Abstract
Poly-ortho-aminophenol (PoAP) and multi-walled carbon nanotubes (MWCNTs) were deposited on the platinum electrode using cyclic voltammetry technique to form the Pt/PoAP/MWCNTs nanosensor for the electrochemical determination of oxytetracycline as analyte. This electrochemical nanosensor with good uniformity and high surface area was prepared in the presence of an ionic surfactant (sodium dodecyl sulfate) as electrolyte to suspend carbon nanotubes within the PoAP and improve the stability and electroactivity of the composite film. The surface morphology of the prepared nanosensor was characterized by scanning electron microscopy and showed a three-dimensional network structure. The influence of several parameters such as number of potential cycles, scan rate and pH of the solution on the electrochemical response of the resultant electrode was investigated. The prepared electrode functioned as a selective recognition element for oxytetracycline determination. It showed excellent electrochemical response to oxytetracycline at low oxidative potential in buffer solution of pH 2.0, with good stability and sensitivity. Under the optimal experimental conditions, the electrochemical response of the sensor was linear with respect to the concentration of oxytetracycline in a dynamic range of 0.2 μM–0.25 mM. The detection limit of the fabricated nanosensor was calculated as 0.10 μM (signal/noise = 3). This sensor was used successfully for the oxytetracycline determination in real samples with recoveries of 96.9–103.5 %.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Conductive polymers, such as polypyrrole [1], poly-ortho-aminophenol (PoAP) [2, 3], polyaniline and their copolymers [4–6] have been extensively studied because of their various applications including corrosion inhibitors, batteries, organic electronics, electrochromic devices, sensors, etc. [7]. Multi-walled carbon nanotubes (MWCNTs), owing to their high surface area, electrical conductivity, chemical stability and mechanical strength have been used in electrochemical sensors [8, 9]. The high surface area and conductivity of MWCNTs may improve the redox properties of conductive polymers and facilitate the electron transfers [10–12]. Therefore, composites of conductive polymers and MWCNTs have been proposed for fabrication of a higher recognition capacity and sensitivity of the electrochemical sensor.
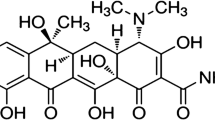
Oxytetracycline (OTC) as a drug (Scheme 1), is widely used for prevention and therapy of human and animal infection diseases and has a great activity against bacteria due to its broad-spectrum activity, good oral absorption, relatively low toxicity and low cost [13–15]. So, this drug is licensed for using in a variety of food-producing animals including cattle, sheep, pig and fish [16]. It is used as animal feed additive and in veterinary medicine as antibiotic. It is accumulated in diary food products, such as meat [17], honey [18], egg [19] and milk [20] which can be directly toxic or cause allergic reactions in some hypersensitive individuals. This massive use causes concerns for consumer’s health. The main concerns are: (a) the possible allergic sensibility of exposed individuals, (b) the selective pressure that antimicrobial drug residues may exert over human gut microflora and (c) the possibility of selecting antibiotic-resistant bacteria [21]. They have been of great interest to researchers to safeguard human health for tetracyclines detection. So, there have been many detection methods such as HPLC [22, 23], capillary electrophoresis [24], chemiluminescence [25], colorimetric sensors [26, 27] and bioluminescent biosensors [28] that are expensive, time-consuming and not useful for field analysis. But, electrochemical sensors play an important role in analytical determination of some toxic compounds due to their simple design and fast response to analyte.
In this research, the combination of MWCNTs and PoAP has been proposed for achieving an electrochemical nanosensor for oxytetracycline (as analyte) that has the capability of offering amplified sensitivity and real-time response as a result of enhanced interaction among PoAP/MWCNT composite and the analyte. It was fabricated by electro-polymerization of ortho-aminophenol (oAP) monomer and MWCNT nanoparticles in the presence of sodium dodecyl sulfate (SDS) as surfactant on a platinum electrode surface. The prepared nanosensor was characterized by scanning electron microscopy (SEM) and cyclic voltammetry (CV) techniques. The influence of several parameters such as number of potential cycles, scan rate and pH of the solution on the electrochemical response of the nanosensor was studied. A linear relationship between the concentration of oxytetracycline and electrochemical response of the nanosensor was reported. Under the optimized conditions, the nanosensor exhibited excellent recognition capacity for oxytetracycline.
