 456 (2011)
S.S.M. Hassan, S.A. Marei, I.H. Badr, H.A. Arida, Anal. Chim. Acta 427, 21 (2001)
S.A. Nabi, S.A. Ganai, M. Naushad, Ads. Sci. Tech. 26, 463 (2009)
G. Sharma, D. Pathania, Mu. Naushad, N.C. Kothiyal. Chem. Eng. J. 251, 413 (2014)
Z.A. ALOthman, Inamuddin, Mu. Naushad, Chem. Eng. J. 166, 639 (2011)
Z.A. ALOthman, Inamuddin, Mu. Naushad, Chem. Eng. J. 169, 38 (2011)
S.A. Nabi, Mu. Naushad, Inamuddin, J. Hazard Mater. 42, 404 (2007)
S.A. Nabi, Mu. Naushad, Chem. Eng. J. 158, 100 (2010)
O.M. Vatutsina, V.S. Soldatov, V.I. Sokolova, J. Johann, M. Bissen, A. Weisswnbacher, React. Funct. Polym. 67, 184 (2007)
L. Wang, L. Yang, Y. Li, Y. Zhang, X. Ma, Z. Ye, Chem. Eng. J. 163, 364 (2010)
L.C. Lin, R.S. Juang, Chem. Eng. J. 132, 205 (2007)
J. Peric, M. Trgo, N.V. Medvidovic, Water Res. 38, 1893 (2004)
Mu. Naushad, Inamuddin, T.A. Rangreez, Z.A. ALOthman, J. Electroanal. Chem. 713, 125 (2014)
Z. Ujang, G.K. Anderson, Water Sci. Technol. 38, 521 (1998)
A. Dabrowski, Z. Hubicki, P. Podkoscielny, E. Robens, Chemosphere 56, 91 (2004)
M.R. Awual, M.M. Hasan, A. Shahat, M. Naushad, H. Shiwaku, T. Yaita, Chem. Eng. J. 265, 210 (2015)
Z.A. ALOthman, M.M. Alam, Mu. Naushad, J. Ind. Eng. Chem. 19, 956 (2013)
K.G. Varshney, M.Z.A. Rafiquee, A. Somya, M. Drabik, Indian J. Chem. 45A, 1856 (2006)
K.G. Varshney, M.Z.A. Rafiquee, A. Somya, Coll. Surf A Physiochem. Eng. Asp. 301, 224 (2007)
N. Iqbal, M. Mobin, M.Z.A. Rafiquee, Chem. Eng. J. 169, 43 (2011)
K.M. El-Azony, A.M. Ismail, A.A. El-Mohty, J. Radioanal. Nucl. Chem. 289, 381 (2011)
A. Mohammad, Inamuddin, A. Amin, J. Therm. Anal. Calorim. 107, 127 (2012)
L.L. Schramm, E.N. Stasiuk, D.G. Marangoni, Annu. Rep. Prog. Chem. Sect. C Phys. Chem. 99, 3 (2003)
S. Tagashira, S. Kimoto, K. Nozaki, Y. Murakami, Anal. Sci. 25, 723 (2009)
N. Li, N. Bai, Sep. Purif. Technol. 42, 237 (2005)
M.M. Rao, A. Ramesh, G.P.C. Rao, K. Seshaiah, J. Hazard Mater. B 129, 123 (2006)
S.A. Nabi, S. Usmani, N. Rahman, Ann. Chim. Sci. Fr. 21, 521 (1996)
W. Kossel, Ann. Phys. 49, 229 (1916)
L. Paulling, J. Am. Chem. Soc. 51, 1010 (1929)
F.A. Miller, C.H. Wilkins, Anal. Chem. 24, 1253 (1952)
S.A. Nabi, M. Naushad, Coll Surf. A Phys. Eng. Asp. 293, 175 (2007)
C. Dual, Inorganic thermogravimetric analysis (Elsevier, Amsterdam, 1963)
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding the work through the research group project No. RGP-VPP-130.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Naushad, M., ALOthman, Z.A., Alam, M.M. et al. Synthesis of sodium dodecyl sulfate-supported nanocomposite cation exchanger: removal and recovery of Cu2+ from synthetic, pharmaceutical and alloy samples. J IRAN CHEM SOC 12, 1677–1686 (2015). https://doi.org/10.1007/s13738-015-0642-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-015-0642-8