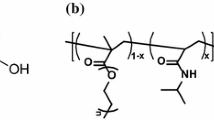
Abstract
This study comprises synthesis and characterization of azocalix[4]arene compounds having copolymeric structures. The novel bisazocalixphenol-A and copolymer containing pendant azocalix[4]arene units with ester (5) and ketone (6) functionalities at their lower rim have been synthesized via diazo-coupling reaction. The phase transfer study is performed by liquid–liquid extraction method. It has been deduced from the observations that their precursors 25,26,27-tribenzoyloxy-28-hydroxy-5-(4-aminophenylazo)calix[4] arene (3), 25,27-diacetoniloxy-26,28-dihydroxy-5,17-(4-aminophenylazo)calix[4]arene (4) and bisazocalixphenol-A (5) show a good phase transfer affinity towards selected transition metal cations, unlike copolymer (6). These compounds are studied by the selective extraction of Fe3+ cation from the aqueous phase into the organic phase and it is carried out using compounds 1–6. It is observed that the reduced azocalix[4]arene 4 is the most efficient (82.93 %) carrier of all compounds (3–6) in the extraction of Fe3+ at pH 5.4.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There has been recent interest in the design of new stimuli responsive host systems. The development of chromogenic ionophores has been a very active research area in a supramolecular chemistry, since it can be applied to many useful and important chemical technologies. Calix[n]arenes (n = 4–20, where n is the number of aromatic rings joined by methylene bridges) are phenolic [1n]-metacyclophanes that can be easily obtained by base catalysed condensation of p-substituted phenols with formaldehyde or paraformaldehyde [1].
Calix[n]arenes, a class of macrocyclic compounds, grows technical importance in various areas. Parent calix[n]arenes have received intense attention over the past 25 years [2, 3]. Most of this interest has focused on the calix[4]arene series containing four phenolic residues that are the subject of our present contribution. They are known to provide useful building blocks for hollow molecular scaffolds with easily functionalizable hydrophilic and hydrophobic groups at either the “lower rim” oxygens or the “upper rim” carbons, respectively [4, 5].
Azocalix[n]arenes, which are generated by the insertion of nitrogen atoms into the p-position units of the calix[n]arene structures, have several isomers based on the position of nitrogen atoms and ring size. The first reported calix[4]arene diazo coupling process is the reaction of p-nitrobenzenediazonium tetrafluoroborate with calix[4]arene [6].
Azocalixarenes, containing conjugated chromophore azo (–N=N–) groups in p-positions, are synthesized via “one-pot” procedures with satisfactory yields [5]. Introduction of azo groups into the calix[n]arenes has been studied by various researchers recently for their use in ionic and molecular recognition recently [7]. Despite such studies, predictive information on the efficiency of coupling reaction between calix[n]arene and substituted aromatic amines is very limited. For example, it has been reported that the reaction of calix[4]arene with substituted benzene diazonium flouroborate in the presence of pyridine provides tetrakis(phenylazo)calix[4]arene with higher yield, but the same reaction with benzene diazonium salt derived from aniline gives a very poor yield of the related product [6, 8].
With these subjects in mind, we have synthesized several azocalix[n]arene derivatives [9–12]. We have found that these azocalix[n]arenes act as excellent host molecules for the selective binding of metal ions [13–15]. They also act as excellent enol–keto tautomers. This article briefly discusses various molecular designs of azocalix[n]arene-type macrocycles for metal ions, and gives examples on the relationship among the structure, complexing, metal selectivity and thermal behaviours of them [16].
Bisphenol-A (BPA, 2,2′-bis(4-hydroxyphenyl)propane) is widely used in manufacturing epoxy resins and polycarbonate, and detected in water and sediments everywhere on the earth. BPA is listed as one of the so-called “environmental estrogens (endocrine disrupters)”, and its toxicity (BPA is sometimes used as a fungicide) [17] and biological effects have been reported. Recently, a migration of BPA from epoxy coating into infant formula liquid concentration has been observed [18–20].
Following a recent communication from this laboratory [12], we report here the synthesis of macromolecular characterization of bisazocalixphenol-A (5) and copolymer (6) in which mono- and di-amino azocalix[4]arene groups are incorporated in the main chains (Scheme 1). In this paper, diazo-coupling reactions between mono- or di-amino azocalix[4]arene compounds and bisphenol-A are described. Finally, the transition metal cation-binding ability of the copolymers is illustrated through the picrate extraction procedure and compared to those of nitro (1 and 2) and amino (3 and 4) azocalix[4]arene derivatives.
