Abstract
We demonstrate herein the modification of magnetic nanoparticles and their use as a magnetic nanocatalyst in direct coupling reactions of aryl halides with terminal alkynes. Magnetite particles were prepared by simple co-precipitation method in aqueous medium, and then Fe3O4@ SiO2 nanosphere was synthesized by using nano-Fe3O4 as the core, TEOS as the silica source and PVA as the surfactant. Fe3O4@SiO2 was coated with polymeric N-heterocyclic carbene/Pd. The samples were characterized by X-ray diffraction, Fourier transform infrared spectroscopy, transmission electron microscopy, field emission scanning electron microscopy, dynamic light scattering, thermogravimetric analysis, vibration sample magnetometer and N2 adsorption–desorption isotherm analysis. Poly (N-vinyl imidazole) functionalized Fe3O4@SiO2 nanoparticle was found to be an efficient nanocatalyst in Sonogashira–Hagihara cross-coupling reactions. The nanocatalyst can be easily recovered by a magnetic field and reused for six runs without appreciable loss of its catalytic activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During the past decade, scientists have developed techniques for synthesizing and characterizing many new materials with at least one dimension on the nanoscale, including nanoparticles, nanolayers and nanotubes [1]. Nanostructured materials, a new branch of materials research, are attracting a great deal of attention because of their potential applications in areas such as catalysis [2], electronics [3], ceramics [4], optic [5], magnetic data storage [6] and nanocomposites. One of the most fundamental characteristics of nanometer-sized particles is their very high surface-to-volume ratio. This can lead to novel and unexpected atomic arrangements and may also have dramatic effects on other physical or chemical attributes [7].
In recent years, the synthesis of superparamagnetic nanoparticles has been intensively developed not only for its fundamental scientific interest, but also for many technological applications: catalytic [8], biosensing [9], magnetic storage media [10], medical applications, such as contrast agents in magnetic resonance imaging (MRI) [11–14], targeted drug delivery [15, 16], magnetic inks for jet printing [17], tissue repairing [18], immunoassay and [18] hyperthermia treatment of cancer cells [19], detoxification of biological fluids [18] and cell separation [18]. These applications are mainly based on the magnetic feature of the solid phase that enables achieving a rapid and easy separation from the reaction medium in a magnetic field [20].
Nanoscale magnetite (Fe3O4) is cheap, non-toxic, biocompatible and easy to prepare [21]. Many methods have been developed for the preparation of Fe3O4 such as sonochemical synthesis [22], thermal decomposition [23], microemulsions [24] and chemical co-precipitation [25, 26]. Among these methods, chemical co-precipitation may be the most promising, because of its simplicity and productivity.
Recently, magnetic core–shell nanostructures have attracted more attention due to their unique magnetic properties. These core–shell nanostructure magnetic catalysts can be easily retrieved under the influence of a magnetic field and used in subsequent reactions. Due to this property, using magnetic core–shell structure composites as catalysts has been recommended in many studies [27]. Also, several methods have been developed to prepare polymer coatings on magnetite nanoparticles such as physical adsorption of polymers and emulsion polymerization in the presence of nanoparticles, the so-called “grafting-to” and “grafting-from” methods [28, 29].
Obviously, homogeneous catalysts show higher catalytic activities than their heterogeneous counterparts because of their solubility in reaction media, which increases catalytic site accessibility for the substrate. However, recycling homogeneous catalysts is often tedious and time consuming and there is also the issue of product contamination observed when these catalysts are used [27]. Immobilization of homogeneous catalysts on various insoluble supports, especially porous materials with high surface areas, is usually the method of choice, since the immobilized catalysts can be facilely recovered via a simple filtration process after reactions [30]. Therefore, a number of functionalized Fe3O4 nanoparticles have been employed in a range of organic transformations, and several studies on immobilization of metal and organo-catalysts on silica-coated iron oxide nanoparticles have been reported [31–34].
