Abstract
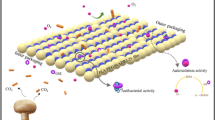
Biodegradable polymers are a suitable alternative for replacing traditional food packaging with environmental concerns. Poly(butylene adipate-co-terephthalate) (PBAT) is a synthetic biodegradable polymer with high flexibility and a high potential for functionalization, aiming at bactericidal, antimicrobial, or antifungal properties. In this work, the casting method was used to prepare PBAT films containing Moringa oleifera (MO) seed powder. The MO was added in different contents of 0, 1, 3, 5, and 10% (wt) to functionalize the film properties and its efficiency as strawberry packaging. The PBAT films containing 1% (wt) of MO were the most suitable for packaging applications, considering the mechanical properties and thermal properties. MO did not present significant interactions with the PBAT matrix and reduced the PBAT crystallinity, leading to a decrease in gas and water vapor permeabilities. The PBAT-1% MO films showed good performance as biodegradable packaging for strawberry storage, prolonging their storage time and reducing their vulnerability to fungal attack. Besides, the MO decreased the fungal contamination of strawberries when compared to those stored in neat PBAT. The developed films performance suggests that the PBAT/MO films have good potential to be used as active food packaging.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Packaging plays a fundamental role in the storage, transportation, and maintenance of food quality. Fresh foods, such as fruits and vegetables, are known for their short shelf life, resulting in high losses in relatively short times (~ 10 days) [1]. Among the primary packaging used by the food industry, commodity polymers are mainly used, with a production of 360 million tons worldwide every year [2]. The basic polymers used for packaging are polyethylene, polypropylene, polyvinyl chloride, polystyrene, and polyethylene terephthalate. Despite being low-density and low-cost materials, these polymers present significant ecological issues for their disposal due to their high degradation time [3]. As an alternative to the top plastic generation with environmental problems in the packaging sector, biodegradable polymers have proven to be good alternatives in replacing the materials currently used [4, 5].
Biodegradable polymers have been studied as food packaging and coatings, as they have good mechanical and thermal properties and are non-toxic. As viable choices to produce the films, poly(butylene adipate-co-terephthalate) (PBAT) is a synthetic biodegradable polymer with high flexibility and a high potential for application in food packaging, as cited in the literature [6]. Due to differences in chemical structure, crystallinity, and barrier properties, in general, biodegradable films are functionalized, aiming to add function and improve performance [7, 8].
Several active agents are added among active or functional packaging materials, aiming at bactericidal, antimicrobial, or antifungal properties [1]. Among the primary materials used, TiO2, Ag, and Au nanoparticles are the main antimicrobial agents. However, various natural fibers have been used to prepare PBAT compounds, such as manga wastes, essential oregano oil, and natural fibers from the Amazon rainforest [9,10,11]. Moringa oleifera seed powder has stood out in recent years [12,13,14]. This material contains high-quality protein (~ 52%) and essential amino acids, which confers antimicrobial properties. Also, the seeds are rich in oils and glycosylates [14, 15]. The bactericidal effects of these proteins, contained in the powder, were confirmed for several microorganisms [16,17,18,19].
In this work, the PBAT/M. oleifera (MO) seed powder composites with different MO contents were prepared by film extension (casting) method for application in food packing for strawberry. Morphology, chemical composition, thermal stability, and crystallinity evaluated the MO properties. Various techniques characterized the films, including mechanical tests, SEM, FT-Raman, TGA, DSC, XRD, oxygen and water vapor permeability, and their strawberry application performance. The overarching aims of this research were twofold: (i) examine the effect of MO content on PBAT/Moringa composites, and (ii) to evaluate the performance of the developed composites as food packaging.
Experimental
Materials
Poly (butylene adipate-co-terephthalate) (PBAT) (Mw 66,500 g/mol) was purchased from BASF (São Paulo, Brazil) under the trade name Ecoflex. M. oleifera seeds were purchased from a local producer (Casa Nova-Bahia, Brazil). Chloroform (1.48 g/cm3 density at 25 °C) was purchased from Synth (Diadema-SP, Brazil).
Preparation of M. oleifera powder
The seeds were dried for 24 h at 60 °C. The dried seeds were peeled and reduced to powder using a home-made processor (Mondial Power 21,500 W) for 10 min. A magnetic sieve separated the resulting powder for 10 min, and the opening, selected for separation of the powder size, was mesh #200 (75 μm). The sample was named MO.