Experimental
All reagents used are purchased from Merck or Carlo-Erba and chemically pure. Merck PF254 silicagel is used for all forms of chromatography. The drying agent employed is anhydrous magnesium sulphate. Melting points are measured using an Electrothermal IA 9100 digital melting point apparatus in capillaries sealed under nitrogen and uncorrected. 1H-NMR spectra are referenced to tetramethylsilane (TMS) at 0.00 ppm as internal standard and recorded on a Bruker 400-MHz spectrometer at room temperature (25 ± 1 °C) in CDCl3. IR spectra are recorded on a Mattson 1000 FT-IR spectrometer using KBr pellets. UV–vis spectra are obtained on a Shimadzu UV-1601 UV–visible recording spectrophotometer. Osmometric molecular weight determination is carried out on a Knauer vapor pressure osmometer at concentration of ca. 10−3 M CHCl3. The elemental analyses are performed in TUBITAK (The Scientific and Technological Research Council of Turkey) Laboratories.
All aqueous solutions are prepared with de-ionized water that have been passed through a Human Power I Plus I + UV water purification system.
Preparation of the ligands
p-tert-Butylcalix[4]arene [21], calix[4]arene [22], 25,26,27-tribenzoyloxy-28-hydroxy-5-(4-nitrophenylazo)calix[4]arene (1) and 25,27-diacetoniloxy-26,28-dihydroxy-5,17-(4-nitro phenylazo)calix[4]arene (2) are synthesized as described by a previously reported method [23, 24].
Reduction of the ligands 1 and 2
25,26,27-Tribenzoyloxy-28-hydroxy-5-(4-aminophenylazo)calix[4]arene (3)
0.95 g (1.11 mmol) 25,26,27-tribenzoyloxy-28-hydroxy-5-(4-nitrophenylazo) calix[4]arene (1) and 2.30 mL (47 mmol) hydrazine hydrate (80 %) were dissolved in 15 mL THF. Then, 2.34 g (41 mmol) of dried Raney–Ni was added to solution. This mixture was stirred until the gases depart from the solution. The mixture was allowed to warm to room temperature and stirred for additional 2 h and set aside throughout the day. It was filtered. The solution was concentrated under vacuum to a volume of 5–8 mL and treated with petroleum ether. The resulting pale brownish powder was filtered, treated with petroleum ether and yielded as 0.48 g (53 %). m.p. 192 °C [Found: C 77.03; H 4.97; N 4.66. C55H41N3O7 requires C 77.18; H 4.83; N 4.91]. λmax (ε): 460 (7,920). νmax: 3,528, 1,726, 1,451, 1,267 cm−1. 1H-NMR (DMSO-d6, 25 °C): δH: 10.38 (broad, 2H, –NH2); 7.76 (s, 1H, OH); 6.96 (m, 4H, ArH–NH2); 6.58 (m, 11H, ArH); 6.56 (m, 15H, ArH–CO); 3.55–4.15 (s, 8H, Ar–CH2–Ar).
This compound (3) was soluble in diethyl ether, acetone, acetic acid, CHCl3, DMSO, benzene, but insoluble in water, EtOH.
25,27-Diacetoniloxy-26,28-dihydroxy-5,17-(4-aminophenylazo)calix[4]arene (4)
The reduction reaction of 25,27-diacetoniloxy-26,28-dihydroxy-5,17-(4-nitrophenyl azo)calix[4]arene (2) with Raney–Ni was carried out according to the procedure above [25]. Yield, 0.80 g (48 %), m.p. 285 °C [Found: C 71.08; H 5.63; N 10.39. C46H42N6O6 requires C 71.30; H 5.46; N 10.85]. λmax (ε): 395 (5,610). νmax: 3,423, 1,736, 1,466, 1,246 cm−1. 1H-NMR (DMSO-d6, 25 °C): δH: 10.30 (s, 4H, –NH2); 7.96 (s, 2H, OH); 7.10 (m, 8H, ArH–NH2); 6.60 (m, 10H, ArH); 3.47–4.15 (s, 8H, Ar–CH2–Ar); 2.10 (s, 4H, –CH2–); 1.16 (s, 6H, CO–CH3).
This compound (4) was soluble in EtOH, diethyl ether, acetone, acetic acid, CHCl3, DMSO, benzene, but insoluble in water.
