Abstract
The synthesis and characterization of three novel Schiff bases of azocalix[4]arene and their selective copper extraction are described. The preparation of azocalix[4]arenes (1c, 2c and 3c) has been achieved by the condensation of 2-hydroxy-3-methoxybenzaldehyde with the amine group of upper rim mono-, di- and tetra-(aminophenylazo) calix[4]arene in ethanol. The synthesized products (1c, 2c and 3c) are characterized by both 1H and 13C NMR techniques, IR spectroscopy and elemental analysis. Extraction capabilities of interested derivatives of azocalix[4]arene from aqueous phase into organic phase are also examined. While extraction of Ag+, Hg+ and Hg2+ cations gave strong complexes with azo groups, extraction of Cu2+ cation is found to be highly effective with –CH=N– and neighboring –OH groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Calix[n]arenes, the third generation of supramolecules, after crown ethers and cyclodextrins, are phenol-based macrocyclics, which are able to form stable and selective complexes with cations, anions and neutral molecules. They are highly specific ligands and their potential applications as hosts and sensing agents for various analytes have received increasing interest. With appropriate appended groups, Schiff bases combining with calix[4]arene framework are good candidates as probe molecules for various species as they selectively entrap specific cations, anions and neutral molecules [1, 2].
Azocalixarenes, both single conjugated chromophore azo (–N=N–) group in p-positions and ester or ether subunits at either the lower or the upper rims of the calixarene macrocyclic ring may have potential applications in preparation, separation, and analysis of enantiomers. In this regard, investigation on the synthesis and characterized recognition properties of azocalix[4]arene derivatives has attracted considerable attention [3–7].
Attention has been focused primarily on attachments to the p-carbons of aryl groups at the upper rim and the oxygen at the lower rim, and only a few attempts have been reported concerning the replacement of hydroxyl groups with other groups. Replacement of one or two of the hydroxyl groups with amino and mercapto groups has been accomplished recently [8, 9]. Synthesis, spectral characterization and biological evaluation of some lanthanite(III) complexes of Schiff bases of carbostyril derivatives have been reported by Dhumwad and coworkers [10].
Metal complexes have proven their significance by entering into the field of diagnosis of a wide variety of disease states ranging from heart diseases, brain disorders, cancer and diabetes. They are also able to determine specific aspects of disease such as tissue hypoxia, as well as to detect molecular phenomena such as multi-drug resistance [11].
Various studies on the synthesis of azocalix[n]arenes for related applications have been arisen in the literature. In our laboratory, azocalix[n]arene derivatives have been synthesized for liquid–liquid extraction of transition metal ions (Ag+, Hg+ and Hg2+) [12–16]. Lu et al. [17] have obtained calix[n]arene carboxyphenylazo derivative as a diagnostic for lead. Potassium and cesium selective azocalix[n]arene derivatives have been reported by Kim et al. [18] and Chawla et al. [19], while a nickel selective azocalix[n]arene derivative has been reported by Ma et al. [20].
Much of our earlier work in this area has concentrated on calix[n]arenes substituted with mono oxime, vic-dioxime, polymeric and azo groups [21–23]. Extraction, transport and stability constant augmented by spectrophotometric studies have provided evidences that many of these lower rim derivatives have very significant ionophoric properties for cations, several with good selectivity within groups of metals [24–26].
The most important contributions in this field are made by our first work [6]. We have embarked on an ambitious program that focuses on applications of calix[n]arenes and azocalix[n]arenes. Herein, we report the synthesis of new azocalix[4]arene Schiff base derivatives (1c, 2c and 3c) and their metal cation extraction abilities (Fig. 1).
Experimental section
Reagents
All chemicals used were of analytical grade purity and used without further purification. Some of the solvents in crystallization were retained in the analytical samples, best fits between the analytical values and appropriate fractional increments of solvents were used. All aqueous solutions were prepared with deionized water that had been passed a Human Power I Plus I + UV water purification system.
