Abstract
An efficient procedure has been developed for one-pot synthesis of N-acyle-2-aryl-1,2-dihydro-(4H)-3,1-benzoxazin-4-ones in the presence of Preyssler-type heteropolyacid acid modified nano-sized TiO2 as catalyst. The reactions proceed smoothly at 25 °C to afford the products in high yields. The catalyst is easily separated and re-used for the next successive reactions without significant loss of its activity.
Graphical Abstract
An efficient procedure has been developed for one-pot synthesis of N-acyle-2-aryl-1,2-dihydro-(4H)-3,1-benzoxazin-4-ones in the presence of Preyssler-type heteropolyacid acid modified nano-sized TiO2 as catalyst. The reactions proceed smoothly at 25 °C to afford the products in high yields. The catalyst is easily separated and re-used for the next successive reactions without significant loss of its activity.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
4H-3,1-Benzoxazin-4-ones are an important class of heterocyclic compounds [1], which are of significant biological activities used as potent inactivators of chymotrypsin [2–4], inhibitors of human leukocyte elastase [5, 6], and HSV-1 protease [7]. They also act as key intermediates in synthetic organic reactions [1]. In addition, these compounds are valuable precursors for the synthesis of various heterocycles, such as 2,3-disubstituted quinazolin-4(3H)-ones [8–15]. A wide variety of such heterocyclic compounds occur in nature [16, 17], and are employed as linking units in polymer chemistry [18]. The chemistry of benzoxazin-4-one is anticipated to play a vital role in the coming years in the synthesis of medicinal and natural products [1]. To the best of our knowledge, only few routes have been evolved for the synthesis of N-substituted-1,2-dihydro-(4H)-3,1-benzoxazin-4-ones [19–21]. Wiklund and co-workers reported a method for the synthesis of N-acetyl-1,2-dihydro-4H-3,1-benzoxazin-4-one via the reaction of N-acylated anthranilic acids with paraformaldehyde in refluxing AcOH [22]. However, most of the reported methods are subject to certain drawbacks, such as longer reaction times, low yield, and harsh reaction conditions. Therefore, development of new and benign approaches still appears as important experimental challenge. Of the most commonly used catalysts, heteropolyacids (HPAs), as supported or in bulk form, have exhibited high activities as solid acid catalysts in various reactions [23]. Preyssler-type heteropolyacid-mediated-nano TiO2 has been proved as a very efficient and environmentally benign solid acid catalyst to effect various chemoselective reactions in the liquid phase [24]. Recently, we have reported an efficient and facile ultrasound-accelerated one-pot synthesis of novel derivatives of N-acetyl-2-aryl-1,2-dihydro-(4H)-3,1-benzoxazin-4-ones [25].
The aim of this study is to investigate the catalytic capability and enhancement of the efficiency of the nano-sized TiO2 for the synthesis of N-acetyl-2-aryl-1,2-dihydro-(4H)-3,1-benzoxazin-4-ones by supporting the Preyssler-type heteropolyacid on TiO2 nanoparticles.
Results and discussion
Effect of the catalyst particle size on the reaction
In continuation of our interest in exploring new and convenient methods for the synthesis of various heterocyclic compounds, and also regarding the lack of sufficient reports on the synthesis of substituted 1,2-dihydro-(4H)-3,1-benzoxazin-4-one [21], herein, we are encouraged to study the hitherto unreported application of Preyssler-type heteropolyacid-mediated-nano TiO2 as a convenient and recyclable heterogeneous catalyst for the synthesis of N-acetyl-2-aryl-1,2-dihydro-(4H)-3,1-benzoxazin-4-ones at 25 °C. We preliminary examined the condensation reaction of benzaldehyde with anthranilic acid in the presence of acetic anhydride as the model reaction (Scheme 1).
