Abstract
In the present study, attempts were made to study the effect of a hybrid UV stabilizing system on high-density polyethylene (HDPE). For this purpose, high molecular weight PE was used to prepare samples containing different amounts of hindered amine light stabilizer (HALS), carbon black (CB) and HALS/CB systems as UV stabilizers. All samples were exposed to UV irradiation simulating 4 and 8 years of exposure to solar irradiation in central part of Iran (Yazd). FTIR results were used to estimate the carbonyl index (CI) of the samples. It showed that CI increased as UV exposure time increased. However, it was found out that in the samples having both stabilizers (HALS/CB), CI value was much less compared to other samples indicating that the presence of hybrid system would show a synergism effect on UV stabilization of HDPE. The gel content of all samples was measured and it confirmed the same results. The PECH sample (containing HALS and CB) showed the least gel content after equivalent time of 4 and 8 years of exposure (2 and 3.5 wt%, respectively) which was in accordance with CI result. Furthermore, the effect of different UV stabilizing systems on the mechanical properties of HDPE was studied. For this purpose, elastic modulus, elongation-at-break and yield stress of the samples were measured. It was found out that HALS/CB hybrid system preserved the mechanical properties of HDPE much better than the other systems, which was attributed to the synergistic effect of the simultaneous use of HALS and CB.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyolefins are the most used polymers with vast outdoor demands in urban and municipal, energy and industrial applications. Since there is a high probability of exposure to solar UV irradiation in outdoor uses, these materials should be stabilized against UV irradiation due to their vulnerability against this kind of radiation. UV irradiation can lead to degradation of polyethylene which generally initiates from the surface and continues by progression into the bulk of the material [1]. The mechanisms of photodegradation and stabilization of polyolefins have been studied comprehensively and it has been shown that their photodegradation in the presence of oxygen is an autocatalytic process [2–4]. Furthermore, different strategies of photostabilization of polymeric materials, particularly polyolefins, have been developed [5]. Rabello et al. [6] studied the effect of polymer chain structure on the photodegradation of polypropylene (PP). It was shown that increasing the crystallinity of PP, controlled by processing conditions, may notably increase its UV stability. Yang et al. [7] studied the effect of nano-ZnO, silica and alumina nano-particles on the photostability of linear low-density polyethylene (LLDPE). They showed that the presence of nano-ZnO induced a good UV stability while alumina nano-particles had the least effect. In addition to mineral micro- and nano-particles, organic compounds are also employed as UV stabilizing agents.
The influence of UV irradiation on the PE products such as PE pipes has received great attention and it is reported that UV radiation could cause the loss of antioxidant which results in lower oxidation induction time (OIT) [8]. The studies have demonstrated that the presence of some materials could hasten the photodegradation process of the polyethylene [8]. Eltayeb et al. [9, 10] showed that the presence of cobalt naphthenate would enhance the degradation of LDPE, while the presence of 2-hydroxy-4-methoxybenzophenone and 2-benzoylbenzoic acid decreased and retarded the photooxidation process. Carbon black (CB) has been recognized as an effective UV stabilizer for many years [11]. It has been a well-accepted fact that CB acts as a physical screen, UV absorber, a radical trapper and a terminator of the free radicals [11, 12]. Liu et al. [13] showed that the size of CB particles had a great impact on their performance as a UV stabilizer. They suggested that smaller particles could be more effective than larger sizes in improving the UV stability of LLDPE films. The hindered amine light stabilizers (HALS) are also used as UV stabilizers [14–16]. Since the introduction of different types of UV stabilizers including UV screens, absorbers and stabilizers, the synergism or antagonism of combined stabilizers has always been a challenge confronted by researchers. The combination of different HALS compounds was also studied, and it was shown that its behavior could not be predicted in a general manner [17]. Kurumada et al. [18] showed that the simultaneous use of HALS and an UV absorber such as calcium stearate or octadecyl 2-(3,5-di-tert-butyl-4-hydroxyphenyl) propionate exhibited synergistic effect on PP, ABS and HDPE. It was found that there was an optimum combination of HALS and UV absorber specific for each material in order to show its synergistic effect. Butola et al. [19] showed that the UV degradation of HDPE filaments could be greatly suppressed by using different types of the HALS and their mixtures. Although, the photodegradation behavior of polyolefins such as PE has been the subject of many research works, the effect of hybrid system of a quencher and a UV screener has not received much attention.
