Abstract
In the present study adsorption behavior of methylene blue and rhodamine B from aqueous solution using adsorbent prepared from “Hyptis suaveolens” (Vilayti Tulsi) was investigated as a function of parameters such as initial concentration, adsorbent dose, pH, contact time and temperature. The adsorption process was pH dependent. The thermodynamic parameters such as \( {{\Updelta}}G,{{\Updelta}}H \,{\text{and}} \,{{\Updelta}}S \) were calculated to investigate the nature of adsorption, their values indicate that the adsorption process is favorable. The first-order, second-order and intra-particle diffusion models were used to describe the kinetic parameter. The Freundlich and Langmuir adsorption models were applied to describe the adsorption equilibrium. Column study was conducted for both dyes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many countries face a common problem of disposal of liquid effluents from various industries viz. paper, leather, textile, etc. For industrial effluents, color is the first contaminant to be recognized due to its visibility. Color affects the nature of water and inhibits sunlight penetration into the stream and reduces photosynthesis [1]. Most of the dyes are resistant to biological degradation and some of dyes are carcinogenic and mutagenic [2]; they affect the biological oxygen demand and natural beauty of rivers [3]. Over 90 % of some 4,000 dyes tested in an Ecological and Toxicological Association of the Dyestuff survey (ETAD) had LD50 values greater than 200 mg kg−1. The highest rate of toxicity was found amongst basic and diazo direct dyes [4]. Thus, the removal of dyes from colored effluents is one of the major environmental concerns these days [5–7].
The available methods for removal of dye from effluents are flocculation, electro flotation, precipitation, electrokinetic coagulation, ion exchange, membrane filtration, irradiation, and adsorption. Each method has its own advantages and disadvantages, but adsorption has been found to be superior to other techniques in terms of simplicity of design, ease of operation and insensitivity to toxic substances [8]. It was observed that though activated carbon is the most effective adsorbent for adsorption of dyes, it is quite expensive and hence there is an increasing need for equally effective but cheaper adsorbents [5, 7]. Literature reveals that large number of waste by-products are used as adsorbents, some of them are orange pill [2], clay [1, 3], peanut hull [9], sand [10], bio-solid [11], coir pith [12, 13], rise husk, teak wood bark, cotton waste, hair [14], chitin [15], chitosan [16], bagasse pith [17], red mud [18], neem leaf powder [19], banana pith [20], etc. New economical, easily available and highly effective adsorbents are still needed.
Hyptis suaveolens (Vilayti Tulsi), an erect, woody, sweet-scented tropical American weed, has now widely spread in India. It is available along the roadside, waste lands, etc. round the year. Methylene blue (MB) is mainly used on bast (soft vegetable fibers such as jute, flax, and hemp) and to a lesser extent on paper, leather, and mordant cotton. It dyes silk and wool but it causes serious health problems like hypertension, pericardial pain, dizziness, mental confusion, anemia, nausea, bladder irritation and cancer. So it acts as environment pollutant and can present in effluent of paper and pulp industries, textile industries, etc. Rhodamine B (RDB) is used as a fluorescent dye and it is used in biotechnology. The adsorbent shows good result with these dyes and it is easily available in India. So these dyes are selected for sorption study. The adsorption of these dyes on untreated adsorbent gives poor result. The NaOH-treated adsorbent found to improve the adsorption.
The aim of the present work was to study the adsorption capacity of sodium hydroxide-treated H. suaveolens (Vilayti Tulsi) plant powder for removal of MB and RDB from aqueous solutions under different experimental conditions.
Experimental
Preparation of sorbent
The stem and branches (without leaves) of fully grown plants of H. suaveolens (Vilayti Tulsi) were collected in and around Aurangabad city (India), they were cut into small pieces of 2–3 cm and dried in sunlight, washed with distilled water 3–4 times and then with 0.01 M NaOH solution followed by distilled water to remove excess of alkali, dried at 353 K in hot air oven, and ground and sieved. The particle size was in the range of 20–53 μm. The material was placed in an airtight container for further use.
Preparation of sorbet
The basic dyes rhodamine B (CI-45170) (RDB) and MB (CI-52015) (MB) of Loba chem. have been used in the present study. An accurately weighed quantity of the dye was dissolved in double distilled water to prepare the stock solution (1,000 mg L−1). Experimental solutions of desired concentrations were prepared by successive dilutions.