To optimize the conditions of the reaction, we prepared TiO2 of various particle sizes via sol–gel method according to the reported procedure [26]. The particle size distribution obtained was estimated to be 15, 30 and 50 nm as determined by transmission electron microscopy (TEM) images.
First, we examined the effect of minimizing the particle size of nano-TiO2 on the rate and yield of the model reaction and the results are schematically shown in Fig. 1. As shown in this figure, the reaction rate and yield are both found to be improved with decreasing the particle size of the nano-TiO2 catalyst. When the reaction was performed using micro TiO2 or in the absence of this catalyst, no completion of conversion was observed even after 4 h.
Effect of supporting the Preyssler acid (H14[NaP5W30O110]) on nano-TiO2
As previously shown [24], supporting the homogeneous Preyssler-type acid may alter the efficiency of the catalyst as to improve the reaction yield. This could be possibly attributed to the addition of Brøncted acid character of Preyssler heteropolyacid to Lewis acid character of nano-TiO2 and enhancement of the acidic character of the combined catalyst in the present work and we supported the Preyssler-type acid on the nano-TiO2 of the favorite particle size (15 nm). To find the most effective molar ratio of the Preyssler acid to nano TiO2, we examined the thermodynamic properties of the Preyssler adsorption on nano-TiO2 of the favorite particle size.
Equilibrium studies
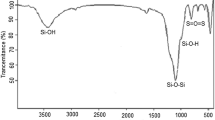
Different volumes of Preyssler acid and water (30 mL) were placed in seven separate conical flasks (Table 1). To each flask was added the adsorbent nano-TiO2 (1.0 g) with strongly shaking the flask. Then, these conical flasks were sealed with rubber stoppers and let to stand for almost 24 h with intermittent shaking. Finally, the content of each flask was centrifuged and the separated solution was titrated against 0.01 N aqueous NaOH solution using phenolphthalein with taking three readings in each case. It is previously shown that no significant differences were observed in the TEM images, and also the IR spectrum of the Preyssler acid-modified nano-sized TiO2 clearly shows the characterization bands [24].
The amount of the adsorbed Preyssler acid per unit mass of the adsorbent TiO2 (mg/g) was calculated using the Eq. (1).
where C o and C e are initial and final concentrations of the Preyssler acid, respectively.
Adsorption isotherms
The extent of adsorption of the Preyssler acid on nano TiO2 is represented by the adsorption isotherm. Among the several possible isotherm equations available, two have been commonly applied for this study, i.e. the Freundlich and Langmuir isotherms. The experimental data were fitted nonlinearly to Freundlich and Langmuir isotherms. At a given temperature, the mass of a solute adsorbed on a solid adsorbent at various concentrations is calculated from the Freundlich Eq. (2) [27]:
where K and n are Freundlich constants. The calculated and experimental values of q and C e obtained at different initial Preyssler concentrations are shown in Table 1.
The Langmuir adsorption model [28] is based on the assumption that the maximum adsorption corresponds to a saturated monolayer of solute molecules on the adsorbent surfaces. The nonlinear expression of the Langmuir model is given by the Eq. (3) as follows:
where a and b are the Langmuir constants related to the capacity and energy of adsorption respectively, and can be determined from nonlinear fitting. The results summarized in Table 2 and shown in Fig. 2 indicate that the experimental data reported in Table 1 fit better with the Langmuir isotherm.
Optimizing the reaction conditions
To establish the reaction conditions, the effect of catalyst loading on the model reaction was studied under conventional condition in the presence of acetic anhydride (1 ml) at 25 °C using the neat nano TiO2 as well as the nano-TiO2-supported Preyssler acid with different molar ratios (Table 3).
The results summarized in Table 3 clearly indicate the enforcing effect of the Preyssler acid on the yield of the reaction when supported on nano-TiO2 in comparison with the yield (75 %) obtained using the neat nano-TiO2 as the catalyst (entry 2). In addition, the importance of the catalyst in this reaction was approved with conducting the reaction under the catalyst-free condition or using the neat Preyssler acid that resulted in very low yields of 20 and 50 %, respectively (entries 1 and 3). The best result in terms of the reaction yield (95 %) was obtained when the reaction was conducted with using the Preyssler acid/nano-TiO2 catalyst in 0.0061 mmol/g ratio (entry 9).