Apparatus
Melting points were determined on a Schorp-APA II digital melting point apparatus without correction. 1H NMR spectra were referenced to tetramethylsilane (TMS) at 0.00 ppm as an internal standard and recorded on a Bruker 200 MHz spectrometer at room temperature (25 ± 1 °C). 13C NMR spectra were referenced to either CDCl3 (77.00 ppm) or TMS (0.00 ppm) and also recorded at room temperature (25 ± 1 °C). IR spectra were recorded on a Mattson 1000 FTIR spectrometer using KBr pellets. UV-vis spectra were obtained on a Shimadzu 160A UV-Visible recording spectrophotometer. The elemental analyses were performed in the laboratories of TUBITAK (Center of Science and Technology Research of Turkey).
Preparation of the ligands
p-tert-Butyl calix[4]arene [27], calix[4]arene [28], 25,26,27-tribenzoyloxy-28-hydroxy calix[4]arene [29], 25,27-diethylacetonyloxy-26,28-dihydroxycalix[4]arene [29], 25,26,27-tribenzoyloxy–28-hydroxy-11-(4-nitrophenylazo)calix[4]arene (1a) [14], 25,27-diethylacetoxy-26,28-dihydroxy-5,17-bis(4-nitrophenylazo)calix[4]arene (2a) [13], and (4-nitrophenylazo) calix[4]arene (3a) [23] are synthesized as described by previously reported method.
Synthesis of 25,26,27-tribenzoyloxy–28-hydroxy-11-(4-aminophenylazo)calix[4]arene (1b)
General reduction procedure [30]: A solution of 0.50 g (0.057 mmol) of 1a and 0.32 g (1.41 mmol) of SnCl2.2H2O in 20 mL MeOH was refluxed for 4 h. The solution was respectively cooled, poured onto ice, and neutralized (pH 7–8) by the addition of 1% NaOH solution, and extracted with CH2Cl2 twice. The organic phase was separated, washed with brine and dried (Na2SO4) and evaporated to leave an orange solid. Trituration with 75 mL of CH3OH followed by crystallization of the insoluble material from i-PrOH gave us 0.33 g (69%) of (1b), mp. 255 °C. [Found: C 77.62; H 4.95; N 4.82. C55H41N3O7 calcd: C 77.18; H 4.83; N 4.91%]. IR (KBr) υmax: 3,528, 3,060, 1,726, 1,451 cm−1. 1 H NMR (CDCl 3 , 25 °C): δ H: 3.50–4.00 (d,8H, J = 13.3 Hz, Ar–CH2–Ar), 6.60–6.90 (s, 4H, Ar–H), 7.00–7.60 (m, 26H, Ar–H), 7.80 (s, 1H, –OH), 8.10 (s, 2H, –NH2).
Synthesis of 25,27-diethylacetoxy-26,28-dihydroxy-5,17-bis(4-aminophenylazo) calix[4]arene (2b)
Compound (2b) was prepared as described above, using 40 ml EtOH with 2a and obtained as a dark brown solid, which was filtered and washed with water and MeOH. Yield, 0.35 g (73%) as a pale reddish-brown solid, m.p. 150 °C; [Found: C 69.17; H 5.68; N 9.92. C48H46N6O8 calcd: C 69.05; H 5.55; N 10.07%]. IR (KBr) υmax: 3,381, 2,926, 1,740, 1,467 cm−1. 1 H NMR (CDCl 3 , 25 °C): δ H: 1,10 (s, 6H, –CH3), 3,2 (q, 4H, CH2–CH3), 4,6 (s, 4H, –O–CH2) 3.40–4.40 (d, 8H, J = 13.1 Hz, Ar–CH2–Ar), 6.60–7.70 (m, 8H, Ar–H), 7.10–8.00 (m, 10H, Ar–H), 8.30 (s, 2H, –OH), 9,1 (4H, broad, –NH2).