Copolymerization
Bisazocalixphenol-A 5 from 25,26,27-tribenzoyloxy-28-hydroxy-5-(4-aminophenylazo) calix[4]arene (3) with bisphenol-A
Diazonium chloride solution of 25,26,27-tribenzoyloxy-28-hydroxy-5-(4-amino phenylazo)calix[4]arene, which was prepared by 3 (1.00 g, 1.20 mmol), sodium nitrite (0.20 g, 2.90 mmol) and conc. HCl (2 mL) in acetic acid (5 mL), was added slowly into a cold (~0 °C) solution of bisphenol-A (0.12 g, 0.53 mmol) in aqueous NaOH and sodium acetate trihydrate (4.08 g, 30 mmol) in DMF–MeOH (26 mL, 8:3, v/v) to give a red suspension. After standing for 2 h at room temperature, the suspension was acidified with aqueous HCl (50 mL, 0.25 %). The resulting mixture was first warmed to 60 °C and kept at that temperature for 30 min to give 5 (yield 1,02 g, 93 %) as a reddish solid, and then filtered and washed with water and MeOH, respectively. A sample for analysis was obtained as follows: compound (5) was dissolved in 100 mL of a hot NaHCO3 (4.2 g) solution. To this, solution was added activated charcoal (1 g). After the charcoal was filtered, the filtrate was cooled (room temperature) and acidified with conc. HCl (1 or 2 mL). The solution was heated (60 °C) again for 30 min and cooled. The resulting solid was filtered, washed with water, and dried. Yield, 0.90 g (82 %), m.p. 241 °C; osmometric molecular Mn (CHCl3, 37 °C), 1,950 (calculated: 1,962). [Found: C 76.34; H 4.87; N 5.56. C125H92N8O16 requires C 76.52; H 4.73; N 5.71]. λmax (ε): 426 (8,790). νmax: 3,529–3,435, 1,727, 1,451, 1,268 cm−1. 1H-NMR (DMSO-d6, 25 °C): δH: 11.40 (s, 2H, bis-OH); 8.68 (s, 2H, OH); 7.92 (m, 6H, bis-ArH); 7.86 (m, 8H, ArH–N=N); 7.64 (m, 30H, ArH–CO); 7.41 (m, 22H, ArH); 3.52–3.92 (s, 16H, Ar–CH2–Ar); 1.93 (s, 6H, –CH3).
This compound (5) was soluble in CHCl3, DMSO, benzene, but insoluble in water, EtOH, diethyl ether, acetone, acetic acid.
Copolymer 6 from 25,27-diacetoniloxy-26,28-dihydroxy-5,17-(4-aminophenylazo) calix[4]arene (4) with bisphenol-A
The diazo-coupling reaction of 25,27-diacetoniloxy-26,28-dihydroxy-5,17-(4-amino phenylazo)calix[4]arene (1.0 g, 1.30 mmol) (4) with bisphenol-A (0.4 g, 1.80 mmol) was carried with the above-described procedure. Usual workup provided 6. Yield, 1.0 g (77 %), m.p. > 360 °C (dec); osmometric molecular Mn (CHCl3, 37 °C), 5,450 (calculated: 5,472) [Found: C 70.17; H 5.18; N 11.93. (C61H52N8O8) n requires C 70.46; H 4.97; N 12.28]. λmax (ε): 410 (3,680). νmax: 3,300–3,232, 1,728, 1,465, 1,243 cm−1. 1H-NMR (DMSO-d6, 25 °C): δH: 9.20 (s, 2H, OH); 8.05 (s, 2H, OH); 7.20–7.00 (m, 14H, ArH); 6.70 (m, 10H, ArH); 3.35–4.05 (s, 8H, Ar–CH2–Ar); 2.30 (s, 4H, –CH2–); 2.00 (s, 6H, Ar–CH3), 1.50 (s, 6H, CO–CH3).
Compound (6) was soluble in DMSO, and slightly soluble in CHCl3, and insoluble in water, EtOH, acetone, acetic acid, benzene, diethyl ether.
Liquid–liquid extraction [15]
A chloroform solution (10 mL) of ligand (1.10−3 M) and an aqueous solution (10 mL) containing metal ions in 2.10−5 M picric acid were shaken together at 298 K for an hour. An aliquot of the aqueous solution was taken and the UV spectrum was recorded. A similar extraction was performed in the absence of picrate ion in the aqueous solutions. This extraction procedure was repeated three times. The extractability (Ex %) of the metal cations is expressed by means of the following equation:
where A 0 and A are the absorbancies in the absence and presence of ligands, respectively.
