Abstract
An intumescent flame retardant (IFR), a Schiff-base polyphosphate ester (PAB)-functionalized montmorillonite (PAB-MMT) was combined with PAB to adopt into ethylene–vinyl acetate copolymer (EVA) by melting intercalation. The synergistic effect between PAB-MMT and PAB was evaluated by thermogravimetric analysis (TGA), transmission electron microscopy (TEM), limiting oxygen index (LOI), vertical burning test (UL-94), microscale combustion colorimeter (MCC) and scanning electron microscopy (SEM). The results showed that when 5.0 wt% PAB-MMT replaced the same amounts of Na-MMT in the composite, the flame retardancy of EVA/PAB composite was improved. For this composite, the LOI value was increased and the ignition time in UL-94 rating was shortened compared to pure EVA or composites containing PAB or Na-MMT/PAB. The MCC results indicated that the peak heat release rate (PHRR) and total heat release (THR) were significantly reduced in comparison with other EVA nanocomposites. Meanwhile, the TGA data showed that the EVA/PAB/PAB-MMT nanocomposite had higher char residue than the EVA/PAB and EVA/PAB/Na-MMT nanocomposites. The TEM and dispersibility measurement results showed that PAB-MMT had better dispersion than Na-MMT. The SEM results demonstrated that the minimal loading levels of PAB-MMT in EVA/PAB/PAB-MMT composite had a well-structured and strong char which had better ability to endure heat erosion. A good synergistic effect between PAB-MMT and PAB was constructed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, polymers are versatile materials and are used massively in our everyday life due to their remarkable combination of properties. However, the lacking in flame retardancy is the common problem of polymers [1]. The incorporation of flame retardant additives has proved to be an effective way to reduce the flammability of polymers [2–4]. Intumescent flame retardant (IFR) materials have found a place in polymer science as a method of providing flame retardancy to polymeric materials [5]. However, IFRs also have drawbacks, for instance, low flame retardant efficiency and low thermal stability [6]. Recently, much attention has been devoted to the use of nanoparticles such as layered silicates, graphite oxide, layered double hydroxides and layered metal phosphates [7]. Among them, montmorillonite (MMT) is the most commonly used layered compound which improves polymeric materials’ flame retardancy, particularly in reducing peak heat release rate (PHRR) during burning when is evaluated by cone calorimetry [8]. The most popular accepted mechanism to explain the fire retardancy of polymer–clay nanocomposites is based on barrier effects [9]. MMT has been identified as a promising synergistic agent when combined with polymer intumescent systems [10, 11].
Natural MMT has usually been modified by organic intercalation agents to obtain the good affinity of MMT with organic polymers [12]. However, organic intercalation agents are combustible, resulting in negative effect on flame retardancy [13]. So the further improvement of the flame retardancy of composites is limited.
As to develop a functionalized MMT with high flame retardancy, it can be achieved by incorporating a flame retardant as a replacement for alky ammonium salts in functionalizing MMT [14, 15]. In our previous study [16, 17], we synthesized an IFR (PAB) and utilized it as an organophilic-modification agent to modify MMT (PAB-MMT). It was found that the PAB-MMT, having a larger interlayer spacing, could improve the flame retardancy of EVA nanocomposites.
To further improve the flame retardancy of EVA and to reduce the amount of IFR in polymer composites, the combination of the PAB-MMT and the flame retardants (PAB) was used. In this work their synergistic effect has been evaluated through transmission electron microscopy (TEM), thermogravimetric analysis (TGA), limited oxygen index (LOI), vertical burning test (UL-94) and microscale cone calorimeter test (MCC). Moreover, scanning electron microscope (SEM) was employed to characterize the morphology of the char of EVA/PAB/PAB-MMT composites.