Synthesis of (p-aminophenylazo)calix[4]arene (3b)
Compound (3b) was prepared as described above, using 60 ml EtOH with 3a and obtained as a dark brown solid, which was filtered and washed with water and MeOH. Yield, 0.30 g (63%) as a brown solid, m.p. 120 °C (dec.); [Found: C 69.18; H 5.08; N 18.49. C52H44N12O4 calcd: C 69.32; H 4.92; N 18.66%]. IR (KBr) υmax: 3,379, 1,620, 1,558, 1,469 cm−1. 1 H NMR (CDCl 3 , 25 °C): δ H: 3.90–4.70 (d, 8H, J = 13.2 Hz, Ar–CH2–Ar), 6.00–6.20 (m, 16H, Ar–H), 7.00–7.40 (m, 8H, Ar–H), 7.80 (s, 4H, –OH), 8.20 (s, 8H, –NH2).
Synthesis of 11-(2-hydroxy-3-methoxyphenylimin-o-phenylazo)-25,26,27-tribenzoyloxy-28-hydroxycalix[4]arene (1c)
General Schiff base procedure [31]: To a solution of 0.15 g (0.18 mmol) of 1b in ethanol (30 mL) was added 2-hydroxy-3-methoxybenzaldehyde 0.026 g (0.18 mmol) and the mixture was refluxed for 24 h. After cooling the reaction mixture, the yellow-colored ligand was precipitated by adding some drops of water. The 1c was recrystallized in ethanol. Yield, 0.12 g (71%) as a dark brown solid, m.p. 263 °C; [Found: C 76.28; H 4.86; N 4.12. C63H47N3O9 calcd: C 76.43; H 4.78; N 4.24%]. IR (KBr) υmax: 3,530, 3,061, 1,727, 1,601, 1,592, 1,451, 1,268, 707 cm−1. 1 H NMR (CDCl 3 , 25° C): δ H: 2.45 (s, 3H, –OCH3), 3.40–3.70 (d, 8H, J = 13.1 Hz, Ar–CH2–Ar), 6.40 (s, 1H, calix–OH), 6.50–6.70 (m, 15H, Ar–C=O), 7.18–7.36 (m, 3H, Ar-OCH3), 7.40–7.70 (m, 4H, N–Ar–N=N), 7.60–7.88 (m, 11H, Ar-calix), 7.78 (s, 1H, CH=N), 11.93 (s, 1H, HO–Ar–CH3). 13 C NMR (CDCl 3 , 25 °C): δ C: 206.1, 160.2, 159.1, 151.2, 149.4, 145.8, 144.6, 139.1, 135.7, 134.3, 132.2, 126.4, 124.3, 123.5, 120.2, 56.2, 33.6, 13.0.
Synthesis of 5,17-(2-hydroxy-3-methoxyphenyliminphenylazo)-25,27-diethylacetoxy-26,28-dihydroxycalix[4]arene (2c)
Compound (2c) was prepared as described above, using 30 ml EtOH with 2b and obtained as a dark brown solid, which was filtered and washed with water and MeOH. Yield, 0.09 g (46%) as a dark brown solid, m.p. 300 °C. [Found: C 69.52; H 5.44; N 7.49. C64H58N6O12 calcd: C 69.68; H 5.30; N 7.62%]. IR (KBr) υmax: 3,374, 3,176, 2,933, 2,838, 1,615, 1,592, 1,464, 1,254, 735 cm−1. 1 H NMR (CDCl 3 , 25 °C): δ H: 2.10 (t, 6H, –CH3), 2.40 (s, 6H, –OCH3), 2.75 (q, 4H, –CH2–), 2.82 (s, 4H, –CH2–CO), 3.45–3.80 (d, 8H, J = 13.1 Hz, Ar–CH2–Ar), 6.60 (s, 2H, calix–OH), 6.95–7.20 (m, 6H, Ar–OCH3), 7.30–7.65 (m, 8H, N–Ar–N=N), 7.80–8.25 (m, 10H, Ar–calix), 8.80 (s, 2H, CH=N), 10.25 (s, 2H, HO–Ar–CH3). 13 C NMR (CDCl 3 , 25 °C): δ C: 203.1, 162.2, 160.2, 152.1, 150.4, 146.3, 145.2, 139.6, 135.3, 133.1, 131.2, 126.1, 124.1, 122.4, 120.2, 57.3, 34.0, 13.1.
