Abstract
Thrombotic microangiopathy (TMA) syndromes can be secondary to a multitude of different diseases. Most can be identified with a systematic approach and, when excluded, TMA is generally attributed to a dysregulation in the activity of the complement alternative pathways—atypical hemolytic uremic syndrome (aHUS). We present a challenging case of a 19-year-old woman who presented with thrombotic microangiopathy, which was found to be caused by methylmalonic acidemia and homocystinuria, a rare vitamin B12 metabolism deficiency. To our knowledge, this is the first time that an adult-onset methylmalonic acidemia and homocystinuria presents as TMA preceding CNS involvement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thrombotic microangiopathy (TMA) syndromes are a rare and potentially fatal group of diseases. While their etiologies can be surprisingly diverse, TMAs share a similar pathophysiological pathway of small vessel narrowing and occlusions caused by microthrombi, resulting in the typical clinical picture of microangiopathic hemolytic anemia (MAHA), consumptive thrombocytopenia, and ischemic lesions of small-vessel-rich organs, such as the brain and the kidney. Reflecting the common final phenotype of endothelial cell injury, renal morphology features are also fairly alike among the many causes of TMA [1, 2].
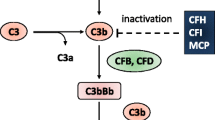
Despite these homogenous clinical features, TMAs’ treatment differs substantially depending on its root cause. This implies diagnostic tools to distinguish the many different causes that have so far been described—including secondary to autoimmune diseases, malignant hypertension, HIV, drugs, shiga toxin-producing Escherichia coli infection, or the more recently recognized ‘atypical HUS (aHUS)’, a TMA driven by inherited complement alternative pathway dysregulation [2].
The authors report a case of a 19-year-old white female patient displaying combined methylmalonic acidemia and homocystinuria, a defect in cobalamin metabolism, which manifested as a thrombotic microangiopathy.
Case report
A 19-year-old woman, who had been irregularly followed by a pediatric psychiatrist for the previous 3 years due to depression and learning difficulties, was observed by her general practitioner complaining of menorrhagia. She had a normocytic anemia of 10 g/L and a normal platelet count of 270 × 109/L. She was also found to be hypertensive at that time, and was started on a beta-blocker and an oral contraceptive pill. A few weeks later, she presented to the emergency department, complaining of nausea, fatigue, abdominal pain, and generally feeling unwell. At admission, she had a macrocytic anemia (5.4 × 10 g/L), platelets 82 × 109/L, LDH 901 U/L (reference value 250–450), haptoglobin < 0.07 g/L (reference value 0.8–2.0), presence of schizocytes, and a serum creatinine of 2.85 mg/dL (252 µmol/L).
Microangiopathic hemolytic anemia along with acute kidney injury due to TMA was diagnosed and the patient was started on plasma exchange (PLEX), while secondary causes of TMA were being excluded. She had normal ADAMTS13 activity, with no auto-antibodies against ADAMTS13; antinuclear antibodies, anti-dsDNA, anti-Scl70, phospholipid syndrome antibodies, and Coombs test, search for human immunodeficiency; hepatitis B and hepatitis C virus were all negative; C3 and C4 were within the normal range; Shiga toxin search was negative; and pregnancy was also excluded.
Despite five consecutive days of PLEX, the patient’s clinical parameters remained unstable, requiring several blood transfusions and urgent dialysis was started. As our investigation for secondary causes of TMA was negative, the patient was assumed to have aHUS refractory to PLEX and was started on eculizumab. Despite eculizumab therapy for 3 weeks, with efficient complement blockade (measured by CH50), hemolysis parameters remained high, requiring frequent blood transfusions along with undetectable haptoglobin levels. It was at this point that the clinical features changed, as the patient started showing neuropsychiatric symptoms (compulsive behavior and visual hallucinations, ataxic gait, drooling, and extreme somnolence), along with no improvement in renal function.
At this point, rare secondary causes were considered—the neurologic involvement prompted us to evaluate serum total homocysteine levels, that were found to be extremely elevated (434 μmol/L—reference value < 20), establishing the diagnosis of a vitamin B12 metabolism error, confirmed by elevated serum methylmalonic acid (9.3 µmol/L—reference value < 0.27; gas chromatography–mass spectrometry) and low methionine (6 μmol/L—reference value 11–37; gas chromatography–ion detector)—the methylmalonic acidemia and homocystinuria diagnosis would later be confirmed by molecular studies showing compound heterozygous mutations in the MMACHC gene (mRNA accession number NM_015506.2)—c.271dupA (p.Arg91Lysfs*14) and c.565C>A (p.Arg189Ser); initial metabolic serum and plasma evaluation were all performed in samples collected before PLEX was initiated. Transthoracic echocardiogram showed no signs of pulmonary hypertension.