There are many research works on the hybrid systems of the same kind; however, the hybrid system of different stabilizers acting through different mechanisms has received less attention. In the present study, attempts were made to investigate the effect of simultaneous utilization of CB (a UV screener and absorber) and HALS (as a quencher) as a multifunctioning hybrid stabilization system on the UV stabilization of HDPE. The UV dosage and exposure time were chosen to reflect the real outdoor conditions for a harsh environment. Carbonyl index, gel content and mechanical properties of the samples were evaluated.
Experimental
Materials
A commercial grade of high-density polyethylene, HDPE, (TAISOX 8001, MFI = 0.24 g/10 min at 190 °C, 5 kg) were used as the base polymer. Furthermore, carbon black, CB (N330), supplied by Iran Carbon Co. and a HALS (Chimassorb 944) supplied by CIBA Co. were used as UV stabilizers.
Sample preparation
The samples containing UV stabilizer(s) were prepared in a batch internal mixer (Brabender, W50EHT) at 180 °C and with a rotor speed of 60 rpm. HDPE was loaded at the beginning of the mixing process and UV stabilizer system (CB, HALS or CB/HALS) was added after 2 min and the mixing was continued up to 10 min. For comparison, the neat HDPE sample was also processed under the same condition. The samples were compression-molded at 180 °C into the tensile test bar (ISO 527, type 2) using a hot press. The compositions and their corresponding codes for different prepared samples are listed in Table 1.
UV exposure condition
Test bars were exposed to UV irradiation in a box at 32 ± 1 °C equipped with a UV-A black-light blue fluorescent tube (Philips, 18 W) for 516.8 and 1,033.6 h, equivalents to sunlight exposure time of 4 and 8 years at temperate latitude, respectively (UV irradiation dosage data was used from Sabziparvar [20] for the city of Yazd, in the central region of Iran, latitude of 31°54′N and altitude of 1,230 m).
Characterization
In order to evaluate the carbonyl index of the specimens before and after exposure, ATR-FTIR spectroscopy was carried out using an FTIR instrument (Brucker, Tensor 27). The carbonyl index (CI) was calculated using Eq. (1):
The state of crosslinking due to degradation was evaluated using gel content measurement. The gel content of the samples was measured according to ASTM D2765-90: 300 ± 5 mg of the material (initial weight) was extracted in 400 mL of boiling xylene for 24 h. The remaining material (gel) was dried for 3 h at 140 °C in a vacuum oven and re-weighed. The gel content was calculated using Eq. (2):
Tensile test was conducted according to ISO 527 using bar-shaped (type 2) specimens on Zwick/Roell tensile testing machine (Z 1010), at room temperature with a fixed crosshead speed of 50 mm/min.
Results and discussion
Carbonyl index
The carbonyl indexes (CI) of the samples before and after exposure to UV irradiation are presented in Table 2 and the ATR spectra of the samples are shown in Fig. 1. The carbonyl index value was determined by the absorbance of the carbonyl group at 1,710–1,730 cm−1 normalized by the absorbance peak of the alkane C–H stretching at 2,915–2,925 cm−1 which was believed to be constant during photodegradation of HDPE. All the samples showed non-zero carbonyl index value before the exposure which could be related to the thermal and mechanical degradation of HDPE during the processing and/or storage in the presence of oxygen. Comparing the results presented in Table 2 showed that the carbonyl index of the samples was decreased as HALS content was increased up to 0.2 % at all exposure times. The lower value of the carbonyl index in the presence of HALS, observed for unexposed samples, could be due to the heat stabilizing effect of HALS [21]. It was observed that the addition of carbon black (CB) increased the carbonyl index for the unexposed samples (about twice the CI of PE) which was due to the presence of carbonyl groups in the structure of CB [22]. The addition of HALS along with CB lowered the carbonyl index in unexposed samples from 0.04768 (PEC) to 0.031 (PECH). As it was explained above, the heat stabilizing effect of HALS could be responsible for the observed result.