Synthesis of 5,11,17,23-(2-hydroxy-3-methoxyphenyliminphenylazo)-25,26,27,28-hydroxy calix[4]arene (3c)
Compound (3c) was prepared as described above, using 30 ml EtOH with 3b and obtained as a dark brown solid, which was filtered and washed with water and MeOH. Yield, 0.09 g (35%) as a dark brown solid, m.p. 325 °C (dec.). [Found: C 70.06; H 4.92; N 11.54. C84H68N12O12 calcd: C 70.18; H 4.77; N 11.69%]. IR (KBr) υmax: 3,425, 3,188, 2,940, 1,591, 1,467, 1,257, 754 cm−1. 1 H NMR (DMSO- d 6 , 25 °C): δ H: 2.45 (s, 12H, –OCH3), 3.25–3.65 (d, 8H, J=13.2 Hz, Ar–CH2–Ar), 5.96 (s, 4H, calix–OH), 6.60–7.15 (m, 12H, Ar–OCH3), 7.40–7.80 (m, 16H, N–Ar–N=N), 7.90–8.15 (m, 8H, Ar–calix), 8.25 (s, 4H, CH=N), 10.18 (s, 4H, HO–Ar–CH3). 13 C NMR (DMSO- d 6 , 25 °C): δ C: 160.2, 159.4, 151.6, 149.0, 145.3, 144.1, 139.3, 135.2, 134.2, 132.1, 126.3, 124.5, 123.26, 120.1, 56.0, 33.1, 13.0.
Solvent extraction
A chloroform solution (10 mL) of ligand (1 × 10−3 M) and an aqueous solution (10 mL) containing 2 × 10−5 M picric acid and 1 × 10−2 M metal nitrate were shaken at 25 °C for 1 h contact time. An aliquot of the aqueous solution was taken and the ultraviolet spectrum was recorded. For each cation–azocalix[n]arene system, the extraction experiments and the absorbance measurements were repeated twice. Blank experiments showed that no picrate extraction occured in the absence of an azocalix[n]arene. The extractability of the metal cations is expressed by means of the following equation:
where A 0 and A are the absorbances in the absence and presence of ligands, respectively.
Results and discussion
Results and characterization of the products
Different functionalized azocalixarenes are excellent potential starting materials for the selective design of new materials. Preparation of mono-, di- and tetra amine functionalized azocalix[4]arenes 1b–3b and their conversion to the 2-hydroxy-3-metoxy benzaldehyde Schiff bases 1c–3c have been reported in this work. Metal extraction studies of interested Schiff base compounds are also reported. Schiff bases are potentially capable of forming stable complexes with metal ions [9].
Azocalixarenes have been widely used as three-dimensional building blocks for the construction of artificial molecular receptors capable of recognizing cations. Thus, azocalix[4] arene Schiff base compounds reported here were designed to take advantage of the well-established binding interactions and spectroscopic properties of azomethine groups. The synthesis of compounds 1a–3a (Fig. 2) was described in literature [13, 14, 23], whereas the three novel Schiff base forms of azocalix[4]arenes from 1a–3a to 1c–3c are synthesized for the first time.
Formerly, our group has synthesized according to the method of Gutsche et al. [27, 28]. In previous work, we investigated the azo coupling reactions of three benzoyl and diethylester calix[n]arene with 4-nitrobenzenediazonium chlorides [13, 14, 23]. The series of three novel azocalix[4]arene derivatives described herein were synthesized according to the method described by Deligöz and Ercan [6]. All reactions proceeded smoothly and the resulting corresponding azo compounds are in good yields.