Experimental
Chemicals
All chemicals used in this work [ortho-aminophenol, sodium dodecyl sulfate, perchloric acid and oxytetracycline] were purchased from Merck (Germany) and used without further purification. Typical MWCNTs (Reinste Nano Ventures Private, India) with outer diameter in the range of 5–20 nm and length measured to several hundreds of nanometers were used. Oxytetracycline hydrochloride tablets (Damloran Razak Pharmaceutical Company, Iran) were used in this study. All aqueous solutions were prepared with double-distilled water.
Measurements
All electrochemical measurements were carried out in a conventional three-electrode cell powered by a μ-Autolab potentiostat/galvanostat (Metrohm, model 12/30/302, The Netherlands). PoAP/MWCNTs composites electrodeposited on a platinum disk electrode of 0.0314 cm2 area (Metrohm, The Netherlands) that was employed as working electrode. Ag/AgCl electrode (Metrohm, The Netherlands) and a platinum wire were used as reference and counter electrodes, respectively. Since MWCNTs are insoluble in most solvents, ultrasonication (Sigma-Aldrich-Bandelin Sonopuls, Germany) is required during preparation to effectively disperse the nanotubes. The surface morphology of the obtained films was characterized by scanning electron microscopy (model KYKY-EM3200, China). The pH values of the solutions were determined using a pH meter (model 827, Metrohm, The Netherlands). All the experiments were carried out at 22 ± 1 °C.
Preparation of Pt/MWCNTs electrode
The MWCNTs (1 % by w/w) were dispersed into a solution of 5 mM SDS by ultrasonic agitation for 40 min to obtain a homogeneous MWCNTs suspension. SDS was used as an additive to suspend MWCNT particles and improve the stability and electroactivity of the resulting films [29]. The modification of platinum electrode by MWCNTs was performed via dropping method. The suspension was coated on the surface of a platinum electrode and the Pt/MWCNTs electrode was obtained after 5 h of drying at room temperature.
Preparation of Pt/PoAP/MWCNTs modified electrode
Before each experiment, the bare platinum electrode was polished with 0.05 μm α-Al2O3 on a piece of leather and thoroughly washed with distilled water and then rinsed ultrasonically in ethanol and water for 5 min. The Pt/PoAP electrode was prepared by electro-polymerization of oAP monomer (5 mM) on a bare platinum electrode between −0.1 and +0.8 V at 100 mV s−1 in a solution of 0.6 M HClO4 containing 5 mM SDS for 50 cycles. The electrode was washed by distilled water several times to remove the remaining oAP monomers adsorbed on the surface of the electrode. The anodic peak current (IPa) of oxytetracycline was dependent on the thickness of PoAP/MWCNTs composite film which was easily adjusted by controlling the number of cycles during the electro-polymerization and also on monomer concentration. The maximum anodic peak current of oxytetracycline was observed when 50 potential cycles were used for the fabrication of modified electrode. The voltammogram response of the Pt/PoAP/MWCNTs electrode to oxytetracycline was found to increase with an increase in monomer concentration up to 5 mM.
The Pt/PoAP/MWCNTs electrode was fabricated by the same procedure as Pt/PoAP electrode, but with addition of 1 % MWCNTs. The stability of Pt/PoAP and Pt/PoAP/MWCNTs electrodes was examined by means of a cycle life test performed for 25 cycles at scan rate of 100 mV s−1.
Real sample preparation
To analyze the oxytetracycline hydrochloride tablets, the average mass of five tablets was determined. The tablets were finely powdered and homogenized in a mortar. An accurately weighed amount of the homogenized powder was transferred into a 100-mL calibrated flask containing 50 mL of buffer solution. The contents of flask were sonicated for 10 min. The undissolved excipients were removed by filtration and then diluted to volume with the same buffer solution. The desired concentration was obtained by accurate dilution with the buffer.
Results and discussion
Characterization of Pt/PoAP/MWCNTs modified electrode
Cyclic voltammogram of the PoAP/MWCNTs composite film (Fig. 1b) showed a pair of broad peak at around −0.1 to +0.3 V related to the redox process of the phenoxazine units [30–32]. The redox peak currents were stronger than pure PoAP film (Fig. 1a), but the voltammetric behavior of both films was similar.