Preparation of composite films by extension method
PBAT was first dried at 50 °C for 24 h before use. Then, 20 g of PBAT was dissolved in 100 mL of chloroform with constant magnetic stirring. The polymeric solution was then applied to a glass plate (13 × 18 cm) and extended in film form using a wire extender TKB Erichsen (200 µm). The plates were air-dried for 1 min, in order to partial evaporation of the solvent (flash-off). The plates were then immersed in demineralized water, in polyethylene trays of 24 × 32 cm (internal dimensions) to perform a coagulation bath. Films were detached from the glass plate’s surface (peel-off effect) and removed from the solution to dry. For composites, MO powder was incorporated after the solubilization of PBAT in different contents of 1, 3, 5, and 10% (wt). Figure 1 shows the methodology schematically.
Characterization
Optical microscopy and scanning electron microscopy
The MO power was evaluated by optical microscopy (ZEISS microscope, model AXIO Scope A1), with 50-fold magnification. A granulometric analysis measured the particles’ sizes. An MEV FEI Quanta 250 scanning electron microscope examined the Moringa powder’s morphological characteristics and the developed films. The samples were previously covered with gold by the sputtering technique (Leica ACE200) with a 20 nm gold deposition on the samples’ surface.
Fourier transform infrared spectroscopy (FTIR)
An equipment Frontier 94,942 (PerkinElmer, USA) collected the FTIR data, in the range of 400–4000 cm−1, with 64 scans and a 4 cm−1 resolution, equipped with an attenuated total reflectance (ATR) accessory with diamond. Origin 8 software processed the data.
Fourier transform Raman spectroscopy (FT-Raman)
The samples were analyzed using an FT-Raman equipment (MultiRaman, Bruker Optics) equipped with a 1064 nm wavelength and a 150 W power laser. The data acquisition parameters were: 600–4000 cm−1, 32 scans, and 4 cm−1 spectral resolution.
X-ray diffraction (XRD)
A D8 Focus diffractometer (Bruker AXS-Karlsruhe, Germany) collected the data using monochromatic CuKα1 radiation λ = 1.54056 Å, operating at 40 kV and 40 mA, with a step width of 0.015° and counting time of 100 s at each 0.5º, from 2θ of 10º–50º. The crystallinity index was calculated by Eq. (1) [20]:
I200 is the peak of highest intensity (2θ ~ 22.5), and Iam is the peak lowest intensity (2θ ~ 18).
Thermogravimetric analysis (TGA)
The thermogravimetric analysis was conducted on an STA 6000 equipment (PerkinElmer, USA), using alumina pans. The samples were heated from 30 to 600 °C at a rate of 10 °C/min under nitrogen atmosphere with a flow rate of 20 mL/min.
Mechanical tests
The tensile tests were investigated by an equipment Instron 3367 (Norwood, USA) at room temperature by the standard ASTM D638-14, with a load cell of 50 N and a test speed of 50 mm/min. The compositions were tested in quintuplicate.
Differential scanning calorimetry (DSC)
A DSC Q-series (TA Instruments) (New Castle, Delaware, USA) collected the DSC data under the nitrogen atmosphere (50 mm/min). The samples were heated to 190 °C, cooled to − 60 °C and, then heated to 200 °C. Equation (2) describes the calculation of the degree of crystallinity (Xc), where ΔHm is the experimental fusion enthalpy, and ΔH100% is the theoretical enthalpy of PBAT (114 J/g).
Oxygen and water vapor permeability
The oxygen transmission rate (O2TR) was determined according to the ASTM D 3985-17 at 23 ± 0.5 °C in a coulometric sensor equipment (Mocon, Oxtran model ST, IL, USA). The film outer side was placed in contact with the permanent gas (100% O2). The readings were corrected to 1 atm of partial pressure gradient of permanent gas and oxygen partial pressure gradient between the two surfaces of film. This gradient corresponds to the driving force for oxygen permeation through the film.
Once O2TR was determined, the oxygen permeability coefficient was calculated by Eq. (3):
where, O2P is the oxygen permeability coefficient (mL (CNTP) mm m−2 day−1 atm−1), O2TR is the oxygen transmission rate (mL (CNTP) m−2 day−1), L is the mean film thickness (µm), and P is the oxygen partial pressure in the gas chamber permeating the diffusion cell (1 atm). The partial pressure of O2 in the carrier gas chamber (N2 + H2) is considered null.
The water vapor transmission rate (WVTR) followed the ASTM E 96/E 96M-05 methods at 25 ± 0.5 ºC, and anhydrous calcium chloride (0% RH) filled the cells. The cells were covered with the films, sealed, and placed in a climatic chamber (Votsch, VC 0063 model, German) at 75 ± 2% RH. Once the WVTR was determined, Eq. (4) determined the water vapor permeability coefficient.
where, WVP is the water vapor permeability coefficient (g water mm−2 day−1 mmHg−1), WVTR is the water vapor transmission rate through a film (g water m−2 day−1), L is the mean film thickness (µm), PWVS is the partial water vapor saturation pressure at the test temperature (mmHg). RH1 is the chamber relative humidity (0.75), and RH2 is the relative humidity inside the capsule with desiccant (0% RH).