Palladium-catalyzed Sonogashira–Hagihara cross-coupling is one of the most widely used carbon–carbon forming reactions [35, 36] and has been widely applied in areas such as natural products [37], pharmaceuticals [38] and biologically active molecules [39]. It provides an efficient route to obtain materials for nonlinear optical and molecular electronics [40], alkenyl- and arylacetylenes, substituted alkynes [41], conjugated oligomers and polymers [42] and symmetrical diynes [43]. The Sonogashira coupling reaction of terminal alkynes with aryl halides can occur in the presence of catalysts such as MCM-41-S–Pd(0) [44], Pd/C [45], CELL–Pd(0) [46], Pd(dmba)Cl(PTA) [47], PdCl2(PCy3)2 [48] and PdCl2(PPh3)2 [49]. In addition, the use of transition metals such as Co [50], Fe [51, 52], Ni [53], In [54] and Ru [55] has been also reported for these reactions. Although these methods are valuable, many of these procedures have significant drawbacks such as tedious workup procedures, low yields, long reaction times, high temperatures and non-recoverable catalysts. Thus, the development of a new procedure for the Pd-catalyzed Sonogashira–Hagihara reaction is still a desirable goal.
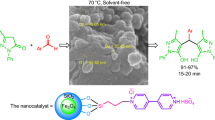
In this work, we report the preparation of Fe3O4@SiO2–polymer-imid–Pd nanocatalyst as illustrated in Scheme 1 and its use as a magnetic nanocatalyst in direct coupling reactions of aryl halides with terminal alkynes.
Experimental
General
All the chemicals reagents used in our experiments were purchased from the Merck Chemical Company in high purity. All the solvents were distilled, dried and purified by standard procedures. Fourier transform infrared (FT-IR) spectra were obtained using a Shimadzu FT-IR 8300 spectrophotometer. NMR spectra were recorded on a Bruker Avance DPX 250 MHz spectrometer in CDCl3 or DMSO-d6 using tetramethylsilane (TMS) as an internal reference. X-ray powder diffraction (XRD) spectra were taken on a Bruker AXS D8-advance X-ray diffractometer with Cu Kα radiation (λ = 1.5418). Transmission electron microscopy (TEM) images were obtained on a Philips EM208 transmission electron microscope with an accelerating voltage of 100 kV, and field emission scanning electron microscopy (FE-SEM) images were obtained on HITACHI S-4160. The BET surface area and porosity of catalysts were determined from nitrogen physisorption measured on a Micromeritics ASAP 2000 instrument at 196 °C. TGA thermograms were recorded on an instrument of Perkin Elmer with N2 carrier gas and with the rate of temperature change of 20 °C min−1. Magnetic properties were obtained on a BHV-55 vibrating sample magnetometer (VSM) and dynamic light scattering (DLS) was recorded on a HORIBA-LB550. Pd loading and leaching test was carried out with an inductively coupled plasma (ICP) analyzer (Varian, vista-pro). Mass spectra were obtained at 70 eV. All products were identified by comparison of their spectral data and physical properties with those of the authentic sample and all yields refer to isolated products. The progress of the reaction was monitored by TLC and purification was achieved by silica gel column chromatography.
General procedure
Preparation of Fe3O4@SiO2 core–shell
The core–shell Fe3O4@SiO2 nanospheres were prepared by a modified Stober method in our previous work [27]. In a typical procedure, the mixture of FeCl3·6H2O (1.3 g, 4.8 mmol) in water (15 mL) was added to the solution of polyvinyl alcohol (PVA 15000), as a surfactant, and FeCl2·4H2O (0.9 g, 4.5 mmol) in water (15 mL), which was prepared by completely dissolving PVA in water followed by addition of FeCl2·4H2O. The resultant solution was left to be stirred for 30 min in 80 °C. Then, hexamethylenetetraamine (HMTA) (1.0 mol/L) was added dropwise with vigorous stirring to produce a black solid product when the reaction media reached pH 10. The resultant mixture was heated on a water bath for 2 h at 60 °C, the black magnetite solid product was filtered and washed with ethanol three times and was then dried at 80 °C for 10 h. Then, Fe3O4 nanoparticle (0.50 g, 2.1 mmol) was dispersed in the mixture of ethanol (50 mL), deionized water (5 mL) and tetraethoxysilane (TEOS) (0.20 mL), followed by the addition of 5.0 mL of NaOH (10 wt%). This solution was stirred mechanically for 30 min at room temperature. Then the product, Fe3O4@SiO2, was separated by an external magnet and was washed with deionized water and ethanol three times and dried at 80 °C for 10 h.