Experimental
Materials
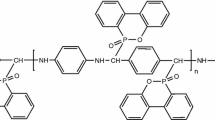
Elvax® 360 is an EVA copolymer containing 25 wt% of vinyl acetate with a melt flow index of 2 g/10 min, a density of 0.948 g/cm3 and was provided by DuPont, USA. Pristine Na-MMT, with a cation exchange capacity of 110 meq/100 g, was supplied by Zhejiang Fenghong Clay Products (Anji County, Zhejiang Province, China). PAB was self-made by interfacial polycondensation according to the procedures reported previously [16]. The chemical structure of PAB is shown in Scheme 1. PAB was reacted with Na-MMT to form the modified clay (PAB-MMT) [17]. All materials were dried in vacuum oven at 80 °C for 24 h before use.
Preparation and characterization of composites
The flame retardant EVA composites were prepared via melt compounding at 120 °C in Thermo Haake Rheomixer (Polylab, Germany) with a rotor speed of 60 rpm for 8 min. The formulations of EVA samples prepared are shown in Table 1. The samples were hot-pressed at 120 °C under 8 MPa for 8 min into sheets for testing.
TG analysis was performed on a TA STD Q600 thermal analyzer (USA) at a heating rate of 20 °C/min in N2. LOI values were determined using an HC-2 oxygen index instrument (China) according to ASTM D2863 with the sample sheets of dimension 150 × 6 × 3 mm3. UL-94 test was conducted in a CZF-Ш horizontal and vertical burning tester (Jiangning Analysis Instrument Company, China) according to UL-94 test ASTM D3801-1996 standard, on the sheet of 127 × 12.7 × 3 mm3. The TEM micrographs of the composites were obtained with a JEM-1200EX electron microscope (JEOL Corporation, Japan). MCC tests were carried out on a Govmak MCC-2 microscale combustion colorimeter (USA) with a heating rate of 1 °C s−1 in a stream of nitrogen flowing at 80 cm3 min−1. The volatile thermal degradation products in the nitrogen gas stream were mixed with a 20 cm3 min−1 stream of pure oxygen prior to entering a 1,000 °C combustion furnace. The SEM observations of the char residues were carried out on an S-4800 microscope (HITACHI, Japan) under an accelerating voltage of 3 kV.
Results and discussion
Morphology
To investigate the dispersion of clay mineral layers in the matrix, the TEM images of EVA/Na-MMT and EVA/20 wt% PAB-MMT composites were carried out. The TEM images are presented in Fig. 1. From Fig. 1a it could be found that Na-MMT was unexfoliated and agglomerated and the layers in the EVA/Na-MMT composite maintained stacking structure. While in the EVA/20 wt% PAB-MMT composite (Fig. 1b), the size of PAB-MMT particles was much smaller and the PAB-MMT particles were dispersed uniformly in the matrix. As denoted by the arrows, the exfoliated and intercalated PAB-MMT sheets could be observed.
Thermal stability
Figure 2 shows the TG and DTG curves of the EVA composites. The detailed TG and DTG data are listed in Table 2. The pure EVA resin began to decompose at 336 °C. When the temperature was further increased, the weight loss increased rapidly, without leaving any char residue at 600 °C in N2. Compared with pure EVA, the decomposition temperature of 5 % weight loss (T 5 %d) of EVA/20 wt% PAB was lower. This phenomenon was attributed to the decomposition of ethyl ester groups in PAB at relatively low temperature [18]. However, the EVA/20 wt% PAB sample could obtain much residual char about 6.6 %. Based on the results of Fig. 2 and Table 2, the residual char of EVA/PAB/PAB-MMT was 16 %, while the EVA/PAB/Na-MMT sample had a residual char of about 11 %. This indicated that the functionalized MMT and PAB had a synergistic effect on improving the thermal stability of EVA only at high temperature and the char forming ability on EVA resin. This phenomenon may attribute to the fact that PAB-MMT is intercalated by PAB which is an IFR and containing phosphorus [14]. It is well known that phosphorus is a promoter for “char-formation” [18] and it is clear that the decomposition of PAB could generate phosphinic acid and led to the formation of char residue which could protect the resin underlying substrates.