Sorbet quantification
For the adsorption studies, 50 mL dye solution of known concentration and pH was added to 0.1 g of adsorbent in 250 mL glass stopper flask at room temperature and the contents were shaken thoroughly using a mechanical shaker rotating with a speed of 1,200 rpm for 5 min, the absorbance of supernatant solution was estimated to determine the concentration. For preparation of standard curve, λ max for RDB is 555 nm and MB is 665 nm. The concentration range is 1–9 ppm for both dyes. Initial pH was controlled by the addition of 0.5 M HCl and 0.5 M NaOH. Effect of adsorbent dosage was studied with different adsorbent dosages (0.05–0.5 g) and 50 mL of 20 mg L−1 of RDB and 50 mg L−1 of MB dye solutions agitated for 30 min.
Langmuir and Freundlich equations were employed to study the equilibrium. For temperature effect studies, 50 mL dye solution of 50 mg L−1 was shaken with 0.1 g adsorbent in thermo-stated rotary shaker at 313, 323 and 333 K. The column study was performed using down flow technique. Adsorbent (2 g) was packed in column of length 0.32 m (internal diameter 0.01 m), dye solution of optimum pH and concentration (50 mg L−1 for RDB and 150 mg L−1 for MB) was fed into the column at the flow rate of 3 mL min−1. The process was continued until effluent concentration reaches the influent concentration.
Results and discussion
Neither changes were observed in adsorption spectrum nor additional peak formed for dye solution after shaking it with the adsorbent. This indicates that there were no breakdown product(s) of the dye and also supported the fact that the dye removal from the solution in this study was through the mechanism of adsorption.
SEM
SEM is widely used to study the morphological features and surface characteristics of the adsorbent material [21]. In the present study, scanning electron microscopic photograph (Fig. 1) of adsorbent reveals its texture and porosity.
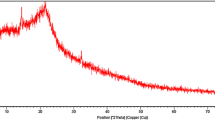
FTIR analysis
The FTIR analysis is carried out to find probable functional groups. FTIR analysis of the saturated adsorbent with dyes is not carried out. The FTIR spectrum (Fig. 2) shows the typical pattern for cellulose-containing materials. The band at 3,344–3,404 cm−1 shows –OH stretching frequency, 1,606, 1,633, 1,722 cm−1 shows the presence of carbonyl and C=C and 1,055 cm−1 shows C–O–C linkage in the compound.
Effect of pH
The hydrogen ion concentration is an important factor in controlling the adsorption. pH affects the degree of ionization of dyes and the surface properties of the adsorbent. The initial pH value of the solution has more influence on the process of adsorption than the final pH [22]. To study the effect of pH on adsorbents of RDB and MB; the experiments were carried out at 50 mg L−1 initial dye concentration with 0.1 g adsorbent dosage for 30 min.
The variation in adsorption of dyes on adsorbents as a function of pH is shown in Fig. 3. The dye adsorption was found to be dependent on the pH. It is evident from the IR spectrum that the adsorbent has functional groups such as –OH. At acidic pH these groups are protonated, an increase in pH from 4.0 to 9.0 increases adsorption of MB blue (20.5–22 mg g−1). The optimum pH value for the adsorption of RDB is 8.0 and for MB it is 9.0.
Effect of initial dye concentration
The effect of initial dye concentration on the rate of adsorption on the adsorbent was studied and the results are shown in Fig. 4. The experiments were carried out at fixed adsorbent dose (0.1 g), pH (8.0 for RDB and 9.0 for MB) and at different initial concentrations ranging from 20 to 50 mg L−1. The adsorption efficiency of adsorbent decreases with increase in initial dye concentration.
Though the percentage adsorption decreases with increase in initial dye concentration, the actual amount of dye adsorbed per unit mass of adsorbent increases with increase in dye concentration in solution. The unit adsorption increased from 6.55 to 13 mg g−1 as the concentration of RDB increased from 20 to 50 mg L−1 (Fig. 4a). Similarly unit adsorption increased from 9.5 to 23.05 mg g−1 as the MB concentration in solution is increased from 20 to 50 mg L−1 (Fig. 4b).
Effect of adsorbent dosage
The adsorption of RDB and MB on adsorbent was studied by varying the adsorbent quantity (0.05–0.5 g) in test solution keeping the initial dye concentration (20 mg L−1 for RDB and 50 mg L−1 for MB), temperature and at optimum pH constant for 30 min time intervals and results are shown in Fig. 5. For RBD the amount of adsorption increased from 13 to 1.43 mg g−1 and for MB the increase is from 41 to 4.7 mg g−1, as the adsorbent dosage increased from 0.05 to 0.5 g. Increase in adsorption with adsorbent dose can be attributed to increased adsorption surface area and availability of more adsorption sites resulting from increased dose and conglomeration of adsorbent. But unit adsorption decreased with increase in adsorbent dose. For RDB the unit adsorption decreased from 13.0 to 1.43 mg g−1 while for MB it decreased from 41.0 to 4.7 mg g−1, as the adsorbent dose increased from 0.05 to 0.5 g.