Synthesis of poly (N-vinylimidazole) functionalized Fe3O4@SiO2 nanoparticle (Fe3O4@SiO2–polymer-imid)
Fe3O4@SiO2 (1 g) was added to the solution of 3-aminopropyl (triethoxy) silane (1 mmol, 0.176 g) in ethanol (10 mL) and the resultant mixture was kept under reflux for 12 h. The solvent was removed and the resulting solid (Fe3O4@SiO2–NH2) was dried at 80 °C overnight. The product was washed with ethanol and water to remove unreacted species and dried at 80 °C for 6 h. Fe3O4@SiO2–NH2 (1 g) was suspended in dry THF (15 mL) and the suspension cooled down to 0 °C. Triethylamine (0.151 g, 1.5 mmol) was added, followed by addition of acryloyl chloride (0.109 g, 1.2 mmol) over a period of 1 h. Then, the resultant mixture was stirred at 0 °C for a further 4 h and the modified Fe3O4@SiO2 was isolated by external magnetic field and washing with THF (10 mL), water (2 × 10 mL) and acetone (10 mL). The solid obtained was then dried at 80 °C for 12 h. To the resultant mixture (1.0 g), N-vinylimidazole (2 mL) and recrystallized benzoyl peroxide (0.025 g) were added and the mixture was heated at 80 °C for 12 h. The poly (N-vinylimidazole) functionalized Fe3O4@SiO2 nanoparticle (Fe3O4@SiO2–polymer-imid) was separated by an external magnet, washed with deionized water and ethanol three times and dried at 80 °C for 10 h.
Synthesis of polymer-imid–Pd functionalized Fe3O4@SiO2 nanoparticle (Fe3O4@SiO2–polymer-imid–Pd)
Fe3O4@SiO2–polymer-imid (1 g) was dispersed in the DMF solution (15 mL), ultrasonically for 15 min. Then, methyl iodide (2.5 mmol, 0.155 mL) was added, the mixture was stirred at 80 °C for 16 h and filtered in an external magnetic field. The product was washed with DMF and ethanol to remove any reacted species and dried at 70 °C for 6 h. The resultant solid product stirred in NaCl solution (5 %) (30 mL) at room temperature for 24 h. The mixture was filtered off, washed thoroughly with excess H2O and then dried in an oven under vacuum at 70 °C for 8 h (Fe3O4@SiO2–polymer-imid–S). The chloride ion capacity of imidazolium-type Fe3O4@SiO2-polymer was found using an argentometric titration method (0.1 g of Fe3O4@SiO2–polymer-imid–S was suspended in 10 mL of 0.1 M HNO3. After adding 1 mL of 0.1 M AgNO3, the mixture was stirred for 10 h at room temperature. The chloride counterions precipitated as AgCl. The remaining Ag+ was back titrated using 0.1 M HCl. The permanent charge density of imidazole groups was calculated to be 1.31 mmol/g). The resulting Fe3O4@SiO2–polymer-imid–S (1.0 g) was reacted with PdCl2 (3 mmol) in the presence of Et3N (6 mmol) as a base and DMF (15 mL) as a solvent at 80 °C for 16 h. The mixture (Fe3O4@SiO2–polymer-imid–Pd) was filtered in an external magnetic field, washed thoroughly with DMF (2 × 5 mL) and H2O (2 × 5 mL) and dried at 70 °C for 8 h.
General procedure for the Sonogashira–Hagihara reaction using Fe3O4@SiO2–polymer-imid–Pd magnetic nanocatalyst
A mixture of ArX (1.0 mmol), alkyne (1.2 mmol), Fe3O4@ SiO2-polymer-imid–Pd magnetic nanocatalyst (0.03 g), Et3N (2 mmol) and DMF (4.0 mL) was stirred at 80 °C in an oil bath. The progress of the reaction was monitored by TLC or GC. After completion of the reaction and separation of Fe3O4@SiO2–polymer-imid–Pd using a magnetic field, 15 mL of water was added and the mixture extracted with Et2O. The combined organic phases were washed with water (2 × 5 mL) and dried over anhydrous Na2SO4. Then, the solvent was removed under reduced pressure. The resulting crude product was purified by flash chromatography to give the desired pure coupling products in high to excellent isolated yields.