Flammability
The results of LOI values and UL-94 ratings of the samples are listed in Table 3. The LOI of the pure EVA was only 19.7 with dripping, which indicated the EVA was an easily flammable polymeric material. The addition of 20 wt% PAB increased the value of LOI to 23.0, while the EVA/PAB/Na-MMT sample had an LOI value of 23.4. Moreover, combining of PAB-MMT and PAB in EVA resin could increase the LOI value to the optimal result of about 25.0. V-2 rating in the UL-94 rating tests could be obtained for the EVA/IFR composites. However, the average flaming times after the first and the second ignition were different and were in accordance with the results of the LOI values. The results of LOI value and UL-94 rating indicated that the PAB-MMT had a better synergistic effect with PAB compared to Na-MMT. In the UL-94 test, though the EVA/PAB/PAB-MMT blend failed to prevent flame dripping, there was no dripping after the first application of the flame. This may attribute to the increased melt viscosity of the system due to the intercalated structure in the PAB-MMT. To inhibit the dripping behavior of EVA/PAB/PAB-MMT blend, 0.3 wt% PTFE (polytertrafluoroethylene) was added into the system. And the EVA/PAB/PAB-MMT/PTFE blend was found to reach UL-94 V-0 rating without dripping.
The combustibility of the blends was evaluated using microscale combustion calorimeter (MCC). The curves of heat release rates (HRR) and detailed MCC data are presented in Fig. 3 and Table 4. The peak heat release rate (PHRR) of pure EVA was 882 kW/m2, while the PHRR of EVA/PAB was 718.1 kW/m2, a reduction of about 18 %. Compared with the EVA/PAB blend, the PHRR of EVA/PAB/Na-MMT and EVA/PAB/PAB-MMT was further reduced, about a reduction of 1.6 and 8.2 %, comparatively. The total heat release (THR) of pure EVA was 41.2 kJ/g. The addition of PAB and Na-MMT in EVA material could also descend the value of THR to 35.4 kJ/g, which is a reduction of about 14 % compared to that of EVA. As to the EVA/PAB/PAB-MMT sample, the THR value was further decreased. The lower THR value meant that a part of the EVA sample had not combusted completely, indicating better flame retardancy.
Analysis of the char residue
The SEM images of the char after the combustion of the EVA composites are shown in Fig. 4. It can be seen from Fig. 4a that the blend without MMT shows swollen chars on the surface. The surface of EVA/PAB/Na-MMT (Fig. 4b) was more compact and denser than that of the EVA/PAB sample, which was very important in improving the flame retardancy of EVA as it could provide a barrier to protect the underlying resin effectively. As to the EVA/PAB/PAB-MMT sample, it showed more continuous and thick char layer and this was in accordance with the results of LOI values and UL-94 rating.
Conclusions
The PAB-functionalized montmorillonite (PAB-MMT) exhibited a synergistic effect with IFR (PAB) on the EVA composite compared with the EVA/PAB/Na-MMT sample, giving the best LOI value and UL-94 rating. The improvement of flame retardancy was mainly induced by the introduction of PAB-MMT into the EVA/PAB sample, which was attributed to the improvement of char forming ability and a denser char barrier.