Extraction of Fe3+ cation [26]
A 5 mL solution of chloroform containing 1–5 (5.3 × 10−4 M, 1.2 × 10−2 g 6) and a 25 mL aqueous solution containing a metal salt (1.06 × 10−4 M) were placed in a flask. The aqueous solution was adjusted to pH 2.2 (0.01 M NaNO3/HNO3, μ = 0.1 with KCl), or buffered to pH 3.8, 4.5 and 5.4 (0.01 M CH3COONa/CH3COOH, μ = 0.1 with KCl). The reported pH values are those of corresponding buffers without individual pH measurement in equilibrated solutions. The mixture was shaken for an hour at room temperature. The extraction was not affected by further shaking, indicating that the equilibrium has been attained within an hour. The extractability (Ex %) was determined by below formula:
where (metal)blank and (metal)water denote the metal concentrations in the aqueous phase, before and after extraction with pure chloroform solution containing extractants, respectively.
Results and discussion
Results and characterization of the products
In recent years, various attempts have been undertaken to incorporate azocalix[n]arenes into different polymer including self-assembled systems. There are two main strategies to prepare diazo coupled calixarene-based polymers. The first one is the attachment of the amino calix[n]arene by diazotization reaction with a suitably functionalized polymer or oligomer. The second one is the reaction of calix[4]arene with the substituted benzene diazonium flouroborate in the presence of pyridine. The former approach gives better defined products since there is no risk of incomplete substitution of the functionalized polymer. Thus, azocalix[n]arene monomers have been incorporated into dimer backbones by utilizing functional groups [12].
Azocalix[n]arenes containing p-nitrophenylazo-chromogen group with one or two free phenolic groups were synthesized through the diazo-coupling reaction of corresponding diazotized amines with calix[4]arene derivatives.
The main focus of this work is the design of new calixarene-based polymers those are easily accessible, with effective binding character for a particular set of cations, and useful for multiple applications, such as laboratory, clinical, environmental, and industrial analyses. To achieve the desired goal, we have attempted to synthesize p-tert-butylcalix[4] arene, calix[4]arene, 25,26,27-tribenzoyloxy-28-hydroxy-5-(4-nitrophenylazo)calix [4]arene (1) and 25,27-diacetoniloxy-26,28-dihydroxy-5,17-(4-nitrophenylazo) calix[4]arene (2) according to the stated methods [21–24]. Recently, we have demonstrated the high selectivity of azocalix[4]arene derivatives toward Ag+, Hg+ and Hg2+ cations [15]. With the present work, we extended our previous studies by coupling 5 and 6 with azocalix[4]arene and bisphenol-A (Scheme 2).
The diazo-coupling reactions were carried out as described in the experimental section. The synthesis of 25,26,27-tribenzoyloxy-28-hydroxy-5-(4-aminophenylazo)calix [4]arene (3) and 25,27-diacetoniloxy-26,28-dihydroxy-5,17-(4-aminophenylazo)calix[4] arene (4), their conversion to bisazocalixphenol-A (5) and copolymer (6) by the diazo coupling reaction with bisphenol-A in DMF/MeOH with the presence of NaNO2 and HCl/CH3COOH were performed according to the literature methods [10]. The bisazocalixphenol-A 5 and copolymer 6 were obtained in 82 and 77 % yield, respectively.
The synthesis of azocalix[4]arene containing one free phenolic group (5) was performed with moderate yields (82 %) by reacting tribenzoylated calix[4]arene at 0–5 °C with 1 equivalent of diazonium salt.
Polymeric azocalix[4]arene (6) containing two free phenolic groups was synthesized by the diazonium coupling reaction of diazotized aromatic amines and calix[4]arene [23, 24]. The identities of purified products were confirmed by spectral data (IR, NMR).
The IR spectra of synthesized amino azocalix[4]arenes (3 and 4) showed absorptions for OH as a broad band at a considerable higher frequency (3,450–3,200 cm−1) than parent calix[4]arenes [10] (~3,120 cm−1) indicating that hydrogen bonds are comparatively weaker in amino azocalix[4]arenes. An asymmetric stretching vibration for the –N=N– group appeared in the 1,600–1,550 cm−1 range.
The synthesized azocalix[4]arenes (3 and 4) could be characterized by analyses of their 1H-NMR spectra. The position of NMR signals for methylene carbons in the δ 3.47–4.15 ppm range for synthesized compounds allowed us to conclude that these derivatives were present in their cone conformation. This conclusion is in accordance with the results reported in the literature [6]. The chemical shift values and splitting pattern of synthesized bisazocalixphenol-A and copolymer are listed in the experimental section.