Results and discussion
The FT-IR spectrum of Fe3O4, Fe3O4@SiO2, Fe3O4@SiO2–NH2, Fe3O4@SiO2–polymer-imid, Fe3O4@SiO2–polymer-imid–S and Fe3O4@SiO2–polymer-imid–Pd nanoparticles are shown in Fig. 1a–f. In Fig. 1a, the absorption band at 559 cm−1 is assigned to the stretching vibration of the Fe–O band of Fe3O4. Also, the peaks at around 3,400 and 1,620 cm−1 in Fig. 1a are due to the adsorbed water in the sample. The surfaces of pure Fe3O4 nanoparticles were readily covered with SiO2 layers. In Fig. 1b, the presence of vibration bands at 561, 1,000–1,150 and 3,400 cm−1, due to Fe–O, Si–O–Si, and –OH, respectively, demonstrates the existence of Fe3O4@ SiO2. Figure 1c shows the FT-IR spectrum of Fe3O4@SiO2–NH2 nanoparticles; the peaks at 560, 1,000–1,150, 1,400–1,410 and 1,543 cm−1 are attributed to Fe–O (stretching vibration), Si–O–Si (asymmetric stretching), C–N(stretching vibration) and N–H (bending), respectively. Also, the presence of several bands with medium intensity in 28,010–2,986 cm−1 and 3,050–3,250 regions are allocated to C–H stretching of the propyl group and N–H stretching (Fig. 1c). The presence of vibration bands at 559, 1,000–1,150, 1,445, 1,649 and 2,792–2,985, due to Fe–O, Si–O–Si, C=N, C=O and CH, respectively, demonstrates the existence of Fe3O4@SiO2–polymer-imid in the spectrum (Fig. 1d). The FT-IR spectrum of Fe3O4@SiO2–polymer-imid–S exhibits a band at 1,518 cm−1 assigned to the C–H bending of the imidazolium group (Fig. 1e). The absorption bands at 562, 1,000–1,150, 1,653 and 2,850–3,000 cm−1 are in correspondence with vibrations of Fe–O, Si–O–Si, C=O and C–H stretching in Fe3O4@SiO2–polymer-imid–Pd (Fig. 1f). It also shows a band in the region of 790 cm−1 which confirms the presence of C–Pd absorption frequency in Fe3O4@SiO2–polymer-imid–Pd (Fig. 1f).
Structural investigation of samples was carried out by powder X-ray diffraction (XRD) technique. Figure 2 shows the XRD patterns for pure Fe3O4, Fe3O4@SiO2 and Fe3O4@SiO2-polymer–Pd nanoparticles. As shown in Fig. 2a, the Fe3O4 nanoparticle has highly crystalline cubic spinel structure, which agrees with the standard Fe3O4 (cubic phase) XRD spectrum (PDF#88-0866). The characteristic peaks at 2Θ = 30.1°, 35.4°, 43.1°, 53.4°, 57° and 62.6° for pure Fe3O4 nanoparticles, which were marked, respectively, by their indices (220), (311), (400), (422), (511) and (440), were also observed for Fe3O4@SiO2 and Fe3O4@SiO2-polymer–Pd nanoparticles (reference JCPDS card no.19-629). This revealed that the surface modification and conjugation of the Fe3O4 nanoparticles do not lead to their phase change. From Fig. 2b, we can see the XRD pattern of Fe3O4@SiO2 showing an obvious diffusion peak at 2Θ = 15–25°, generally considered as the diffusion peak of amorphous silica. For Fe3O4@SiO2–polymer-imid–Pd nanoparticles, the broad peak was transferred to lower angles due to the synergetic effect of amorphous silica and polymer. Also, the XRD pattern of Fe3O4@SiO2–polymer-imid–Pd nanocatalyst, showing the peaks of both Fe3O4 and Pd and the diffraction peak at 39.79°, indicates that the Pd particles are in a metallic state. The broadening of each peak in XRD mean crystallite size was calculated by applying Scherrer’s equation: D = 0.9 λ/β cosΘ, where D is the average diameter in A°, λ is the wavelength of the X-rays, β is the broadening of the diffraction line measured at half of its maximum intensity in radians and Θ is the Bragg diffraction angle. The mean crystallite size superparamagnetic nanocatalyst was found to be around 80 nm.