Films performance as packaging for strawberries
Strawberries were purchased from a local farm (Santo André-SP, Brazil) and immediately treated with sodium hypochlorite (250 ppm), and then dried at room temperature. Fruits of uniform size and free of fungal actions or physical damage were selected. The washed fruits were put in PBAT films and their composites and sealed by a manual plastic packaging sealer at a temperature of 120 °C (TECNAL STN, São Paulo, Brazil). Low density polyethylene (LDPE) and polyvinyl chloride (PVC) films were used to compare commercial materials with the new formulation [21, 22]. A refrigerator at 4 °C stored the samples. Photographs were taken from the fruits at the beginning of the experiment, and after 3, 7, 10, and 15 days of storage [23].
Results and discussion
M. oleifera seed powder characterization
Granulometry and morphological analysis
The optical microscopy evaluated the morphology and regularity of the power, as shown in Fig. 2a. The particles were analyzed by the Image J software to measure the particle diameter, and the average diameter was 40.9 ± 9.7 µm. Figures 2b, c show the scanning electron micrographs of MO power. This material is heterogeneous and has a relatively porous matrix [24]. This porosity, according to Shirani et al. contributes to the efficiency of this biopolymer in removing bacteria and fungi [25].
a Optical microscopy image of the Moringa oleífera seed power. b, c Scanning electron microscopy with different zooms of the MO sample. d FTIR spectrum zoom between 1800 and 800 cm−1. e FT-Raman spectrum of MO with inserts from the 2950–2800 cm−1 and 1700–800 cm−1 regions. f XRD. g TGA of M. oleifera powder
FTIR and FT-Raman
Infrared spectroscopy investigated the MO powder structure in the dried state. The typical spectrum (Fig. 1S, Supplementary Material) showed peaks between 4000 and 1000 cm−1. The peak in the region of 3500–3200 cm−1 indicates alcohol or phenol, a functional group predominant in the structures of proteins and fatty acids present in M. oleifera seeds [26]. The peak below 3400 cm−1 indicates NH elongation, and intense peaks at 3398 cm−1 are associated with –OH elongation [27]. Bands between 2800 and 2900 cm−1 are associated with the extent of CH vibration, and their high intensity is also associated with the presence of long carbon chain compounds, that is, the methylene groups in lipid and cellulose molecules [28]. The presence of bands between 1800 cm−1 and 1500 cm−1, observed in Fig. 2d, is related to proteins and amino acids. The peak at 1748 cm−1 represents carboxylic groups. The band between 1669 and 1527 cm−1 provides the stretching vibration added to the carbonyl group (C=O) [29].
M. oleifera active groups are mainly proteins. Under suitable conditions, these proteins can be extracted from the seed to obtain a concentrated extract, or they can also be obtained from the seed in nature through the exudation process, which occurs naturally. According to Kwaambwa and Maikokera, the organic groups of MO protein are characteristic of amide I and II [30]. The spectra showed two strong absorption bands of 1658 and 1544 cm−1 of amides I (protein α-coil helix) and II, respectively, which confirmed the structure of the protein present in the Moringa seeds [31, 32].
Flexion vibrations between 1449 and 1338 cm−1 represented O–H and C–N (from aromatic amines) [29]. The peaks at 1230, 1150, and 1050 cm−1 represented acetyl groups present in the lignin structure, and C–O and C=O elongation of ether, ester, and phenol bonds, respectively [33].
Raman spectroscopy was used as a complementary technique to FTIR to analyze the structure and functional groups of the ground Moringa seed (Fig. 2e). The peaks 2930, 2910, and 2850 cm−1 were assigned to =CH2 stretch. The peaks of 1650 and 1590 cm−1 corresponded to amide groups I and II, respectively [34]. The peaks at 1450 and 1300 cm−1 were associated with C–NH vibrations of tertiary amides [35], while at 1060 and 852 cm−1 they were associated with C–C=CH2 stretches and the CCO– vibration, respectively.
X-ray diffraction (XRD)
Figure 2f shows the diffractograms of MO powder. The seeds are composed of a high amount of oil, protein, and a cellulose fraction. The characteristic XRD peak at 22.5° is associated with the plane (002), and the amorphous region was verified at 12.5° [34]. Segal equation analyzed the crystallinity index (CI), and a CI value of 56% was obtained, which corroborates the literature [28].
Thermogravimetric analysis (TGA)
Figure 2g shows the MO thermogram. The sample has three main events of weight loss: the first occurs between 30 and 130 °C and is associated with loss of water, volatiles, and low molar mass components. The second is associated with the loss of gases, such as CO2 and NH3, resulting from the protein amine groups [34]. The third event occurs from 300 to 428 °C, which corresponds to the MO powder [36, 37].