The value of the carbonyl index of the samples exposed for 500 and 1,000 h (equivalents to 4 and 8 years of UV exposure, respectively) are also presented in Table 2 and the percentage change in carbonyl index (with respect to the unexposed sample) is plotted in Fig. 2. It can be seen that the carbonyl index of the neat PE is increased by about 131 and 236 % for 500 and 1,000 h of exposure, respectively. Since no photostabilizer was used in PE sample, the increase of the carbonyl index due to photodegradation was inevitable. The addition of 0.05 wt% HALS (PE-0.05 samples) resulted in lower carbonyl index; from 0.0638 to 0.0286 and 0.0931 to 0.0324 for 500 and 1,000 h of exposure time in comparison to neat PE. Increasing the HALS content from 0.05 up to 0.1 wt% decreased CI by about 12.6 and 14.1 % for 500 and 1,000 h of exposures, respectively. It is worth noting that the increasing the HALS content not only reduced the CI, but it also decreased the rate of CI increment showing enhanced UV stability of the compound [18]. It was observed that increasing the HALS content from 0 to 0.2 wt% decreased the rate of CI increment from 0.032 to 0.002 (4 years−1). The obtained results for PEC showed that although its carbonyl index was increased, as stated above, the rate of CI increment was reduced dramatically and it was almost the same as that seen for PE-0.05. It can be concluded that the presence of about 1 wt% of CB produces the same performance of about the 0.05 wt% of HALS, but it is somehow better for longer exposure time (equivalent to 8 years) as the rate of CI increment showed a plateau for PEC after 500 h of exposure. Nevertheless, it must be noticed that CB and HALS act through completely different mechanisms in UV stabilization of the matrix. HALS stabilizes PE through reacting with free radicals but CB stabilizes the PE through acting as a UV screen, absorbing UV irradiation and preventing photodegradation of polyethylene [18]. When CB and HALS were used simultaneously (as PECH sample), it was observed that CI value decreased compared to PEC but it was still higher that the other samples, because the presence of CB increased the initial CI of the sample. But, from Fig. 2, it can be seen that the rate of CI increment was at the lowest level for PECH in comparison to the other samples. It can be concluded that the simultaneous use of HALS (Chimassorb 944) and CB (N330) exhibited a synergistic effect in the UV stabilization of HDPE. This could be explained in terms of coupling two different mechanisms of UV stabilization; UV screening (CB) and free radical deactivation (HALS).
Sol–gel analysis
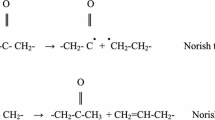
Since the degradation of polyethylene undergoes crosslinking, study on the gel content of the samples can provide useful information regarding its photostabilization. The gel content of the samples with different exposure times is shown in Fig. 3. As it is depicted in Fig. 3, the PE shows the highest gel content among all other samples, representing the greater extent of degradation, as expected. As it can be noticed, PE sample shows higher increase in gel content after the first 500 h of exposure (0–28.5 %) compared to the next 500 h (28.5–35.7 %). This may indicate that the rate of crosslinking during the first period of exposure was higher than that of the second exposure, for two reasons: first, the concentration of uncrosslinked chain in the adjacent layers of the surface was reduced, so that the increase of the crosslink density and hence the gel content would not be the same as the first period (first 500 h). Second, the chain scission of PE macromolecules might occur through Norrish (type I and II) reaction in the later stages of the exposure due to the presence of carbonyl groups, formed as the result of photodegradation of HDPE. This could suppress the formation of the gel which could lead to having a lower increment rate of the gel content in the second period in comparison to the first period.