The morphology and sizes of (a) Fe3O4 and (b) Fe3O4@SiO2 particles were observed by transmission electron microscopy (TEM) as shown in Fig. 3. As shown in Fig. 3a, the Fe3O4 nanoparticles prepared by the chemical coprecipitation are quasi spherical with an average of about 12 nm. Figure 1b clearly displays that Fe3O4 nanoparticles have been successfully encapsulated into the SiO2 shell, and Fe3O4@SiO2 nanoparticles were obtained with a diameter of about 20 nm due to the agglomeration of Fe3O4 inside nanospheres and surface growth of silica on the shell. The mesoporous silica shell on the surface of Fe3O4 is quite homogeneous and exhibits good monodispersity with an estimated thickness of 8 nm.
The morphology of Fe3O4@SiO2–polymer-imid–Pd nanoparticle was also observed by FE-SEM (Fig. 3c). The Fe3O4@SiO2–polymer-imid–Pd nanoparticles are spherical in shape with a smooth surface morphology. The diameter of the nanoparticles is found to be approximately 90 nm. The FE-SEM images indicate the successful coating of the magnetic Fe3O4 particles.
The hydrodynamic diameter of Fe3O4, Fe3O4@SiO2 and Fe3O4@SiO2–polymer-imid–Pd nanoparticles is determined by the DLS technique (Fig. 3). This size distribution is centered at a value of 12, 20 and 85 nm for Fe3O4, Fe3O4@SiO2 and Fe3O4@SiO2–polymer-imid–Pd, respectively (Fig. 3d–f). The theoretical curve of standard distribution from our studies was calculated by means of Microsoft Excel.
N2 adsorption–desorption isotherm analysis provides information on the specific surface area and porosity of the prepared samples. The surface areas and average pore radius were measured by N2 adsorption and results are shown in Table 1.
The results of magnetization measurements as a function of applied magnetic field are shown in Fig. 4a. It indicated that all products had superparamagnetism. The magnetization curve and demagnetization curve are coincident, no hysteresis phenomenon is found, and remanent magnetization and coercivity are equal to zero. As shown in Fig. 4a(a), the saturation magnetization of Fe3O4 is 65.8 emu/g, while for Fe3O4@SiO2–polymer-imid–Pd with a 75 nm shell 27.3 emu/g at 300 K [Fig. 4a(b)]. These results indicated that the magnetization of Fe3O4 decreased considerably with the increase of SiO2 and polymer. Nevertheless, the polymer–Pd supported on Fe3O4@SiO2 can still be separated from the solution by using an external magnetic field [Fig. 4a(b)].
Determination of Pd content was performed by inductively coupled plasma (ICP) analyzer. According to the ICP analysis, the Pd content in the magnetic nanocatalyst was determined, which revealed the presence of 0.33 mmol/g for this catalyst.
Thermal analysis was performed to confirm coating formation on the surface of Fe3O4@SiO2. As shown in Fig. 4b, the TGA curve of Fe3O4@SiO2–polymer-imid–Pd shows first weight loss of 2.6 % below 120 °C, which might be due to the loss of adsorbed water in the sample. The mass loss of about 27.74 % by weight in the range of 200–500 °C is attributed to the decomposition of pure polymer and the temperature of the maximum weight loss is 487 °C (Fig. 4b). Below 200 °C, the rate of weight loss is relatively slow owing to the loss of residual water adhering to the sample surface. Thus, the TGA curve confirmed the successful grafting of polymer molecules on the magnetic surface.
The photographs of the dispersions of the Fe3O4@SiO2–polymer-imid–Pd nanoparticles are given in Fig. 5a, which was well dispersed in DMF under normal conditions. On the contrary, the nanoparticles rapidly gathered on the sidewall of the cylinder under a magnetic approach (Fig. 5b). This result indicated that the Fe3O4@SiO2-polymer–Pd nanoparticles can be easily manipulated by an external magnetic field.
To show the merit of application of these magnetic nanoparticles in organic synthesis, we applied them as the catalysts in the Sonogashira–Hagihara cross-coupling reactions. Initial studies were performed upon the reaction of iodobenzene with phenylacetylene as a model reaction and the effects of different solvents, temperature, bases and amount of catalyst were studied for this reaction (Table 2). To elucidate the role of the catalyst, initially the reaction between iodobenzene and phenylacetylene was examined in the presence of varying amounts of the nanocatalysts and the results are presented in Table 2. The best result was achieved by carrying out the reaction with (0.03 g:1:1.2 mmol) ratio of nanocatalyst, iodobenzene and phenylacetylene in DMF at 80 °C (Table 2, entry 6). The use of a higher amount of nanocatalysts did not improve the yield (Table 2, entry 14), while a decrease in the amount of nanocatalysts decreased the yield (Table 2, entries 12 and 13). In the absence of a magnetic nanocatalyst, the reaction did not proceed even after a long reaction time (10 h) (Table 2, entry 17).