Films characterization
Mechanical tests
Mechanical properties are essential for food packaging since good flexibility and mechanical resistance are required to protect the food during handling, transport, and storage. Table 1 shows the mechanical properties of films. The PBAT showed stiffness values of approximately 160 MPa and ε of ~ 125%; these values are consistent with those reported in the literature [38, 39]. The MO addition decreased the E, σ, and ε associated with the weak stress transformation across interphase [40]. However, there is another possibility to explain the mechanical resistance: the composite’s preparation method. During the phase inversion process, the polymer was submerged in water, which induced the formation of pores in the films due to MO’s high hydrophilicity, reducing the final material [39, 41, 42].
According to conventional standards, σ for packaging film must be > 3.5 MPa [43]. Thus, PBAT-1% MO is most suitable for such an application. The other compositions showed a significant decrease in mechanical properties, associated with MO’s high content, leading to possible pores appearance.
Scanning electron microscopy
SEM images reveal information about the morphology of composites and filler dispersion. The neat PBAT showed a smooth and homogeneous surface, as presented in Fig. 3a. After the addition of MO, a porous and highly rough surface was formed. The indicative arrows in the photomicrographs indicate voids formed by the absence of a reinforcement phase. According to Moustafa et al. this behavior can indicate M. oleifera particles [44]. Despite the matrix being porous, Wu et al. stated that variations in oxygen or water vapor permeation occur in active packaging and positively influence food storage [45].
Thermal properties
Figure 3b shows the TGA curves and their derivative thermogravimetric (DTG) curves of all the composites. PBAT showed a T10% of 377.7 °C and Tmax of 402.6 °C, with a single weight-loss event. The values found are similar to those reported in literature [46]. The PBAT thermal degradation is associated with the C–C bonds cleavage present in the polymer structure [47]. After the addition of MO, no separate degradation stage occurred, which indicates that M. oleifera has been entirely covered by PBAT molecules [48].
Table 2 summarizes the DSC results, and Fig. 2S (Supplementary Material) shows the second heating and cooling curves. The PBAT-MO samples have higher Tg values, and PBAT-1% MO presents the high values attributed to the uniform dispersion of MO, and probably this sample shows the best adhesion/cover between PBAT and MO [13]. The M. oleifera restricts the mobility of polymer segments and increases the glass transition temperatures. No significant differences were observed in the melting temperature [11].
The Tc values significantly increased after the MO addition, which means that the MO facilitates PBAT crystallization, acting as a nucleating agent [49]. The slight changes observed in the Xc values are associated with a possible change in polymer crystallization kinetics. Smaller amounts of MO do not affect crystallization, while larger amounts limit the crystallite growth, generating a smaller size crystalline phase, reflecting the decrease in Xc values.
X-ray diffraction
XRD was used to examine the crystallinity of PBAT and the PBAT/MO samples (Fig. 3c). PBAT exhibits five characteristics diffraction peaks at 16.2°, 17.3°, 20.4°, 23.4°, and 25.2°, associated with the planes (011), (010), (110), (100), and (111) [50, 51]. The PBAT diffraction pattern remained unchanged after the MO addition. No transcrystalline phases were created in the system interface [50]. Small shifts were observed for the PBAT structure, emphasizing the 20.4° angle, which shifted to ~ 20.0°. This small variation may indicate a change in the formation of PBAT crystallites. However, a new broad peak at 12° was detected, which can be associated to the MO XRD pattern, confirming MO incorporation in the composites. Table 3 shows the crystallinity values obtained by XRD patterns.
The crystallinity differences observed between the XRD and the DSC can be associated with the test methodology. While the DSC occurs through the polymer melting and cooling, the XRD is performed on the prepared films. Since in the present study, the films were prepared by wire extension, the XRD data is closer to the real crystalline structure found in the developed films.
Oxygen permeability and water vapor permeability
The addition of MO (Table 2) greatly influenced the O2P and WVP values. PBAT is a polymer with high O2P and WVP values due to its chemical structure and physical properties, such as low crystallinity [52, 53]. The addition of M. oleifera decreased the films oxygen barrier properties, which can be attributed to the films lower crystallinity, i.e., the MO reduced the films abilities to act as oxygen barriers [54]. The oxygen permeabilities increased rapidly with the powder Moringa content when the latter is over 3% (wt), resulting in blend films with weaker oxygen barrier properties [55]. The O2P is highly dependent on film structure, thickness, void volume, and arrangement of the polymer chain [56]. As previously described, the addition of Moringa resulted in low interactions between the PBAT matrix and the MO powder. Thus, the presence of pores changed the PBAT chain arrangement. Despite the increase in O2P values, it is important to note that the Moringa seed has known active properties. Thus, with the balance between the OM functionality and the permeability, the developed packaging, mainly the films PBAT-1% MO, is suitable for certain foods, such as strawberries.