Amine functionalization of azocalix[4]arenes 1b–3b was carried out by reduction with SnCl2.H2O. Condensation of mono-, di- and tetra- amino derivatives with 2-hydroxy-3-methoxybenzaldehyde gave respective Schiff bases. Substitution of the hydroxyl groups on the lower rim of the Schiff bases 1c and 2c was carried out by benzoylchloride and ethylbromoacetate. The introductions of tri- and di- ester groups on the lower rim of calix[4]arenes lock the macrocycle in cone conformation. The cone conformation was exclusively achieved by esterification of lower rim hydroxyl groups in DMF/MeOH at room temperature using K2CO3 to impart template effect. In the present investigation, calix[4]arene-based Schiff bases showing liquid–liquid extraction property are reported for the first time as compared with different liquid–liquid extraction compounds of azocalix[4]arene reported formerly.
The formulations and molecular structure of azocalix[4]arene compounds (1c, 2c and 3c) given in Fig. 2 are supported by the data obtained from micro analyses, wherein the percentage of C, H, and N in the analyses conform the calculated values. The structures of novel compounds were also characterized by a combination of 1H and 13C NMR spectra.
According to 1H NMR spectra of the azocalix[4]arenes 1c and 2c, both compounds appeared to exist in cone conformation due to the presence of two sets of characteristic AB systems at 3.30 and 3.70 ppm (J = 13.1 Hz) and 3.45 and 3.80 ppm (J = 13.2 Hz), respectively. On the basis of spectroscopic evidence, the transformation of compound 3c occurs with the retention of the cone conformation of the azocalix[4]arene moiety.
1H NMR data of all compounds (1c, 2c and 3c) showed a peak which can be assigned to azomethine proton (CH=N) δ = 7.78, 8.80 and 8.25 ppm, respectively. The appearance of a peak within the range of δ = 2.82 ppm is due to the presence of proton of methylene (–OCH2–) group for compound 2c.
In the 1H NMR spectra of the ligands 1c–3c, the singlets at 11.93, 10.25 and 10.18 ppm area can be attributed to the proton of the –OH group neighboring the azomethine group. The protons of lower rim –OH groups in azocalix[4]arene 1c–3c are appeared at 6.40, 6.60 and 5.96 ppm as a singlet, respectively. The methoxy (–OCH3) group protons of 1c–3c are shown at 2.45, 2.40 and 2.45 ppm as a singlet.
The formation of Schiff bases (1c and 2c) is confirmed by the sharp singlet at δ 7.78–8.25 due to the azomethine proton. A singlet observed at δ 11.93–10.25 ppm is probably due to phenolic OH group. The sharp multiplet signals of the phenyl protons are found in the region δ 6.50–7.88 to 6.95–8.25 ppm. The 1H NMR spectra of ligand in DMSO-d 6 revealed a multiplet at 6.60–8.15 ppm corresponding to aromatic protons.
13C NMR data of all compounds showed a peak due to the presence of azomethine carbon (CH=N) observable at the chemical shift δ = 160.2 ppm; only compound 2c showed a peak at δ = 152.1 ppm. The aromatic carbon showed peaks observable at δ = 159 to 120 ppm; aliphatic carbon chain showed peaks at δ = 56–33 ppm and methylene bridge carbon showed peak at δ = 13.0 ppm.
All the calix[4]arene Schiff bases obtained are found to be thermally stable. Spectroscopic methods (FTIR, 1H and 13C NMR) have been employed to elucidate the structures of the compounds (1c, 2c and 3c). FTIR data show that the investigated bonds, which can be assigned to the stretching of azomethine (C=N) of all compounds were observed at the frequencies of 1,592–1,670 cm−1. Ether bands corresponding to C–O stretching appear at 1,254–1,268 cm−1.
In order to study the binding of Schiff base ligands with metals by picrate extraction method, the IR spectrum of the free Schiff base ligands was compared with those of the binding ones. IR spectrum of free ligands exhibits bands at 3,374–3,530 cm−1 and 2,933–3,061 cm−1 that are assignable to ν(OH) and ν(Ar–CH). The bands at 1,591–1,592 cm−1 are due to the vibration of the azomethine group ν(–CH=N–) in both type of ligands.