The results showed that among the tested solvents, DMF was more efficient and the desired product was obtained in shorter reaction times (30 min) and higher yields (Table 2, entry 6) than the other solvents under study (Table 2, entries 1–5). In the reactions employing tetrahydrofuran, toluene, water, acetonitrile and dimethyl sulfoxide as solvents, the reactions did not progress efficiently and after 2 h the desired product was obtained in only 66, 41, 79, 83 and 86 % yields, respectively, at 80 °C (Table 2, entries 1–5).
The effect of different bases on the reaction of iodobenzene (1 mmol) with phenylacetylene (1.2 mmol) in the presence of Fe3O4@SiO2-polymer–Pd magnetic nanoparticles (0.03 g) in DMF (4 mL) at 80 °C was studied (Table 2, entries 6, 15-21). We have presented the results of this study in Table 2, which indicates that Et3N is the most suitable among the different bases studied for this purpose (Table 2, entry 6).
Also, the effect of temperature was studied by carrying out the model reaction at different temperatures (room temperature, 40, 60, 80 and 90 °C) in DMF in the presence of Et3N and the best results were obtained at 80 °C (Table 2, entries 6–10). Therefore, we continued the reactions under optimum conditions (Table 2, entry 6).
The generality of the reaction of phenylacetylene with diverse aryl halides was studied under optimum conditions, that is, Fe3O4@SiO2-polymer–Pd (0.03 g), DMF (4 mL) and 2 mmol of Et3N and the results are summarized in Table 3. As expected, the reaction of aryliodides bearing electron-donating groups was completed with longer reaction times (Table 3, entries 2–4) than those with electron-withdrawing groups (Table 3, entries 5, 6, 8 and 9). The coupling of ortho- and meta-substituted iodobenzenes having ortho-methyl, ortho-chloro and meta-chloro groups took place with phenylacetylene to give the corresponding products in 87, 87, and 90 % yield, respectively (Table 3, entries 3, 8 and 9). The coupling reaction of phenylacetylene with both electron- releasing and electron-withdrawing aryl bromides afforded the desired products in high yields (Table 3, entries 10–17). Also, 3-bromopyridine, 5-bromopyrimidine and 2-thiophenyl iodide led to the corresponding arylated alkynes in good yields (Table 3, entries 15–17). Although aryl chlorides are not as reactive and are less likely employed in palladium-catalyzed coupling reactions, Sonogashira–Hagihara reactions could take place using phenylacetylene and aryl chlorides in the presence of catalytic amount of Fe3O4@SiO2–polymer-imid–Pd nanoparticles (Table 3, entries 18-21).

To confirm the reusability and stability of the magnetic nanocatalyst, it was separated from the reaction mixture after its first use in the Sonogashira–Hagihara reaction. The recovered catalyst was found to be reusable for six cycles with a slight loss in activity (Fig. 6).
Conclusions
Fe3O4 was successfully prepared by co-precipitation with FeCl2 and FeCl3 as reaction substrate, polyvinyl alcohol (PVA 15000) as surfactant and hexamethylenetetraamine as precipitant. The core–shell Fe3O4@SiO2 nanospheres were prepared by a modified Stober method. Then, Fe3O4@SiO2 was coated with polymeric N-heterocyclic carbene/Pd. The nanocatalyst was successfully synthesized and structural, surface, morphological and magnetic properties of these nanoparticles were evaluated. TEM microscopy revealed a very fine layer of SiO2 and polymer on the Fe3O4. The size of the prepared nanoparticles with roughly spherical shapes and core–shell structures was about 20 nm in diameter. Moreover, magnetization curves demonstrated a high degree of superparamagnetism. Magnetic nanocatalyst with saturation magnetization value of about 27.3 emu/g can be readily recovered under an external magnetic field. The amount of polymer capsulated around the magnetite nanoparticles in the prepared magnetic nanocatalyst was estimated to be 27.74 % by weight. The crystallite size obtained from X-ray line profile fitting is comparable with the particle size obtained from TEM. Therefore, considering the importance of this catalyst, we have shown that Fe3O4@SiO2–polymer-imid–Pd is an efficient and stable nanocatalyst and is strongly active in coupling reactions of aryl halides with terminal alkynes. This method gives notable advantages such as easy preparation, heterogeneous nature and easy separation of the catalyst by external magnetic field, excellent yields, short reaction times and simplicity of operation, making it a facile tool in the Sonogashira–Hagihara cross-coupling reaction. In addition, the novel catalyst used is easily recovered by using a permanent magnet and reused without any noticeable loss of activity after at least six times. The formation of the carbene–Pd bond leads to leaching of reduced Pd nanoparticles. Polymer chains acts as spacer between Fe3O4@SiO2 and Pd and leads to the increased catalytic activity of Pd. The ICP analysis does not show any Pd leaching at this stage. This experiment confirmed the heterogeneous character of the catalytically active species.