Table 2 also shows the WVP values of PBAT films without and with MO. The addition of MO powder increased the WVP values, which the interaction between PBAT and powder Moringa can decrease the water vapor barrier properties. The MO is a hydrophilic material that favors transfer of water molecules through the film [57]. Besides, the lower crystallinity of film favors the diffusion of water molecules since the polymer chains are less densely packed due to M. oleifera powder. The PBAT and PBAT-1% MO films showed very similar water vapor permeability coefficients. Furthermore, increasing the powder content increases the WVPs of the films [55].
Films performance as packaging for strawberries
The strawberries are highly susceptible to rapid loss of water owing to respiration and transpiration. Commonly, weight loss occurs during the fruit storage because of its respiratory process, humidity transfer, and oxidation [58]. Figure 4 shows strawberries before packaging after the last test day (day 30). In Fig. 4, it is also possible to observe mass loss suffered by the fruits after 30 days. Figure 3S (Supplementary Material) shows the packaged strawberries and the visual changes observed after 0, 3, 7, 10, and 15 days.
The weight loss harms appearance of strawberry fruits, leading to shrinkage and opaque-looking skin. Fruit storage is an important parameter that controls and slows deterioration [59].
The samples that showed less weight loss were PVC (25.2%) and PBAT-1% MO (53.7%). According to the literature, PVC film used as a control sample has low permeability rates to oxygen and water vapor, which justifies its small mass loss and the absence of fungi visible to the naked eye. Besides, the strawberry did not lose the water, which maintained its weight [60].
Considering the PBAT samples without and with MO, neat PBAT was the one that presented the most significant loss of fruit mass after 30 days of storage. Also, films with 3, 5, and 10% MO showed the highest amounts of visible fungi, while the sample with 1% MO was the one with the least damaged and contaminated strawberries. It can be associated with the agglomeration effect: while 1% showed good dispersion, the other contents may have resulted near of the particles to each other, which limited the release of the active agent. The presence of MO in small amounts can cause cell damage in mold and yeast, altering the synthesis of fungal enzymes [61, 62]. Thus, morphological changes can occur and cause structural changes and molecular disorganization in fungal cells [63] (Fig. 5). As observed in the FTIR analysis, the seeds have distinct groups that show protein in the MO powder. Exudation of Moringa proteins (i.e., active compound) from the powder surface to the films results in damage to microbial cell membranes, minimizing the fungal attack on strawberries.
The biodegradable films effectively delay senescence, making the fruits more vulnerable to pathogenic infection due to loss of cellular or tissue integrity. Therefore, PBAT-1% MO films were useful as biodegradable packaging for storing sensitive fruits, such as strawberries, by prolonging their storage time and reducing their vulnerability to fungal attack. The packaging is biodegradable and is a potential substitute for the commodities currently used, and its performance is comparable to the performance of PE packaging.
Conclusion
PBAT/MO films were successfully prepared using M. oleifera (MO) seeds in the powder form. Samples containing 1% MO showed the best results in their mechanical properties. The maximum stress values correspond to the values of international standards required for packaging applications (above 3.5 MPa). The films showed good thermal stability, only slightly decreased for samples with higher MO content. The PBAT/MO samples showed higher Tg values since the MO particles restricted mobility of polymer segments. No changes were observed on Tm values associated with the absence of chemical interaction between the MO and the PBAT. These results were confirmed by FT-Raman analysis. However, the absence of interactions can be beneficial, considering MO available active sites for subsequent interaction with pathogenic microorganisms. The reinforcement addition reduced the crystallinity of the films, changing the structural organization of PBAT polymer chains. The lower crystallinity reflected in the oxygen and water vapor permeability and the films containing M. oleifera showed high permeability, which increased with MO content. Possibly, due to permeability changes, the films containing MO allowed the strawberries to breathe more efficiently, which delayed their deterioration. Only the 1% MO sample showed consistent results for use in packaging. PBAT-1% MO films showed good performance as packaging for strawberry storage, prolonging their storage time, and reducing their vulnerability to fungal attack.