References
Guo JB, He M, Li QF, Yu J, Qin SH (2013) Synergistic effect of organo-montmorillonite on intumescent flame retardant ethylene-octene copolymer. J Appl Polym Sci 129:2063–2069
Alwaan IM, Hassan A (2014) Effects of zinc borate loading on the thermal stability, flammability, crystallization properties of magnesium oxide/(90/10) mLLDPE/(NR/ENR-50) blends. Iran Polym J 23:277–287
Li L, Qian Y, Jiao CM (2012) Influence of red phosphorus on the flame-retardant properties of ethylene vinyl acetate/layered double hydroxides composites. Iran Polym J 21:557–568
Fuzail M, Shah G, Anwar J (2010) Modification of polyethylene and incorporation of Al(OH)3 for improvement of mechanical properties, burning behaviour and thermal stability. Iran Polym J 19:47–56
Liu H, Zhong Q, Kong QH, Zhang XG, Li YJ, Zhang JH (2014) Synergistic effect of organophilic Fe-montmorillonite on flammability in polypropylene/intumescent flame retardant system. J Therm Anal Calorim 117:693–699
Guo Q, Wei P, Wang C, Qian Y, Liu JP (2013) Study of the effect of organomodified montmorillonite on PP/APP/Si-E system. Polym Plast Technol Eng 52:273–279
Quadrini F, Santo L, Squeo EA (2012) Solid-state foaming of nano-clay-filled thermoset foams with shape memory properties. Polym Plast Technol Eng 51:560–567
Lu HD, Ma YQ, Wu HT, He H, Zhao DF (2013) Flammability performance and char behavior of poly (vinyl alcohol)–zirconium phosphate-montmorillonite composites. Polym Plast Technol Eng 52:827–832
Lecouvet B, Sclavons M, Bailly C, Bourbigot S (2013) A comprehensive study of the synergistic flame retardant mechanisms of halloysite in intumescent polypropylene. Polym Degrad Stab 98:2268–2281
Ren Q, Zhang Y, Li J, Li JC (2011) Synergistic effect of vermiculite on the intumescent flame retardance of polypropylene. J Appl Polym Sci 120:1225–1233
Du BX, Ma HY, Fang ZP (2011) How nano-fillers affect thermal stability and flame retardancy of intumescent flame retarded polypropylene. Polym Adv Technol 22:1139–1146
Zhu HF, Li J, Xu L, Tao K, Xue LX, Fan XY (2011) Synergistic effect between montmorillonite intercalated by melamine and intumescent flame retardant (IFR) on polypropylene. Adv Mater Res 295–297:315–318
Huang GB, Gao JR, Wang X (2012) Preparation and characterization of montmorillonite modified by phosphorus-nitrogen containing quaternary ammonium salts. Appl Surf Sci 258:4054–4062
Lai XJ, Zeng XR, Li HQ, Liao F, Yin CY, Zhang HL (2012) Synergistic effect of phosphorus-containing montmorillonite with intumescent flame retardant in polypropylene. J Macromol Sci B Phys 51:1186–1198
Huang GB, Guo HC, Yang JG, Wang X, Gao JR (2013) Effect of the phosphorus–nitrogen-containing quaternary ammonium salt structure on the flammability properties of poly (methyl methacrylate)/montmorillonite nanocomposites. Ind Eng Chem Res 52:4089–4097
Liu Y, Fang Z P (2014) The effect of a novel intumescent flame retardant-functionalized montmorillonite on the thermal stability and flammability of EVA. J Zhejiang Univ-Sc A, revised
Liu Y, Zhang Y, Cao ZH, Fang ZP (2013) Synthesis and performance of three flame retardant additives containing diethyl phosphite/phenyl phosphonic moieties. Fire Safety J 61:185–192
Liu Y, Zhang Y, Cao ZH, Fang ZP (2012) Synthesis of three novel intumescent flame retardants having azomethine linkages and their applications in EVA copolymer. Ind Eng Chem Res 51:11059–11065
Acknowledgments
Doctoral Program Foundation of East China Institute of Technology (NO. DHBK2013210) and Open Project Foundation of the Key Laboratory of Radioactive Geology and Exploration Technology Fundamental Science for National Defense, East China Institute of Technology, China (RGET1411) are acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Y. The synergistic effect of functionalized montmorillonite with intumescent flame retardant in EVA. Iran Polym J 24, 197–202 (2015). https://doi.org/10.1007/s13726-015-0312-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-015-0312-9