References
C.C. Koch, Nanostructured materials, processing, properties and applications (Norwich, New York, 2002)
J.M. Cowley, Diffraction physics (North-Holland, New York, 1981)
J.M. Cowley, Appl. Phys. Lett. 15, 58 (1969)
J.M. Cowley, Ultramicroscopy. 49, 4 (1993)
E. Zeitler, M.G.R. Thomson, Optik. 31, 258 (1970)
J.M. Rodenburg, R.H.T. Bates, Philos. Trans. R. Soc. 339, 521 (1992)
D. Zanchet, B.D. Hall, D. Ugarte, Characterization of nanophase materials (Wiley, Germany, 2000)
E.W. Wong, M.J. Bronikowski, M.E. Hoenk, R.S. Kowalczyk, B.D. Hunt, Chem. Mater. 17, 237 (2005)
M.M. Miller, G.A. Prinz, S.F. Cheng, S. Bounnak, Appl. Phys. Lett. 81, 2211 (2002)
S. Sun, C.B. Murray, D. Weller, L. Folks, A. Moser, Science. 287, 1989 (2000)
M.M.J. Modo, J.W.M. Bulte, Molecular and cellular MR imaging (CRC Press, Boca Raton, 2007)
C. Burtea, S. Laurent, A. Roch, L. Vander Elst, R.N. Muller, J. Inorg. Biochem. 99, 1135 (2005)
J.W. Bulte, Methods Mol. Med. 124, 419 (2006)
S. Boutry, S. Laurent, L. Vander Elst, R.N. Muller, Contrast Med. Mol. Imaging. 1, 15 (2006)
I. Chourpa, L. Douziech-Eyrolles, L. Ngaboni-Okassa, J.F. Fouquenet, S. Cohen-Jonathan, M. Souce, H. Marchais, P. Dubois, Analyst. 130, 1395 (2005)
J. Javidi, M. Esmaeilpour, Colloid. Surface B. 102, 265 (2013)
S.W. Charles, J. Popplewell, Endeavour. 6, 153 (1982)
A.K. Gupta, M. Gupta, Biomaterials. 26, 3995 (2005)
H. Pardoe, P.R. Clark, T.G. St Pierre, P. Moroz, S.K.A. Jones, Magn. Reson. Imag. 21, 483 (2003)
L. Lei, X. Liu, Y. Li, Y. Cui, Y. Yang, G. Qin, Mater. Chem. Phys. 125, 866 (2011)
B. Sahoo, S.K. Sahu, P. Pramanik, J. Mol. Catal. B Enzym. 69, 95 (2011)
K. Suslick, M. Fang, T. Hyeon, J. Am. Chem. Soc. 118, 11960 (1996)
S. Veintemillas-Verdaguer, M.P. Morales, C.J. Serna, Mater. Lett. 35, 227 (1998)
P. Tartaj, C. Serna, Chem. Mater. 14, 4396 (2002)
D. Kim, Y. Zhang, W. Voit, K. Rao, M. Muhammed, J. Magn. Magn. Mater. 225, 30 (2001)
R. Massart, E. Dubois, V. Cabuil, E. Hasmonay, J. Magn. Magn. Mater. 149, 1 (1995)
M. Esmaeilpour, A.R. Sardarian, J. Javidi, Appl. Catal. A 445–446, 359 (2012)
K. Ohno, K.M. Koh, Y. Tsujii, T. Fukuda, Macromolecules. 35, 8989 (2002)
S. Sun, S. Anders, H.F. Hamann, J.U. Thiele, J.E.E. Baglin, T. Thomson, E.E. Fullerton, C.B. Murray, B.D. Terris, J. Am. Chem. Soc. 124, 2884 (2002)
P.D. Stevens, J. Fan, H.M.R. Gardimalla, M. Yen, Y. Gao, Org. Lett. 7, 2085 (2005)
S. Shylesh, V. Schunemann, W.R. Thiel, Angew. Chem. Int. Ed. 49, 3428 (2010)
A. Schatz, M. Hager, O. Reiser, Adv. Funct. Mater. 19, 2109 (2009)