References
Abral H, Ariksa J, Mahardika M, Handayani D, Aminah I, Sandrawati N (2020) Transparent and antimicrobial cellulose film from ginger nanofiber. Food Hydrocoll 98:105266
Facts P (2019) An analysis of Europeanplastics production, demand and waste data. https://www.plasticseurope.org/application/files/9715/7129/9584/FINAL_web_version_Plastics_the_facts2019_14102019.pdf
Akshaykranth A, Rao TV, Kumar RR (2020) Growth of ZnO nanorods on biodegradable poly (lactic acid) (PLA) substrates by low temperature solution method. Mater Lett 259:126807
Ilyas RA, Sapuan SM, Sanyang ML, Ishak MR, Zainudin ES (2018) Nanocrystalline cellulose as reinforcement for polymeric matrix nanocomposites and its potential applications: a review. Curr Anal Chem 14:203–225
Mangaraj S, Yadav A, Bal LM, Dash SK, Mahanti NK (2019) Application of biodegradable polymers in food packaging industry: a comprehensive review. J Packag Technol Res 3:77–96
Saibuatrong W, Cheroennet N, Suwanmanee U (2017) Life cycle assessment focusing on the waste management of conventional and bio-based garbage bags. J Clean Prod 158:319–334
Hasheminejad K, Montazeri A (2020) Enhanced interfacial characteristics in PLA/graphene composites through numerically-designed interface treatment. Appl Surf Sci 502:144150
Chiarathanakrit C, Riyajan SA, Kaewtatip K (2018) Transforming fish scale waste into an efficient filler for starch foam. Carbohydr Polym 188:48–53
Nawab A, Alam F, Hasnain A (2017) Mango kernel starch as a novel edible coating for enhancing shelf- life of tomato (Solanum lycopersicum) fruit. Int J Biol Macromol 103:581–586
Cardoso LG, Santos JC, Camilloto GP, Miranda AL, Druzian JI, Guimarães AG (2017) Development of active films poly (butylene adipate-co-terephthalate)-PBAT incorporated with oregano essential oil and application in fish fillet preservation. Ind Crops Prod 108:388–397
Ferreira FV, Pinheiro IF, Mariano M, Cividanes LS, Costa JC, Nascimento NR, Kimura SP, Neto JC, Lona LM (2019) Environmentally friendly polymer composites based on PBAT reinforced with natural fibers from the amazon forest. Polym Compos 40:3351–3360
Takassugui KS, Arantes CC, de Jesus TA (2019) Comparação da remoção de turbidez e cor aparente em filtros lentos de areia com e sem adição de proteína imobilizada de Moringa oleifera. Rev Bras Ciência Tecnol e Inovação 4:169
de Nunes EC, de Souza AG, dos Rosa DS (2019) Use of a chain extender as a dispersing agent of the CaCO3 into PBAT matrix. J Compos Mater 54:1373–1382
Jain A, Subramanian R, Manohar B, Radha C (2019) Preparation, characterization and functional properties of Moringa oleifera seed protein isolate. J Food Sci Technol 56:2093–2104
Chen R, Wang XJ, Zhang YY, Xing Y, Yang L, Ni H, Li HH (2019) Simultaneous extraction and separation of oil, proteins, and glucosinolates from Moringa oleifera seeds. Food Chem 300:125162
Landázuri AC, Villarreal JS, Núñez ER, Pico MM, Lagos AS, Caviedes M, Espinosa E (2018) Experimental evaluation of crushed Moringa oleifera Lam. seeds and powder waste during coagulation–flocculation processes. J Environ Chem Eng 6:5443–5451
Fernandes DM, Sousa RM, de Oliveira A, Morais SA, Richter EM, Muñoz RA (2015) Moringa oleifera: a potential source for production of biodiesel and antioxidant additives. Fuel 146:75–80
Gopalakrishnan L, Doriya K, Kumar DS (2016) Moringa oleifera: a review on nutritive importance and its medicinal application. Food Sci Hum Wellness 5:49–56
Dezfooli SM, Uversky VN, Saleem M, Baharudin FS, Hitam SM, Bachmann RT (2016) A simplified method for the purification of an intrinsically disordered coagulant protein from defatted Moringa oleifera seeds. Process Biochem 51:1085–1091
Segal L, Creely JJ, Martin AE, Conrad CM (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text Res J 29:786–794
Ritchie DA (2012) Ampere using single electron pumps. Nat Commun 3:930–936
Mali S, Grossmann MVE (2003) Effects of yam starch films on storability and quality of fresh strawberries (Fragaria ananassa). J Agric Food Chem 51:7005–7011
Garcia PS, Grossmann MV, Shirai MA, Lazaretti MM, Yamashita F, Muller CM, Mali S (2014) Improving action of citric acid as compatibiliser in starch/polyester blown films. Ind Crops Prod 52:305–312