To establish the generality of the catalyst and to develop the scope of the reaction, we conducted the reaction with a series of aromatic aldehydes 1a–r carrying different substituents under the determined optimum conditions (Scheme 2).
All the reactions proceeded smoothly to afford the respective products 2a–r in quantitative yields as summarized in Table 4. As seen in Table 4, we have noticed that the aldehydes bearing electron withdrawing groups attached to the ring at positions para and/or meta to the aldehyde functional group undergo nucleophilic attack by amino group of the anthranili acid more rapidly to afford improved yields. The electron releasing groups, on the other hand, cause the aldehydes to react more slowly with reduced yields. The possible explanation for the activating effect of the electron-withdrawing groups rests on the dispersal of the negative charge of the carbonyl oxygen over the intermediate anion produced by nucleophilc addition to carbonyl carbon atom. In contrast, the electron-releasing groups tend to intensify the negative charge on the carbonyl oxygen, destabilize the negative ion adduct, and thus cause slower reaction. These activating or deactivating effects of the substituent groups occur through their resonance and/or inductive effects. Moreover, the substituent groups of all kinds situated at position ortho to the aldehyde group, generally retard the reaction because of their sterical hindrance that makes the carbonyl group less accessible to nucleophilic attack (cf. entries 2g–2i, 2j–2k, and 2m–2o). However, the groups such as –OH attached to ortho position which can involve in hydrogen-bonding with carbonyl oxygen exhibit activating effect by increasing the tendency of the aldehyde towards nucleophilic attack (cf. entry 2c with entry 2e).
With respect to the aforementioned effects on the reaction parameters, this reaction can possibly proceed through the intermediacy of a corresponding imine derivative (A) produced from the reaction of anthranilic acid with catalyst-activated aromatic aldehyde 1 as depicted in Scheme 3. The subsequent acetylation of this intermediate with the n-TiO2-activated Ac2O followed by cyclization to provide the product 2. To approve this mechanism, the imine (A) was obtained in a separate experiment as a stable compound from the reaction of anthranilic acid with 2-pyrrolecarbaldehyde 2r as the test compounds in MeOH both with using the Preyssler-mediated nano-TiO2 catalyst and without using the catalyst. It was noticed that, the use of the catalyst can cause considerable enhancement in the yield and rate of the reaction. The intermediate A was subsequently treated with Ac2O in the presence of the acid-mediated n-TiO2 catalyst at room temperature and the formation of the expected product 2r in ~80 % yield was resulted after crystallization from EtOH.
In summary, we have explored the application of Preyssler heteropolyacid-mediated nano-TiO2 as a suitable heterogeneous catalyst in a reliable protocol for the synthesis of N-acetyl-2-aryl-1,2-dihydro-(4H)-3,1-benzoxazin-4-ones from the one-pot reaction of aromatic aldehydes with anthranilic acid in the presence of acetic anhydride at 25 °C. The reactions proceed under mild and solvent-free conditions to furnish the products in short reaction times and quantitative yields.
Experimental
Material and instruments
Chemicals used in this work were purchased from Fluka (Buchs, Switzerland) and Merck (Hohenbrunn, Germany) companies and used without purification. Preyssler-type acid was prepared as reported in the literature [29]. IR spectra were recorded on a Perkin Elmer GX FT IR spectrometer from KBr pellets. 1H and 13C NMR spectra were measured for samples in CDCl3 with a JEOL FX 90Q instrument at 90 and 22.5 MHz, respectively, using Me4Si as an internal standard. Mass spectra were recorded with a spectrometer Finnigan-MAT 8430 operating at an ionization potential of 70 eV. Melting points were measured on a SMPI apparatus. Elemental analyses for C, H, and N atoms were performed using a Perkin–Elmer 2400 series analyzer.