Room temperature benzoylation of calix[4]arene with benzoylchloride and pyridine yielded 1, in which methylene protons appeared as a pair of doublets in its 1H-NMR spectrum. When azocalix[4]arenes with two free phenolic groups present in the cone conformation (5) were subjected to etherification in the presence of chloroacetone and K2CO3 at room temperature, they gave 5 and 6 with 82–77 % yield, which exhibited a pair of doublet in the 1H-NMR spectrum.
Extraction studies
Although numerous investigations have recently been reported regarding the extraction of alkaline metals from aqueous phase into organic phase by azocalix[n]arene, information concerning the extraction of transition metals is very limited. In this work, we have investigated the effectiveness of four diazo-coupling calix[4]arenes (3–6) in transferring transition metals (Ag+, Hg+, Hg2+, Co2+, Ni2+, Cu2+, Cd2+, Zn2+, Al3+, Cr3+, La3+) from aqueous phase into organic phase (Table 1).
We initially investigated the transferring ability of dimeric bisazocalixphenol-A (5) and compared the results with the data obtained for azocalix[4]arenes 3. As shown in Table 1, both soft (Ag+, Hg+, Hg2+) and hard (Co2+, Ni2+, Cu2+, Cd2+, Zn2+, Al3+, Cr3+, La3+) metal cations were not extracted by polymeric azocalix[4]arene (6) from the aqueous phase to organic phase. However, with the introduction of tribenzoyl ester groups at the cone conformation on the lower rim of azocalix[4]arene (3), the soft Hg2+ has been transferred more effectively than that of bisazocalix[4]arene 4, but both ligands for the hard metal cations were extracted very poorly.
From the data given in Table 1, it can be seen that polymeric azocalix[4]arene (6) is not effective in transferring heavy metal ions into organic phase, whereas bisazocalix phenol-A (5) and azocalix[4]arene derivatives (3 and 4) are effective in transferring soft (Ag+, Hg+, Hg2+) metal cations. Here, we should note that azocalix[4]arenes (3) and bisazocalixphenol-A (5) in cone conformations show high extraction ability toward Hg2+ (91 %) and Hg+ (81.2 %) metal cations, respectively. The results expressed as percentage of cation extracted (E %) are used to graph Fig. 1.
In our previous work, two-phase solvent extraction of Fe3+ cation from aqueous phase into organic phase was achieved with calix[4]arene and some of its derivatives. In this work, we have synthesized new polymeric compounds containing more than one azocalix[4]arene units by reacting azocalix[n]arene derivatives with bisphenol-A and investigated the extraction abilities of these polymeric azocalix[4]arenes in the water–chloroform system at various pH levels.
Polymeric calixarenes are potentially capable of forming stable complexes with many metal ions. On the basis of the previous experiences, bisazocalixphenol-A (5) and polymeric azocalix[4]arene (6) were constructed as shown in Scheme 1.
Ionophoric polymer-supported azocalixarenes in which their upper rims are coupled via a single site on each azocalix[n]arene were synthesized. The synthesized dimeric azocalix[4]arene (5) and copolymeric azocalix[4]arene (6) were subjected to comparative studies. The results of two-phase extraction measurements of 1–6 with Fe3+ cations in a water–chloroform system at pH 2.2, 3.8, 4.5, and 5.4 are summarized in Table 2.
With all ligands substituted with more than one azocalix[4]arene unit 10.47–25.90 % extraction was accomplished at pH 2.2. When the ketone substituted azocalix[4]arene (4) was used, the extraction ratio was increased significantly (Fig. 2).
These compounds are capable of extracting Fe3+ even at lower pH ranges. This shows that the n electrons on the –N=N– groups are effective in neutral media and extraction depends on the hydrogen atom in acidic medium. Furthermore, the compound 4 extracted 82.93 % of the Fe3+, and this process is due to easy separation of hydrogen atom easily from the compound 4. This phenomenon has also been reported previously [26].
The increase in pH is due to the number of liberated H+ cations, after the complex is formed between the azocalix[4]arene and Fe3+ in DMF. The extraction reaction of the present systems can be expressed by equation.
Based on the above results, polymer-supported azocalix[4]arenes exhibited high levels complexation ability. In addition, it was observed that nitro group substitutions displayed high acidity for Fe3+. Because azocalix[n]arenes are not only available in larger quantities, but also suitable for unlimited chemical modifications. It can be expected that even better extractants or ion carriers can be obtained on the basis of the azocalixarenes.