Araújo CS, Alves VN, Rezende HC, Almeida IL, De Assuncao R, Tarley CR, Segatelli MG, Coelho NM (2010) Characterization and use of Moringa oleifera seeds as biosorbent for removing metal ions from aqueous effluents. Water Sci Technol 62:2198–2203
Shirani Z, Santhosh C, Iqbal J, Bhatnagar A (2018) Waste Moringa oleifera seed pods as green sorbent for efficient removal of toxic aquatic pollutants. J Environ Manage 227:95–106
Matinise N, Fuku XG, Kaviyarasu K, Mayedwa N, Maaza M (2017) ZnO nanoparticles via Moringa oleifera green synthesis: physical properties and & mechanism of formation. Appl Surf Sci 406:339–347
Saran M, Vyas S, Mathur M, Bagaria A (2018) Green synthesis and characterisation of CuNPs: insights into their potential bioactivity. IET Nanobiotechnol 12:357–364
Nayak S, Kumar Khuntia S (2019) Development and study of properties of Moringa oleifera fruit fibers/polyethylene terephthalate composites for packaging applications. Compos Commun 15:113–119
Madubuonu N, Aisida SO, Ali A, Ahmad I, Zhao TK, Botha S, Maaza M, Ezema FI (2019) Biosynthesis of iron oxide nanoparticles via a composite of Psidium guavaja-Moringa oleifera and their antibacterial and photocatalytic study. J Photochem Photobiol B Biol 199:111601
Kwaambwa HM, Maikokera R (2008) Infrared and circular dichroism spectroscopic characterisation of secondary structure components of a water treatment coagulant protein extracted from Moringa oleifera seeds. Colloids Surf B Biointerfaces 64:118–125
Swelam A, Saied S, Hafez A (2019) Removal comparative study for Cd(II) ions from polluted solutions by adsorption and coagulation techniques using Moringa oleifera seeds. Egypt J Chem 62:1499–1517
Prasuna CPL, Chakradhar RPS, Rao JL, Gopal NO (2009) EPR and IR spectral investigations on some leafy vegetables of Indian origin. Spectrochim Acta A Mol Biomol Spect 74:140–147
Bello OS, Adegoke KA, Akinyunni OO (2017) Preparation and characterization of a novel adsorbent from Moringa oleifera leaf. Appl Water Sci 7:1295–1305
Kebede TG, Dube S, Nindi MM (2018) Fabrication and characterization of electrospun nanofibers from Moringa stenopetala seed protein. Mater Res Express 5:125015
Kebede TG, Dube S, Nindi MM (2019) Characterisation of water-soluble protein powder and optimisation of process parameters for the removal of sulphonamides from wastewater. Environ Sci Pollut Res 26:21450–21462
Bhutada PR, Jadhav AJ, Pinjari DV, Nemade PR, Jain RD (2016) Solvent assisted extraction of oil from Moringa oleifera Lam seeds. Ind Crops Prod 82:74–80
Finzi-Quintão CM, Novack KM, Bernardes-Silva AC, Silva TD, Moreira LE, Braga LE (2018) Influence of Moringa oleifera derivates in blends of PBAT/PLA with LDPE. Polímeros 28:309–318
Ferreira FV, Dufresne A, Pinheiro IF, Souza DH, Gouveia RF, Mei LH, Lona LM (2018) How do cellulose nanocrystals affect the overall properties of biodegradable polymer nanocomposites: a comprehensive review. Eur Polym J 108:274–285
Leal IL, da Silva Rosa YC, da Silva PJ, Cruz Correia PR, da Silva MP, Guimarães DH, Barbosa JD, Druzian JI, Machado BA (2019) Development and application starch films: PBAT with additives for evaluating the shelf life of Tommy Atkins mango in the fresh-cut state. J Appl Polym Sci 136:48150
Dou Q, Cai J (2016) Investigation on polylactide (PLA)/poly(butylene adipate-co-terephthalate) (PBAT)/bark flour of plane tree (PF) eco-composites. Materials 9:393
He H, Liu B, Xue B, Zhang H (2019) Study on structure and properties of biodegradable PLA/PBAT/organic-modified MMT nanocomposites. J Thermoplas Compos Mater 6:0892705719890907
Pinheiro IF, Ferreira FV, Souza DH, Gouveia RF, Lona LM, Morales AR, Mei LH (2017) Mechanical, rheological and degradation properties of PBAT nanocomposites reinforced by functionalized cellulose nanocrystals. Eur Polym J 97:356–365
Cheng M, Wang J, Zhang R, Kong R, Lu W, Wang X (2019) Characterization and application of the microencapsulated carvacrol/sodium alginate films as food packaging materials. Int J Biol Macromol 141:259–267
Moustafa H, Guizani C, Dupont C, Martin V, Jeguirim M, Dufresne A (2017) Utilization of torrefied coffee grounds as reinforcing agent to produce high-quality biodegradable PBAT composites for food packaging applications. ACS Sustain Chem Eng 5:1906–1916
Wu CS (2012) Utilization of peanut husks as a filler in aliphatic-aromatic polyesters: preparation, characterization, and biodegradability. Polym Degrad Stab 97:2388–2395