The infrared spectra of the ligands (1c, 2c and 3c) show a broad weak band at 3,530, 3,374, 3,425 cm−1 due to the phenolic OH groups suggesting the intramolecular hydrogen bonding between hydroxyl hydrogen and nitrogen of azomethine group. The absorption peaks at around 1,615, 1,532 and 1,254 cm−1 are due to ν(C=O) ester carbonyl, ν(C=N) azomethine and ν(C–O) phenolic, respectively.
Liquid–liquid extraction
Transportation experiments with metal picrate salts were carried out with a H2O–CHCl3 liquid–liquid phase transfer system using the diazo coupling calix[n]arene and diazo compounds as cation carriers. The results of the cation transportation experiments are in harmony with those of the two-phase extraction measurements.
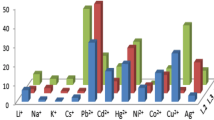
The ionophoric properties of compounds 1c and 2c towards the alkaline-earth and the transition metal cations were first investigated by the picrate extraction method [15] for the first time. The results expressed as a percentage of cation extracted (E%) are collected in Table 1 and shown graphically in Fig. 3a and b.
The extraction of these cations (Ag+, Hg+, Hg2+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+, Cr3+ and Al3+) with ligands 1c and 2c had already been done [15], with same experimental conditions. Even though the azocalix[4]arene derivatives which are used in the previous studies contained –OH functional groups, in this work the azocalix[4]arene derivatives contain benzoyl and ethyl ester derivatives. Ligand 3c was insoluble because it did not have any of the stated groups. Therefore, it was not used in extraction studies. These groups are selected due to their positive effect on solubility of the resulting compound. This situation increases the efficiency of extraction. Some remarks can be made without correcting the data. While the extraction level for Hg2+ (69.51%) is very superior to that of Cu2+ (48.39%), the extraction level for Cr3+ (31.81%) is inferior to that of Cu2+.
Both compounds (1c and 2c) form 1:1 complexes with Hg+, and the cation is believed to hold an encapsulation into the cavity defined by the conjugated chromophore azo (–N=N–) groups. π interactions may play a role in complexation with azocalix[n]arenes. The results have shown that, although these ligands bear hard nitrogen donor atoms, they display a strong affinity towards soft metal cations, like Ag+ and Hg2+.
Our results suggest that the match between the cation and the calix[4]arene Schiff Base derivatives are an evident factor in selectivity. For example, Co2+ and Cu2+ have equal ionic radii and copper is the first on extractability scale, and also Zn2+ and Cd2+ having similar sizes are almost on the similar ends of that scale for both azocalix[4]arene Schiff bases. With other ester ligands, a similar situation is observed for Ni2+, Zn2+ and Co2+. Another important remark is one of the smallest cation Cu2+ which is mostly extracted by ligand 2c and showed a strong peak selectivity for Cu2+ (48.39 %), with nearly double diameter.
A series of homo- and hetero-polynuclear copper(II) complexes of N,N″-bis[1-biphenyl-2-hydroxyimino-2-(4-acetylanilino)-1-ethylidene]-diamines have been prepared and characterized with different physical techniques by Dede et al. [32]. They suggested that dinuclear complexes of the diimine-dioxime ligands have a 2:1, and trinuclear complexes of the diimine-dioxime ligands have a 3:2 metal:ligand ratio. Our proffs are in accordance with the literature for similar compounds.
Both of the IR spectra information supports the suggestion of coordination of the imine nitrogen atom of the azocalixarene moiety and phenolic oxygen atom of the 3-methoxy-2-hydroxybenzaldehyde moiety to the metal ion. According to the above discussion, the general structure for the metal complex is proposed as shown in Fig. 4.