Therapy with intramuscular hydroxocobalamin (5 mg, three times a week), folinic acid (10 mg/day), and levocarnitine (3 g/day) was started, with rapid and significant neurological improvement after just a few days of therapy, as well as progressive resolution of hemolytic parameters. Renal biopsy could then be safely performed and a severe TMA glomerular and vascular lesions were documented (Fig. 1). Meanwhile, the negative search for mutations in the genes encoding the complement regulating proteins by next generation sequencing and multiplex ligation-dependent probe amplification of CFH, CFHR1, CFHR3, CFHR4, CFI, CFB, C3, THBD, and DGKE (as previously described [3]) allowed for a cautious eculizumab suspension; we saw no relapse in hemolysis parameters thereafter. After almost 12 months of effective targeted therapy, despite full neurological and hematological recovery, the patient remains dialysis dependent, and is now being considered for renal transplantation.
Discussion
This is a case of a severe form of TMA, found to be unresponsive to PLEX and with no obvious secondary causes, leading to the diagnosis of aHUS and eculizumab therapy. After 3 weeks of unsuccessful treatment with the complement blocking drug and the development of neuropsychiatric symptoms, methylmalonic acidemia associated with homocystinuria was considered.
Vitamin B12 (also known as cobalamin [Cbl]) has a complex metabolism, as it functions as a cofactor for two enzymes: (1) methyltetrahydrofolate methyltransferase, which catalyzes the conversion of homocysteine to methionine in cytoplasm and (2) methylmalonyl-CoA mutase, which catalyzes the conversion of methylmalonyl-CoA to succinyl-CoA in mitochondria [4]. There can be several errors in Cbl’s metabolism, which result in the combination of accumulation of homocysteine and/or methylmalonic acid (MMA), and a deficiency in methionine. While rare (with an estimated incidence of 1/85,000 in our country [5]), combined methylmalonic acidemia and homocystinuria (also termed CblC type), is the most frequent of these disorders, and is caused by mutations in the MMACHC gene [6, 7].
The pathophysiology of CblC is not fully understood, but it is likely the result of the synergistic effect of the toxicity of homocysteine and methylmalonic acid and the deficiency in methionine. Even less clear, is how it results in TMA—some argue that the toxic homocysteine levels cause endothelial damage, which in turn produces complement activation [8, 9].
Despite exceedingly variable clinical phenotypes, the typical presentation occurs in newborns, manifestations being predominantly neurological. Late-onset manifestations in childhood and adulthood are less frequent, and while neurological manifestations continue to be more frequent, thromboembolic events are also relatively common—TMA being one of them [10]. However, the report of MMACHC mutations has allowed for genotype–phenotype correlations regarding age of onset. The association of two deleterious mutations found in our patient has been previously described, c.271dupA being one of the most common mutation in Portuguese patients [11, 12]. All patients homozygous for the c.271dupA mutation have presented with early onset disease. Conversely, the individuals with compound heterozygous mutations for the c.271dupA mutation and a missense mutation, as our patient, have been correlated with later onset of disease, presenting as late as 10–20 years of age [11]. It is possible that transcripts containing the missense allele are translated into proteins with residual function [6].
Clinical suspicion is fundamental, as it will lead to targeted biochemical testing. In our institution, serum homocysteine is readily available, and allowed for a presumptive diagnosis and therapy start. The search for urine organic acids, serum MMA, and plasma amino acids should also be done. There are an increasing number of reports, suggesting that serum homocysteine and MMA should be requested for every patient presenting with TMA [13]. Of note, vitamin B12 levels are normal and will not help in diagnosis. The screening of mutations in MMACHC will confirm the diagnosis.
The treatment goal is to normalize serum methionine and to lower homocysteine and MMA as soon as possible, which can be achieved through the administration of hydroxocobalamin and betaine; folinic acid and levocarnitine might also be beneficial, but their efficacy is not established. Of note, hydroxocobalamin administration needs to be parental (intramuscular, subcutaneous, or intravenous), as neither oral hydroxocobalamin nor parenteral cyanocobalamin seem to be effective. Serum homocysteine is useful for monitoring metabolic control of the disease and hydroxocobalamin dose management [10].
In our case, the adult-onset TMA manifestation meant that we were ill-prepared for this diagnosis: by the time it was attained and therapy started, the patient had already severely damaged kidney function, and despite a full recovery of neurological and hemolytic parameters, the patient remains dialysis dependent—Fig. 2. In recent years, TMAs have been recognized to represent the interaction of a predisposition background and a triggering event—typically infections or drugs. Compound mutations for CblC help explain the silent course of disease until the patient was 19 years. In our view, beta-blocker and an oral contraceptive pill recent introduction probably represent the trigger for full-blown CblC manifestations. In addition, oral contraceptives are known to potentially cause disturbed B12 absorption [14]. Theoretically, this could further enhance disease manifestations. Learning difficulties had been identified during our patient’s childhood, which could be interpreted as subtle ClbC manifestations; however, we saw no improvement during follow-up, and despite no formal cognitive or neuro-psychological evaluation, we do not think that the patient’s learning difficulties are attributable to CblC.