Sorption dynamics
The first-order kinetic model
The rate constant of adsorption is determined from the first-order rate expression given by Lagergren [23]
where q e and q t are the amount of dye adsorbed (mg g−1) at equilibrium and at time t (min), respectively, and K 1 is the rate constant of adsorption (L min−1). The Lagergren first-order plots for the adsorption of MB and RBD at various initial concentrations are given in Fig. 6a, b. Values of K 1 were calculated from the plot of log (q e − q t) versus t. The study of influence of initial MB and RBD on Lagergren rate constant from Table 1 shows a decrease in rate constant with increase in initial dye concentration. The applicability of Lagergren model suggests the formation of monomolecular layer of dye species on the surface of adsorbent. The values of K 1 and q e at different concentrations calculated from slopes and intercepts of this curve are represented in Table 1. That the correlation coefficient values are not high for all concentrations showed that the pseudo-first-order equation for Lagergren does not fit well with the whole range of contact time and is generally applicable to initial stage adsorption.
The second-order kinetic model
The Lagergren pseudo-second-order kinetic model [24] is expressed as
The initial adsorption rate, h (mg g−1 min) as t → 0 can be defined as
The initial adsorption rate (h), the equilibrium adsorption capacity (q e) and second-order constant K 2 (g mg−1 min) can be determined experimentally from slope and intercept of plot t/q t versus t. The plot for MB is given in Fig. 7a and for RDB is shown in Fig. 7b, these plots show good correlation coefficient when pseudo-second-order equation was employed. The K 2 and h values as calculated from Fig. 7a and b are listed in Table 1. Similar phenomena have been observed in the adsorption of congo red and 2-chlorophenol [25, 26]. Thus, by increasing initial dye concentration the equilibrium adsorption capacity \( q_{\text{e}} \) increases.
Adsorption equilibrium study
Various isotherm equations have been used to describe the equilibrium nature of adsorption. Two of them are used for the present study.
Langmuir isotherm
It is represented by the following equation [27]
where C e is the concentration of dye solution at equilibrium (mg L−1), q e is amount of dye absorbed at equilibrium (mg g−1), q m is Langmuir constant related to adsorption capacity (mg g−1), b is the Langmuir constant related to energy of adsorption (L mg−1).
The plots of \( C_{\text{e}} /q_{\text{e}} \) versus C e for MB is given in Fig. 8a and for RBD is shown in Fig. 8b. The correlation coefficient (i.e. R 2) values are close to unity suggesting the applicability of the above model to the present system, showing the monolayer formation of dyes at the outer surface of adsorbent. The values of \( q_{\text{m}} \) and b were obtained from slope and intercept of plot of \( C_{\text{e}} /q_{\text{e}} \,{\text{versus}}\, C_{\text{e}} \) and are shown in Table 2. The correlation coefficient which approaches unity suggests that the Langmuir isotherm fits well for both MB and RBD dyes. It is clear from Table 2 that the adsorption efficiency \( q_{\text{m}} \) increases from 0.776 to 0.1539 mg g−1 for RBD, while for MB it increases from 0.9089 to 1.609 mg g−1. For RBD the absorption energy b increases from 5.927 to 10.20 L mg−1 and from MB it increases from 17.88 to 19.56 L mg−1.
In order to find out the feasibility of isotherm, R L a dimensionless constant separation factor was determined by the equation [28, 29] as follows
where C 0 is the initial concentration of dye solution and ‘b’ is Langmuir isotherm constant. The parameter R L indicates the nature of the adsorption isotherm. R L > 1 is the unfavorable adsorption, 0 < R L < 1 is favorable adsorption, R L = 0 is irreversible adsorption, R L = 1 is the linear adsorptionThe values of R L lie between 0 and 1 which indicates that the process is favorable adsorption.