The results showed that introducing 0.05 wt% of HALS would dramatically decrease the gel content by a factor of about 2.5 (Fig. 3). It could be observed that by increasing the HALS content up to 0.2 wt%, the gel content decreased by about 50 % compared to PEH-0.05 indicating the photostabilization effect of HALS on HDPE. The results demonstrate that introducing 1.5 wt% of CB also decreased the gel content of over 75 % compared to neat PE samples which shows the photostabilization effect of CB through UV screening. Comparing the results of the PECH with other samples, it was observed that the simultaneous presence of HALS and CB in the PE matrix resulted in lowest gel content among all other samples implying a strong synergistic effect. This finding is in agreement with the results obtained by the carbonyl index values of the samples. As discussed before, the presence of two different photostabilization mechanisms due to the presence of different stabilizers could be responsible for this effect.
Mechanical properties
The mechanical properties of the samples were studied and the elastic modulus (E), tensile strength (σ B) and elongation-at-break (ε B) of the samples are demonstrated in Figs. 4, 5, 6, respectively. The mechanical properties variations are summarized in Table 3. The results indicate that the elastic modulus of neat PE samples increased by 12.49 and 15.73 % for the exposure times of 500 and 1,000 h, respectively, which were the highest change rate in all samples. In the absence of UV stabilizers, crosslinking of the polyethylene chains through photodegradation increased the gel content which led to the changes in the elastic modulus of neat PE for different exposure periods. Introducing 0.05 wt% HALS reduced the changes in elastic modulus by a factor of 0.35, since it prevented the formation of crosslinked chains and reduced the gel content of the samples. Increasing HALS content intensified this effect by suppressing the increase in elastic modulus as expected. Addition of 1.5 wt% CB to a neat HDPE also reduced the elastic modulus variation from 12.49 to 6.57 % and 15.73 to 8.87 % for 500 and 1,000 h of exposures, respectively. Because the CB particles have acted as rigid particles, the elastic modulus was expected to have higher values as depicted in Fig. 4. Comparing the result of PECH with other samples showed that the change in its elastic modulus was at lowest level. Again, it emphasized that the simultaneous presence of CB and HALS indicates the synergistic effect on the UV stabilization of HDPE.
Figure 5 shows the change of stress-at-break (σ B) for the samples. One may notice that the samples containing CB exhibit a little higher σ B in comparison to the samples without CB; meanwhile, Fig. 6 depicts that ε B in PEC and PECH is much lower than other samples. This may imply that, the presence of CB would make the samples to lose their toughness and behave somehow as a brittle material compared to samples without CB. As the results demonstrate, the presence of HALS has had no considerable effect on the mechanical properties of the unexposed samples. The changes in yield stress and elongation-at-break showed the same trend as the elastic modulus of the samples. This could imply that the simultaneous presence of HALS and CB has minimized the change in mechanical properties which may be preserved for a longer time of UV irradiation exposure.
Conclusion
The effects of HALS and CB as UV stabilizers on the properties of high-density polyethylene were studied. It was shown that HALS as a UV stabilizer can effectively reduce the CI of the corresponding samples indicating that it effectively prevented the degradation of the matrix. It was also found that CB had the same effect. It was seen that using a hybrid system of light stabilizers, HALS and CB, could have a synergistic effect. As the results depicted in this study, a hybrid stabilizing system could prevent increases in CI which was an indication of a better stabilization effect. The gel content of the samples was measured and it was seen that pure polyethylene sample showed a high amount of gel formed through photodegradation of polyethylene. It was also observed that the hybrid system displayed the best performance having only 3.5 % of the gel content after 1,000 h (equivalent to 8 years under direct sunlight). Studying the mechanical properties of the samples demonstrated that the use of a hybrid system would suppress any change in the mechanical properties of the PE matrix, preserving its properties for a longer time under UV exposure.