Conclusion
In this study, we presented the preparation of azocalix[4]arene Schiff base derivatives (1c–3c) that can perform complexes with Cu2+ in which two calixarene moieties are united in a single molecule. Presence of two calix[4]arene moieties in single molecule has increased the inclusion ability and other calyx[4]arene-related capabilities of the complex. Furthermore, the Schiff base ligands (1c–3c) and their copper complex, both have solvent-dependent UV/vis spectra (solvatochromicity). They can be used in non-linear optical active (NLO) material applications.
Solvatochromicity was observed for the ligands and their copper(II) complexes, i.e. in different solvents depending on the solvent polarity, their λmax of absorption in electronic spectra appeared in different wavelengths. Therefore, these compounds can be good candidates for NLO applications.
References
C.D. Gutsche, J.F. Stoddart, in Calixarenes revisited. Monographs in supramolecular chemistry (The Royal Society of Chemistry, London, 1998), p.149–167
M. Durmaz, S. Alpaydın, A. Sırıt, M. Yilmaz, Chiral Schiff base derivatives of calix[4]arene: synthesis and complexation studies with chiral and achiral amines. Tetrahedron Asymmetr. 17, 2322–2327 (2006)
H. Deligöz, Azocalixarenes: synthesis, characterization, complexation, extraction, absorption properties and thermal behaviours. J. Incl. Phenom. Macrocyclic Chem., 55, 197–218 (2006) (Review article)
H.M. Chawla, S.P. Singh, S.N. Sahu, S. Upreti, Shaping the cavity of calixarene architecture for molecular recognition: Synthesis and conformational properties of new azocalix[4]arenes. Tetrahedron 62, 7854–7865 (2006a)
H.M. Chawla, S.P. Singh, S. Upreti, Synthesis of cesium selective pyridyl azocalix[n]arenes. Tetrahedron 62, 2901–2911 (2006b)
H. Deligöz, N. Ercan, The synthesis of some new derivatives of calix[4]arene containing azo groups. Tetrahedron 58(14), 2881–2884 (2002)
H. Deligöz, Synthesis and properties of a series of novel calix[6]arene diazo derivatives. J. Incl. Phenom. Macrocycl. Chem. 43(3/4), 285–289 (2002)
H. Shimuzu, K. Iwamoto, K. Fujimoto, S. Shinkai, Chromogenic calix[4]arene. Chem. Lett. 2147–2150 (1991)
A.A. Alemi, B. Shaabani, Synthesis and characterization of a Schiff base of p-tert-butylcalix [4]arene and its complex with copper(II). Acta Chim. Slov. 47, 363–367 (2000)
M.A. Phaniband, P.G. Avaji, S.D. Dhumwad, Synthesis, spectral characterization and biological evaluation of some lanthanite(III) complexes of Schiff bases of carbostyril derivatives”. Main Group Chem. 7(4), 271–283 (2008)
N.H. Patel, H.M. Parekh, M.N. Patel, Synthesis, physicochemical characteristics and biocidal activity of some transition metal mixed-ligand complexes with bidentate (NO and NN) Schiff bases. Pharma. Chem. J. 41(2), 78–81 (2007)
H. Deligöz, M.S. Ak, Azocalixarenes.6: synthesis, complexation, extraction and thermal behaviour of four new azocalix[4]arene. J. Incl. Phenom. Macrocycl. Chem. 59, 115–123 (2007)
Ö.Ö. Karakuş, H. Deligöz, Azocalixarenes.8: synthesis and investigation of the absorption spectra of di-substituted azocalix[4]arenes containing groups. J. Incl. Phenom. Macrocycl. Chem. 61, 289–296 (2008)
Ö.Ö. Karakuş, H. Deligöz, Azocalixarenes7: synthesis and study of the absorption properties of novel mono-substituted chromogenic azocalix[4]arenes. Turkish J. Chem. 35, 87–98 (2011)