Freundlich isotherm
Freundlich [30] isotherm was also applied to plot the equilibrium data of the adsorption.
where q e is the dye concentration in solid at equilibrium (mg g−1), C e the dye concentration in solution at equilibrium (mg L−1), K f the measure of the adsorption capacity and n is the adsorption intensity
The intensity of adsorption is indicative of the bond energies between dye ion and adsorbent. The plot of \( {\text{log }}q_{\text{e}} \) versus \( { \log }\,C_{\text{e}} \) for MB is given in Fig. 9a and for RBD is shown in Fig. 9b. The values of ‘n’ and \( K_{f} \) were calculated from slope and intercept of these plots. The values of \( K_{f} \) and ‘n’ obtained are represented in Table 2. It shows that the values of adsorption intensity 1/n < 1 reveal that applicability of Freundlich adsorption is good for RBD. The correlation coefficient from Table 2 for RBD shows that Freundlich isotherm fits better than Langmuir isotherm.
Effect of temperature
Increase of temperature increases the percentage removal of dye. Changes in standard free energy, enthalpy and entropy of adsorption were calculated using following equations
where K 0 is the equilibrium constant, \( C_{\text{solid}} \) the solid phase concentration at equilibrium (mg L−1) and \( C_{\text{liquid}} \) is the liquid phase concentration at equilibrium (mg L−1)
where R is the gas constant, K 0 is the equilibrium constant and T is the temperature in Kelvin
According to Van’t Hoff equation
Plot of log K 0 versus 1/T is linear (Fig. 10), values of \( {{\Updelta}}H \) and \( {{\Updelta}}S \) were calculated from the slope and intercept of Van’t Hoff plot, respectively (Table 3). The value of \( \Updelta H \) for MB is 6.812 kJ mole−1 and for RDB is 18.17 kJ mole−1. The entropy change for RBD and MB is found to 59.69 and 41.13 J K−1 mole−1, respectively. The positive values of \( {{\Updelta}}H \) show endothermic nature of adsorption and it govern the possibility of physical adsorption. The free energy for RBD changes from −0.627 to −1.836 kJ mole−1 and for MB the energy change is from −6.020 to −6.837 kJ mole−1, respectively. The negative values of \( {{\Updelta}}G \) indicate the spontaneous and highly favorable nature of adsorption. Entropy of activation can be regarded as a measure of the “siddle point energy” over which reactant molecule must pass as activated complex. Thus, \( \Updelta S \) conveys whether a particular reaction proceeds faster or slower than another individual reaction. The positive values of \( {{\Updelta}}S \) suggest the increased randomness at the solid/solution interface during the adsorption of dye. The orientation or restructuring of water around the non-polar solute or surface is very unfavorable in terms of entropy, since it disturbs the existing water structure and imposes a new and more ordered structure on the surrounding water molecules. As a result of adsorption of MB on the adsorbate, the number of water molecules surrounding MB decreases and the degree of freedom of the water molecules increases.
Conclusion
The adsorption of RDB and MB from aqueous solutions was investigated under different experimental conditions. The conditions of maximum adsorption of dyes were optimized. It was observed that under optimum conditions up to 13 mg g−1 of RDB and 23.05 mg g−1 of MB dye can be removed from aqueous solutions. The adsorption data fit the Langmuir and Freundlich isotherms, showing that adsorption was monolayer in nature. The thermodynamic parameters were found to be thermodynamically favorable physical adsorption process. The kinetic parameter with high correlation coefficient shows the second-order nature of adsorption process. Since the adsorbent material is freely available in large quantities, the treatment method seems to be economical.