References
Feldman D (2002) Polymer weathering: photo-oxidation. J Polym Enviorn 10:163–173
Cicchetti O (1970) Mechanisms of photo degradation and stabilization of polyolefins. Adv Polym Sci 7:70–112
Carlsson DJ, Wiles DM (1976) The photo-oxidative degradation of polypropylene part 1: photooxidation and photoinitiation process. J Macromol Sci Part C Polym Rev 14:65–106
Singh B, Sharma N (2008) Mechanistic implication of plastic degradation. Polym Degrad Stab 93:561–584
Andrady AL, Hamid H, Torikai A (2011) Effects of solar UV and climate change on materials. Photochem Photobial Sci 10:292–300
Rabello MS, White JR (1997) The role of physical structure and morphology in the photodegradation behavior of polypropylene. Polym degrad and stab 56:55–73
Yang R, Li Y, Yu J (2005) Photo-stabilization of linear low density polyethylene by inorganic nano-particles. Polym Degrad Stab 88:168–174
Maria R, Rode K, Brull R, Dorbath F, Baudrit B, Bastian M, Brendle E (2011) Monitoring the influence of different weathering conditions on polyethylene pipes by IR-microscopy. Polym Degrad Stab 96:1901–1910
Eltayeb E, Mahdavian AR, Barikani M (2011) Effect of cobalt naphthenate and 2-benzoylbenzoic acid on UV-degradation of LDPE, Iranian. J Chem Eng 8:31–42
Eltayeb E, Barikani M, Mahdavian AR, Honarkar H (2009) The effect of cobalt naphthenate and 2-hydroxy-4-methoxybenzophenone on photo-oxidative degradation of LDPE. Iran Polym J 18(9):753–760
Hawkins WL, Worthington MA, Winslow FH (1960) Rubber Age 88:279–283
Hawkins WL, Worthington MA (1963) Synergistic antioxidant combinations. Carbon black substitutes. J Polym Sci Part A 1:3489–3497
Liu M, Horrocks AR (2002) Effect of carbon black on UV stability of LLDPE films under artificial weathering conditions. Polym Degrad Stab 75:485–499
Gugumus F (1993) Re-evaluation of the stabilization mechanism of various light stabilizer classes. Polym Degrad Stab 39:117–135
Gugumus F (2000) Aspects of the impact of stabilizer mass on performance in polymers: 3. performance of HALS in Polyethylene. Polym Degrad Stab 96:93–104
Gugumus F, Klemchuk P, Pospisil J (1989) Inhibition of oxidation processes in organic materials, vol 2. CRC, Boca Raton 2
Gugumus F (2002) Possibilities and limits of synergism with light stabilizers in polyolefins 1. HALS in polyolefins. Polym Degrad Stab 75:295–308
Kurumada T, Ohsawa H, Yamazaki T (1987) Synergism of hindered amine light stabilizers and UV-absorbers. Polym Degrad Stab 19:263–272
Butola BS, Joshi M (2013) Photostability of HDPE filaments stabilized with UV absorbers (UVA) and light stabilizers (HALS). J Eng Fibers Fabs 8:61–68
Sabziparvar AA (2008) A simple formula for estimating global solar radiation in central arid deserts of Iran. Renew Energy 33:1002–1010
Tolinski M (2009) Additives for polyolefins: getting the most out of polypropylene, polyethylene and TPO. William Andrew, Oxford, UK
Boehm HP (2002) Surface oxides on carbon and their analysis: a critical assessment. Carbon 40:145–149
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Javadi, Y., Hosseini, M.S. & Aghjeh, M.K.R. The effect of carbon black and HALS hybrid systems on the UV stability of high-density polyethylene (HDPE). Iran Polym J 23, 793–799 (2014). https://doi.org/10.1007/s13726-014-0275-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-014-0275-2