Typical procedure for the synthesis of N-acetyl-2-aryl-1,2-dihydro-(4H)-3,1-benzoxazin-4-ones (2)
A mixture of aldehyde 1 (1 mmol), anthranilic acid (1 mmol), acetic anhydride (1 mL) and Preyssler acid-mediated-nano TiO2 (20 mg) was stirred at room temperature for an appropriate time (Table 2), until the reaction was completed as monitored by TLC (n-hexane/EtOAc; 2:1) analysis. The reaction mixture was then centrifuged to separate the solid catalyst, the residue was treated with iced water and filtered to leave a solid product, which was crystallized from ethanol to yield pure product. All the products were characterized by their physical and spectral data (IR, MS, 1H NMR, 13C NMR) and elemental analysis that were in accord with the reported data [25, 30].
Typical synthesis of imine (A)
A mixture of anthranilic acid (0.137 g, 1 mmol) and 2-pyrrolecarbaldehyde 1r (0.095 g, 1 mmol) in MeOH (10 mL) was left to stir at room temperature. Following completion of the reaction within 90 min as monitored by TLC, the solvent was removed under reduced pressure to leave a yellow solid which was crystallized from EtOH to yield pure corresponding imine (A) in 80 % yield. It is interesting to note that the use of acid-mediated nano-TiO2 catalyst in this reaction resulted in the highly increased reaction rate and a moderate improvement in the yield (90 %) of the intermediate (A). The physical and spectral data for (A) are given below:
mp 205–207 °C (dec). 1H NMR spectrum (DMSO-d6, 90 MHz): δ 6.34 (s, 1H, N=CH), 6.95–8.50 (m, 7H, Ar-H), 12.14 (s, 1H, NH), 14.55 (s, 1H, COOH) ppm. 13C NMR (DMSO-d6, 22.5 MHz): δ 111.0, 116.0, 118.0, 119.4, 122.6, 125.5, 126.9, 129.1, 131.0, 133.6, 148.7, 166.7. FT-IR (KBr, cm−1): 3,108, 2,250, 1,650, 1,600, 1,445, 1,263, 1,158, 756.
Recycling potential of the catalyst
To study the stability and reusability potential of the catalyst, the reaction mixture was centrifuged after the completion. The separated solid was washed with ethyl acetate (2 × 5 mL) and dried under vacuum (20 °C). The recovered catalyst was reused for three consecutive fresh runs without any significant loss of activity (Table 4, entry 1). To investigate the possible leaching of heteropolyacid from the recycled nano-catalyst by Ac2O in the course of the reaction, we measured the mass balance of the catalyst after it was recycled. No detectable loss in the weight of the recycled catalyst was observed in comparison with the weight of the freshly used starting catalyst. This observation disapproves the leaching of heteropolyacid from the acid-mediated catalyst during the reaction.