H. Deligöz, M. Yilmaz, Liquid–liquid extraction of transition metal cations by calixarenes-based cyclic ligands. Solv. Extr. Ion Exch. 13(1), 19–26 (1995)
H. Deligöz, E. Erdem, Comparative studies on the solvent extraction of transition metal cations by calixarene, phenol and ester derivatives. J. Hazard. Mater. 154(1/3), 29–32 (2008)
J. Lu, X. Tong, X. He, A mercury ion-selective electrode based on a calixarene derivative containing the thiazole azo group. J. Electroanal. Chem. 540, 111–117 (2003)
J.Y. Kim, C.R. Kim, S.H. Lee, J.H. Lee, J.S. Kim, UV band splitting of chromogenic azo-coupled calix[4]crown upon cation complexation. J. Org. Chem. 68, 1933–1937 (2003)
V. Arora, H.M. Chawla, T. Francis, M. Nanda, S.P. Singh, Synthesis of a new cesium selective calix[4]arene based chromoionophore. Indian J. Chem. 42, 3041–3043 (2003)
Q. Ma, H. Ma, M. Su, Z. Wang, L. Nie, S. Liang, Determination of nickel by a new chromogenic azocalix[4]arene. Anal. Chim. Acta 439, 73–79 (2001)
H. Deligöz, Synthesis of an oligomer and a styrene polymer-supported calix[4]arene derivatives and selective extraction of Fe3+. Supramol. Chem. 15(5), 317–321 (2003)
M. Yilmaz, H. Deligöz, Studies on compounds of uranium(VI) with two vic-dioxime derivatives of calix[4]arene. Synth. React. Inorg. Met. Org. Chem. 28(5), 851–861 (1998)
F. Karcı, I. Şener, H. Deligöz, Azocalixarenes.1: synthesis, characterization and investigation of the absorption spectra of substituted azocalix[4]arenes. Dyes Pigments 59, 53–61 (2003)
H. Deligöz, M. Yilmaz, Synthesis of polymer supported calix[4]arene and selective extraction of Fe3+. React. Funct. Polym. 31, 81–88 (1996)
H. Deligöz, A.I. Pekacar, M.A. Özler, M. Ersöz, Solvent extraction of Fe3+ cation by 25, 26, 27, 28-tetraisonitrosoaceto calix[4]arene and based ligands. Sep. Sci. Technol. 34(16), 3297–3304 (1999)
M. Ak, D. Taban, H. Deligöz, Transition metal cation extraction by ester and ketone derivatives of chromogenic azocalix[4]arene. J. Hazard. Mater. 154(1-3), 51–54 (2008)
C.D. Gutsche, M. Iqbal, p-tert-butylcalix[4]arene. Org. Synth. 68, 234–238 (1990)
C.D. Gutsche, M. Iqbal, D. Stewart, Calixarenes.18. Synthesis procedures for para-tert-butylcalix[4]arene. J. Org. Chem. 51, 742–745 (1986)
C.D. Gutsche, L.G. Lin, Calixarenes 12. The synthesis of functionalized calixarenes. Tetrahedron 42, 1633–1640 (1986)
C.D. Gutsche, K.A. See, Calixarene.27. Synthesis, characterization and complexation studies of double-cavity calix[4]arenes. J. Org. Chem. 57(16), 4527–4539 (1992)
Z. Asfari, F. Arnaud-Neu, J. Vicens, Schiff base p-tert-butylcalix[4]arenes. Synthesis and metal ion complexation. J. Org. Chem. 59, 1741–1744 (1994)
B. Dede, F. Karipcin, M. Cengiz, Synthesis, characterization and extraction studies of N,N″-bis[1-biphenyl-2-hydroxyimino-2-(4-acetylanilino)-1-ethylidene]-diamines and their homo-and heteronuclear copper(II) complexes. J. Chem. Sci. 121(2), 163–171 (2009)
Acknowledgments
This work was supported by the Research Foundation of Pamukkale University, Denizli, Turkey (BAP 2006FEF005), which is gratefully acknowledged for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karakuş, Ö.Ö., Deligöz, H. Synthesis and characterization of three novel azocalix[4]arene Schiff base derivatives and their selective copper extraction. J IRAN CHEM SOC 9, 93–100 (2012). https://doi.org/10.1007/s13738-011-0014-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-011-0014-y