V. Polshettiwar, R. Luque, A. Fihri, H. Zhu, M. Bouhrara, J.M. Basset, Chem. Rev. 111, 3036 (2011)
A. Schatz, O. Reiser, W.J. Stark, Chem. Eur. J. 16, 8950 (2010)
K. Sonogashira, Y. Tohda, N. Hagihara, Tetradron. Lett. 16, 4467 (1975)
R. Chinchilla, C. Najera, Chem. Rev. 107, 874 (2007)
I. Paterson, R.D.M. Davies, R. Marquez, Angew. Chem. Int. Edit. 40, 603 (2001)
G. Amiet, H.M. Hugel, F. Nurlawis, Synlett. 41, 495 (2002)
N.D.P. Cosford, L. Tehrani, J. Roppe, E. Schweiger, M. Washburn, M.A. Varney, J. Med. Chem. 46, 204 (2003)
P. Li, L. Wang, H. Li, Tetrahedron. 61, 8633 (2005)
G. Altenhoff, S. Wurtz, F. Glorius, Tetrahedron Lett. 47, 2925 (2006)
A. Nagy, Z. Nova’k, A. Kotschy, J. Organomet. Chem. 690, 4453 (2005)
F. Yang, X. Cui, Y. Li, J. Zhang, G. Ren, Y. Wu, Tetrahedron. 63, 1963 (2007)
M. Cai, Q. Xu, J. Sha, J. Mol. Catal. A Chem. 272, 293 (2007)
M.B. Thathagar, G. Rothenberg, Org. Biomol. Chem. 4, 111 (2006)
K.R. Reddy, N.S. Kumar, P.S. Reddy, B. Sreedhar, M.L. Kantam, J. Mol. Catal. A Chem. 252, 12 (2006)
J. Ruiz, N. Cutillas, F. Lopez, G. Lopez, D. Bautista, Organometallics. 25, 5768 (2006)
C. Yi, R. Hua, J. Org. Chem. 71, 2535 (2006)
Y. Liang, Y.X. Xie, J.H. Li, J. Org. Chem. 71, 379 (2006)
L. Feng, F. Liu, P. Sun, J. Bao, Synlett. 10, 1415 (2008)
X. Xie, X. Xu, H. Li, X. Xu, J. Yang, Y. Li, Adv. Synth. Catal. 351, 1263 (2009)
M. Carril, A. Correa, C. Bolm, Angew. Chem. Int. Ed. 47, 4862 (2008)
M. Bakherad, A. Keivanloo, S. Mihanparast, Synth. Commun. 40, 179 (2010)
H.N. Borah, D. Prajapati, R.C. Boruah, Synlett. 655, 2823 (2005)
S. Park, M. Kim, D.H. Koo, S. Chang, Adv. Synth. Catal. 346, 1638 (2004)
H. Firouzabadi, N. Iranpoor, M. Gholinejad, J. Mol. Catal. A Chem. 321, 110 (2010)
T. Suzuka, Y. Okada, K. Ooshiro, Y. Uozumi, Tetrahedron. 66, 1064 (2010)
H. Firouzabadi, N. Iranpoor, F. Kazemi, M. Gholinejad, J. Mol. Catal. A Chem. 357, 154 (2012)
Acknowledgments
The authors are grateful to the council of Iran National Science Foundation and University of Shiraz for their unending effort to provide financial support to undertake this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohsen, E., Jaber, J., Mehdi, M.A. et al. Synthesis and characterization of Fe3O4@SiO2–polymer-imid–Pd magnetic porous nanospheres and their application as a novel recyclable catalyst for Sonogashira–Hagihara coupling reactions. J IRAN CHEM SOC 11, 499–510 (2014). https://doi.org/10.1007/s13738-013-0323-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-013-0323-4