References
G.M. Coppola, J. Heterocycl. Chem. 36, 563 (1999)
R. Alazard, J. Bechet, A. Dupaix, J. Yon, Biochim. Biophys. Acta 309, 379 (1973)
T. Teshima, J.C. Griffin, J.C. Powers, J. Biol. Chem. 257, 5085 (1982)
L. Hedstrom, A.R. Moorman, J. Dobbs, R.H. Abeles, Biochemistry 23, 1753 (1984)
R.L. Stein, A.M. Strimpler, B.R. Viscarello, R.A. Wildonger, R.C. Mauger, D.A. Trainor, Biochemistry 26, 4126 (1987)
A. Krantz, R.W. Spencer, T.F. Tam, T.J. Liak, L.J. Copp, E.M. Thomas, S.P. Rafferty, J. Med. Chem. 33, 464 (1990)
R.L. Jarvest, M.J. Parratt, C.M. Debouck, J.G. Gorniak, L.J. Jennings, H.T. Serafinowska, J.E. Strickler, Bioorg. Med. Chem. Lett. 6, 2463 (1996)
M. Alajarin, A. Vidal, M.M. Ortina, D. Bautista, Synthesis 2426 (2005)
H.M.F. Madkour, Arkivoc 36 (2004)
S.M. Mosaad, K.I. Mohammed, M.A. Ahmed, S.G. Abdel-Hamide, J. Appl. Sci. 4, 302 (2004)
C. Parkanyi, H.L. Yuan, B.H.E. Stromberg, A. Evenzahav, J. Heterocycl. Chem. 29, 749 (1992)
M.J. Kornet, T. Varia, W. Beaven, J. Heterocycl. Chem. 20, 1553 (1983)
L.A. Errede, J.J. McBrady, H.T. Oien, J. Org. Chem. 42, 656 (1977)
A.V. Lygin, A. DeMeijere, J. Org. Chem. 74, 4554 (2009)
A.A. Laeva, E.V. Nosova, G.N. Lipunova, A.V. Golovchenko, N.Y. Adonin, V.N. Parmon, V.N. Charushin, Rus. J. Org. Chem. 45, 913 (2009)
M.L. Bouillant, J. Favre-Bonvin, P. Ricci, Tetrahedron Lett. 24, 51 (1983)
G.J. Niemann, J. Liem, A.V.D.K.V. Hoof, W.M.A. Neissen, Phytochemistry 31, 3761 (1992)
M. Ueda, S. Komatsu, J. Polym. Sci, Polym. Chem. 27, 1017 (1989)
L. Legrand, N. Lozach, Bull. Soc. Chim. Fr. 2067 (1967)
P. Wiklund, I. Romero, J. Bergman, Org. Biomol. Chem. 1, 367 (2003)
N.J. Herib, J.G. Jurcut, D.E. Bergna, K.L. Burgher, H.B. Hartman, S. Kafca, L.L. Kerman, S. Kongsamut, G.E. Roehr, M.R. Szewczak, A.T. Woods-Kettelberger, R. Corbett, J. Med. Chem. 39, 4044 (1996)
P. Wiklund, J. Bergman, Tetrahedron Lett. 45, 969 (2004)
T. Okuhara, N. Mizuno, M. Misono, Adv. Catal. 41, 113 (1996)
M. Rahimizadeh, G. Rajabzadeh, S.M. Khatami, H. Eshghi, A. Shiri, J. Mol. Catal. 323, 59 (2010)
D. Azarifar, D. Sheikh, Heteroat. Chem. 22, 106 (2011)
G. Rajabzadeh, A. Jalalian, Proceedings of the 14th International Sol–gel Conference, Montpellier, France (2007)
H. Freundlich, Z. Phys. Chem. 57, 384 (1906)
I. Langmuir, J. Am. Chem. Soc. 40, 1361 (1918)
M.H. Alizadeh, S.P. Harmalker, Y. Jeannin, M.T. Pope, J. Am. Chem. Soc. 107, 2662–2669 (1985)
D. Azarifar, D. Sheikh, Chem. Heterocycl. Compd. 9, 1372 (2011)
Acknowledgments
The authors wish to thank the Research Council of Bu-Ali Sina University and also the Ministry of Science, Research and Technology of Islamic Republic of Iran for financial support to carry out this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Azarifar, D., Khatami, SM., Zolfigol, M.A. et al. Nano TiO2-supported Preyssler-type heteropolyacid (nanoTiO2/H14[NaP5W30O110]): an efficient and re-usable catalyst for the one-pot synthesis of N-acetyl-2-aryl-1,2-dihydro-(4H)-3,1-benzoxazin-4-ones. J IRAN CHEM SOC 10, 1039–1046 (2013). https://doi.org/10.1007/s13738-013-0242-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-013-0242-4