Conclusion
In this study, two new copolymers have been synthesized from azocalix[4]arene by electrophilic substitution reaction. The copolymers are amorphous and exhibit low Tg. The thermal stability of 6 is up to 320 °C. Both copolymers show a good phase transfer affinities toward selected transition metal cations. Their processability, successful immobilization and special properties could open up a new area of selective, polymer supported reagents for catalysis and metal-ion separation, which enhance their utility in phase transfer reactions, as absorbents, or as potential candidate materials for fabricating membranes and sensors.
References
C.D. Gutsche, in Calixarenes Revisited: Monographs in Supramolecular Chemistry, ed. by J.F. Stoddart (Royal Society of Chemistry, Cambridge, 1998)
Calix11 International Conference on Calixarenes (2011)
V. Böhmer, Angew. Chem. Int. Ed. Engl. 34, 713 (1995)
A. Ikeda, S. Shinkai, Chem. Rev. 97, 1713 (1996)
H. Deligöz (Review Article), J. Incl. Phenom. Macrocyclic Chem. 55, 197 (2006)
S. Shinkai, K. Araki, J. Shibata, O. Manabe, J. Chem. Soc. Perkin Trans. 1, 195 (1989)
H.M. Chawla, S.P. Singh, S.N. Sahu, S. Upreti, Tetrahedron 62, 7854 (2006)
L. Jianquan, T. Xiaoqin, H. Xiwen, J. Electroanal. Chem. 111, 540 (2003)
H. Deligöz, H. Çetişli, J. Chem. Res. 10, 427 (2001)
Y. Morita, A. Toshio, J. Org. Chem. 57, 3658 (1992)
A. Uygun, A.G. Yavuz, S. Şen, F. Deligöz, Ö.Ö. Karakuş, H. Deligöz, J. Appl. Polym. Sci. 115(5), 2697 (2010)
Ö.Ö. Karakuş, H. Deligöz, Turkish J. Chem. 35(1), 87 (2011)
Ö.Ö. Karakuş, S. Elçin, M. Yılmaz, H. Deligöz, Desalinat. Water Treat. 26, 72 (2011)
Ö.Ö. Karakuş, H. Deligöz, J. Appl. Polym. Sci. 122, 76 (2011)
A. Akdoğan, M. Deniz (Tavaslı), S. Cebecioğlu, A. Şen, H. Deligöz, Sep. Sci.Technol. 37, 973 (2002)
H. Deligöz, Ö. Özen, G.K. Çılgı, H. Çetişli, Thermochim. Acta 426, 33 (2005)
H. Kitano, T. Hirabayashi, M. Ide, M. Kyogoku, Macromol. Chem. Phys. 204, 1419 (2003)
J.E. Biles, T.P. McNeal, T.H. Begley, J. Agric. Food Chem. 45, 4697 (1997)
P. Sohoni, J.P. Sumpter, J. Endocrinol. 158, 327 (1998)
R. Steinmetz, N.A. Mitchner, A. Grant, D.L. Allen, R.M. Bigsby, N. Ben-Jonathan, Endocrinology 139, 2741 (1998)
C.D. Gutsche, M. Igbal, Org. Synth. 68, 234 (1990)
C.D. Gutsche, M. Igbal, D.J. Steward, Org. Chem. 51, 742 (1986)
M.S. Ak, H. Deligöz, J. Incl. Phenom. Macrocyclic Chem. 55, 223 (2006)
Ö.Ö. Karakuş, H. Deligöz, J. Incl. Phenom. Macrocyclic Chem. 61, 289 (2008)
S.E. Matthews, M. Saadioui, V. Bohmer, S. Barboso, F. Arnaud-Neu, M.J. Schwing-Weill, A.G. Carrera, J.F. Dozol, J. Prakt. Chem. Chem. Ztg. 341, 264 (1999)
Ö.Ö. Karakuş, H. Deligöz, Anal. Lett. 43(5), 768 (2010)
Acknowledgments
We thank to the Research Foundation of The Pamukkale University of Turkey for the financial support (BAP 2005FBE019).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tunç, M.M., Karakuş, Ö.Ö. & Deligöz, H. Synthesis and characterization of azocalix[4]arene ester and ketone derivatives incorporated in a polymeric backbone with bisphenol-A and their cation-binding properties. J IRAN CHEM SOC 9, 729–735 (2012). https://doi.org/10.1007/s13738-012-0085-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-012-0085-4