Rahimi SK, Aeinehvand R, Kim K, Otaigbe JU (2017) Structure and biocompatibility of bioabsorbable nanocomposites of aliphatic-aromatic copolyester and cellulose nanocrystals. Biomacromol 18:2179–2194
Buffa JM, Mondragon G, Corcuera MA, Eceiza A, Mucci V, Aranguren MI (2018) Physical and mechanical properties of a vegetable oil based nanocomposite. Eur Polym J 98:116–124
Zhang X, Ma P, Zhang Y (2016) Structure and properties of surface-acetylated cellulose nanocrystal/poly(butylene adipate-co-terephthalate) composites. Polym Bull 73:2073–2085
Moustafa H, Guizani C, Dufresne A (2017) Sustainable biodegradable coffee grounds filler and its effect on the hydrophobicity, mechanical and thermal properties of biodegradable PBAT composites. J Appl Polym Sci 134:1–11
Mukherjee T, Tobin MJ, Puskar L, Sani MA, Kao N, Gupta RK, Pannirselvam M, Quazi N, Bhattacharya S (2017) Chemically imaging the interaction of acetylated nanocrystalline cellulose (NCC) with a polylactic acid (PLA) polymer matrix. Cellulose 24:1717–1729
Meng D, Xie J, Waterhouse GI, Zhang K, Zhao Q, Wang S, Qiu S, Chen K, Li J, Ma C, Pan Y (2019) Biodegradable Poly(butylene adipate-co-terephthalate) composites reinforced with bio-based nanochitin: preparation, enhanced mechanical and thermal properties. J Appl Polym Sci 137:48485
Venkatesan R, Rajeswari N (2017) ZnO/PBAT nanocomposite films: investigation on the mechanical and biological activity for food packaging. Polym Adv Technol 28:20–27
Falcão GA, Vitorino MB, Almeida TG, Bardi MA, Carvalho LH, Canedo EL (2017) PBAT/organoclay composite films: preparation and properties. Polym Bull 74:4423–4436
Arfat YA, Ahmed J, Ejaz M, Mullah M (2018) Polylactide/graphene oxide nanosheets/clove essential oil composite films for potential food packaging applications. Int J Biol Macromol 107:194–203
Liu Y, Wang S, Lan W, Qin W (2017) Fabrication and testing of PVA/chitosan bilayer films for strawberry packaging. Coatings 7:109
Siripatrawan U, Vitchayakitti W (2016) Improving functional properties of chitosan films as active food packaging by incorporating with propolis. Food Hydrocoll 61:695–702
Liu Y, Wang S, Lan W (2018) Fabrication of antibacterial chitosan-PVA blended film using electrospray technique for food packaging applications. Int J Biol Macromol 107:848–854
da Silva IS, Neto WP, Silvério HA, Pasquini D, Zeni Andrade M, Otaguro H (2017) Mechanical, thermal and barrier properties of pectin/cellulose nanocrystal nanocomposite films and their effect on the storability of strawberries (Fragaria ananassa). Polym Adv Technol 28:1005–1012
Giuggioli NR, Girgenti V, Briano R, Peano C (2017) Sustainable supply-chain: evolution of the quality characteristics of strawberries stored in green film packaging. CYTA J Food 15:211–219
Zhong Y, Godwin P, Jin Y, Xiao H (2019) Biodegradable polymers and green-based antimicrobial packaging materials: a mini-review. Adv Ind Eng Polym Res 3:27–35
Maqsood M, Qureshi R, Arshad M, Ahmed MS, Ikram M (2017) Preliminary phytochemical screening, antifungal and cytotoxic activities of leaves extract of Moringa oleifera lam. From salt range, Pakistan. Pak J Bot 49:353–359
Jimoh WA, Ayeloja AA, Badmus GO, Olateju KO (2020) Antibacterial and antifungal effect of moringa (Moringa oleifera) seedmeal on marinated smoked African mud catfish (Clarias gariepinus). J Food Saf 4:e12772
Valenzuela C, Tapia C, López L, Bunger A, Escalona V, Abugoch L (2015) Effect of edible quinoa protein-chitosan based films on refrigerated strawberry (Fragaria × ananassa) quality. Electron J Biotechnol 18:406–411
Acknowledgements
The authors thank the financial support provided by CNPQ (305819/2017-8) and FAPESP (2018/11277-7), CNPq, CAPES (Code 001), Revalores Strategic Unit, and the Multiuser Experimental Center of the Federal University of ABC (CEM-UFABC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Availability of data and material
The authors are willing to provide the manuscript’s data, if necessary.
Code availability
Not applicable.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Verdi, A.G., de Souza, A.G., Rocha, D.B. et al. Biodegradable films functionalized with Moringa oleifera applied in food packaging. Iran Polym J 30, 235–246 (2021). https://doi.org/10.1007/s13726-020-00885-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-020-00885-7