References
Y. Nacera, B. Aicha, Kinetic model for sorption of dye from aqueous solution by clay–wood sawdust mixture. Desalination 185, 499–508 (2005)
R. Sivaraj, C. Namasivayam, K. Kadirvelu, Orange peel as an adsorbent in the removal of acid violet. J. Waste Manag. 21, 105–110 (2001)
V.P. Vinod, T.S. Anirudhan, Adsorption behavior of basic dyes on the humic acid immobilized pillared clay. Water Air Soil Pollut. 150, 193–217 (2003)
J. Shore, Advances in direct dyes. Indian J. Fiber Text. Res. 21, 1–29 (1996)
T.A. Khan, V. Singh, D. Kumar, Removal of some basic dyes from artificial textile waste water by adsorption on Akash kinari coal. J. Sci. Ind. Res. 63, 355–364 (2004)
F.C. Wu, R.I. Tseng, R.S. Juang, Adsorption of dyes and phenol from water on the activated carbon prepared from corn cob waste. Environ. Technol. 22, 205–213 (2001)
Hem Lata, V.K. Garg, R. K. Gupta. Removal of basic dye from aqueous solution by adsorption using “Parthenium hysterophorus”: an agricultural waste. Dyes and Pigment. XX, 1–6 (2006)
V.K. Garg, R. Gupta, T. Juneja, Removal of basic dye (Rhodamine B) from aqueous solution by adsorption using timber industry waste. Chem. Biochem. Eng. Q. 18(4), 417–422 (2004)
R. Gong, Y. Sun, J. Chen, H. Liu, C. Yang, Effect of chemical modification on dye adsorption capacity of peanut hull. Dyes Pigment. 67, 175–181 (2005)
S.B. Bukallah, M.A. Rauf, S.S. Alali. Removal of methylene blue from aqueous solution by adsorption on sand. Dyes and pigment. XX, 1–3 (2006)
C.H. Lung, L.K. Hsiung, C.S. Yu, C.C. Guan, P. San De, Dye adsorption on biosolid adsorbents and commercially activated carbon. Dyes Pigment. XX, 1–8 (2006)
D. Kavitha, C. Namasivayam, Recycling coir pith an agricultural solid waste for the removal of procion orange from wastewater. Dyes Pigment. XX, 1–12 (2006)
C. Namasivayan, M.D. Kumar, K. Selvi, R. Beum, T. AshruffunissaVanathi, R.T. Yamuna, Waste coir pith—a potential biomass for the treatment of dyeing waste water. Biomass Bioenergy 21, 477–483 (2001)
G. Mckay, J.F. Porter, G.P. Prasad, The removal of dye colours from aqueous solution b adsorption on low cost materials. Water Air Soil Pollut. 114, 423–438 (1999)
G. Akkaya, I. Uzun, F. Giizel, Kinetic of the adsorption of reactive dyes by chitin. Dyes Pigment. XX, 1–10 (2005)
I. Uzun, Kinetic of the adsorption of reactive dyes by chitosan. Dyes Pigment. 70, 76–83 (2006)
G. McKay, M. El Geundi, M.M. Nassar, Adsorption model for the removal of acid dyes from effluent by bagasse pith using a simplified isotherm. Adsorpt. Sci. Technol. 15, 737–752 (1997)
C. Namasivayam, J.S.E. Arasi, Removal of Congo red from waste water by adsorption onto waste red mud. Chemosphere 34, 401–417 (1997)
K.C. Bhattachara, A. Sharma, Adsorption characteristics of the dye Brilliant green on leaf powder. Dyes Pigment. 7, 211–222 (2003)
C. Namasivayam, D. Prabha, M. Kumutha, Removal of direct red and acid brilliant blue by adsorption on to banana pith. Bioresour. Technol. 64, 77–79 (1998)
I. uzun, Kinetics of the adsorption of reactive dyes by chitosan. Dye. Pigment. 70, 76–83 (2006)
P. Waranusantigul, P. Pokethitiyook, M. Knatrachue, E.S. Upatham, Kinetics of basic dye (Methylene blue) biosorption by giant duckweed (Spirodela polyrhiza). Environ. Pollut. 125, 385–392 (2003)
E. Forgacs, T. Cserhati, G. Oros, Removal of synthetic dyes from wastewater: a review. Environ. Int. 30, 953 (2004)
G. McKay, Y.S. Ho, Pseudo second order model for sorption processes. Process Biochem. 34, 451 (1999)
J.W. Lee, S.P. Choi, T. Ramesh, W.G. Shim, H. Moon, Evaluation of the performance of adsorption and coagulation processes for the maximum removal of reactive dye. Dyes Pigment. 69, 196 (2006)
M. Ozacar, I.A. Segil, Adsorption of reactive dyes on calcined alunite from aqueous solution. J. Hazard. Mater. B98, 211 (2003)
I. Langmuir, The adsorption of gases on plane surface of glass, mica and platinum. J. Am. Chem. Soc. 40, 1361 (1918)
F.J. Ferreo, Hazard Mater. 142, 144 (2007)
G. Mckay, H.S. Blair, J.R. Gardner, J. Appl. Polym Sci. i.27, 3043 (1982)
H.Z. Freundlich, Over the adsorption in solution. J. Phys. Chem. 57A, 385–470 (1906)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pardeshi, S.D., Sonar, J.P., Zine, A.M. et al. Kinetic and thermodynamic study of adsorption of methylene blue and rhodamine B on adsorbent prepared from Hyptis suaveolens (Vilayti Tulsi). J IRAN CHEM SOC 10, 1159–1166 (2013). https://doi.org/10.1007/s13738-013-0256-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-013-0256-y