Eculizumab-resistant TMA due to CblC has been previously reported [15], albeit without neurological symptoms. To our knowledge, this is the first time that an adult-onset methylmalonic acidemia and homocystinuria presents as TMA preceding CNS involvement.
In summary, TMAs are severe life-threatening syndromes, whose secondary causes should be promptly and aggressively investigated, especially in the cases where there is no response to anti-C5 therapy. Methylmalonic acidemia with homocystinuria is a rare disease, usually diagnosed in infancy, manifestations being predominantly neurological. Its manifestation as TMA is unusual, and can easily be ruled out by measuring serum homocysteine. The outcome is usually dismal, but aggressive B12 administration for life can control the disease.
References
Yu X-J, Yu F, Song D, Wang S-X, Song Y, Liu G, et al. Clinical and renal biopsy findings predicting outcome in renal thrombotic microangiopathy: a large cohort study from a single institute in China. Sci World J. 2014;2014:680502.
Fakhouri F, Zuber J, Frémeaux-Bacchi V, Loirat C. Haemolytic uraemic syndrome. Lancet. 2017;6736(17).
Fidalgo T, Martinho P, Pinto S, Oliveira C, Salvado AC, Borràs RN, et al. Combined study of ADAMTS13 and complement genes in the diagnosis of thrombotic microangiopathies using next-generation sequencing. Res Pract Thromb Haemost. 2017;(March):1–12.
Brunelli SM, Meyers KEC, Guttenberg M, Kaplan P, Kaplan BS. Cobalamin C deficiency complicated by an atypical glomerulopathy. Pediatr Nephrol. 2002;17(10):800–3.
Nogueira C, Marcao A, Rocha H, Sousa C, Fonseca H, Valongo C, et al. Molecular picture of cobalamin C/D defects before and after newborn screening era. J Med Screen. 2016;0(0):1–6.
Lerner-Ellis JP, Tirone JC, Pawelek PD, Doré C, Atkinson JL, Watkins D, et al. Identification of the gene responsible for methylmalonic aciduria and homocystinuria, cblC type. Nat Genet. 2006;38(1):93–100.
Weisfeld-Adams JD, Morrissey MA, Kirmse BM, Salveson BR, Wasserstein MP, McGuire PJ, et al. Newborn screening and early biochemical follow-up in combined methylmalonic aciduria and homocystinuria, cblC type, and utility of methionine as a secondary screening analyte. Mol Genet Metab. 2010;99(2):116–23.
Sharma AP, Greenberg CR, Prasad AN, Prasad C. Hemolytic uremic syndrome (HUS) secondary to cobalamin C (cblC) disorder. Pediatr Nephrol. 2007;22(12):2097–103.
Beck BB, van Spronsen FJ, Diepstra A, Berger RMF, Kömhoff M. Renal thrombotic microangiopathy in patients with cblC defect: review of an under-recognized entity. Pediatr Nephrol Pediatric Nephrol. 2017;32(5):733–41.
Carrillo-Carrasco N, Chandler RJ, Venditti CP. Combined methylmalonic acidemia and homocystinuria, cblC type. I. Clinical presentations, diagnosis and management. J Inherit Metab Dis. 2012;35(1):91–102.
Nogueira C, Aiello C, Cerone R, Martins E, Caruso U, Moroni I, et al. Spectrum of MMACHC mutations in Italian and Portuguese patients with combined methylmalonic aciduria and homocystinuria, cblC type. Mol Genet Metab. 2008;93(4):475–80.
Thauvin-Robinet C, Roze E, Couvreur G, Horellou M-H, Sedel F, Grabli D, et al. The adolescent and adult form of cobalamin C disease: clinical and molecular spectrum. J Neurol Neurosurg Psychiatry. 2007;79(6):725–8.
George JN. Cobalamin C deficiency-associated thrombotic microangiopathy: uncommon or unrecognised? Lancet. 2015;386(9997):1012.
Moll R, Davis B. Iron, vitamin B 12 and folate. Medicine (Baltimore). Elsevier Ltd; 2017;45(4):198–203.
Cornec-Le Gall E, Delmas Y, De Parscau L, Doucet L, Ogier H, Benoist JF, et al. Adult-onset eculizumab-resistant hemolytic uremic syndrome associated with cobalamin C deficiency. Am J Kidney Dis. 2014;63(1):119–23.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors have declared no competing interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
About this article
Cite this article
Navarro, D., Azevedo, A., Sequeira, S. et al. Atypical adult-onset methylmalonic acidemia and homocystinuria presenting as hemolytic uremic syndrome. CEN Case Rep 7, 73–76 (2018). https://doi.org/10.1007/s13730-017-0298-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13730-017-0298-6