Figure 2 shows the scanning electron micrographs of pure PoAP, MWCNTs and PoAP/MWCNT films on the platinum electrode. As it is obvious, the tangled MWCNTs with diameter of 5–20 nm and length measured to several hundreds of nanometers were distributed very well (Fig. 2a). It was clearly observed that PoAP/MWCNTs composite film showed a three-dimensional network structure (Fig. 2c) with a larger surface area in comparison with Pt/MWCNTs and Pt/PoAP films (Fig. 2b). PoAP/MWCNTs composite exhibited highly porous morphology with distinct structures of uniform size and seemed to wrap the MWCNTs.
The surface areas of bare and modified electrode were acquired by CV method in 1 mM K3Fe(CN)6 as a probe at different scan rates. The surface area for a reversible process was calculated according to the slope of \( i_{p} - \sqrt \nu \) [33]:
where A is the electroactive surface area (cm−2), n is the number of electrons participated in the redox reaction, D is the diffusion coefficient (cm2 s−1), C is the bulk concentration of the redox probe (mol cm−3) and ν is the scan rate (V s−1). For a solution containing K3Fe(CN)6, the surface areas of the platinum, Pt/PoAP, Pt/MWCNTs and Pt/PoAP/MWCNTs electrodes were calculated as 0.036, 0.061, 0.089 and 0.112 cm2, respectively.
Electrochemical behavior of oxytetracycline with respect to Pt/PoAP/MWCNTs electrode
Figure 3 shows typical cyclic voltammograms for 0.2 μM oxytetracycline solution in hydrochloric acid buffer solution of pH 2.0 at different electrodes. A pair of weak redox peak was observed for bare platinum electrode (Fig. 3a) in comparison with other modified electrodes. An obvious redox peak was observed around −0.7 to 0 V for Pt/PoAP electrode (Fig. 3b). It seemed that the presence of PoAP film improved the electrochemical oxidation of oxytetracycline compared to bare platinum electrode. The anodic and cathodic peak currents of oxytetracycline were increased for Pt/MWCNTs electrode (Fig. 3c) and the oxidation potential was shifted to the positive values. This was attributed to the electronic structure, the nanometer dimension of MWCNT and the topological defects on its surface [34].
The following mechanism could be proposed according to the functional groups in oxytetracycline:
MWCNTs can improve the OTC adsorption on the surface.
A comparison between Pt/PoAP/MWCNTs electrode and other modified electrodes showed that composite had the largest oxidative and reductive current at −0.05 and −0.75 V, respectively. Thus, it exhibited the most sensitive electrochemical response and improved the electrochemical oxidation potential of oxytetracycline (Fig. 3d). This was attributed to the special synergistic enhancement of combined PoAP and MWCNTs and the catalytic activity of MWCNTs within the PoAP film to the oxidation of oxytetracycline.
The influence of the pH of buffer solution (pH 1.5–5.0) on the electrochemical behavior of oxytetracycline with respect to Pt/PoAP/MWCNTs electrode was investigated for obtaining optimal electrochemical behavior of oxytetracycline (Fig. 4). The highest peak current occurred at pH 2.0. Then, the anodic peak current decreased with increasing the pH of solution above 2.0. This was attributed to the structural changes of polymer film and its lower conductivity performance at higher pH [35]. Thus, the buffer solution of pH 2.0 was chosen for the experiments.
Figure 5 shows the voltammograms of the Pt/PoAP/MWCNTs electrode in buffer solution at different concentrations of oxytetracycline. As it can be seen, the Pt/PoAP/MWCNTs electrode exhibits a well-defined catalytic oxidation current increasing linearly as oxytetracycline concentration increases. Calibration plot for analysis of oxytetracycline (Fig. 5) showed a linear dependence of the anodic peak current on oxytetracycline concentrations in the range of 0.20 μM–0.25 mM and a correlation coefficient of 0.9974. A detection limit of 0.1 μM (signal/noise = 3) was obtained. The anodic peak current gradually leveled off when oxytetracycline concentration was greater than 0.25 mM.
Cyclic voltammograms for oxidation of oxytetracycline on the Pt/PoAP/MWCNTs electrode in buffer solution (pH 2.0) with different concentrations of oxytetracycline in the range of 0.2 μM–0.25 mM. The arrow indicates the trends of current during CVs. Inset plot of anodic peak current versus oxytetracycline concentration
Analytical characterization
To examine the practicality of this electrochemical sensor for determination of real samples, detection of oxytetracycline hydrochloride was tested in pharmaceutical formulations. Five oxytetracycline hydrochloride tablets were analyzed by this method (Table 1). The assigned content of oxytetracycline in each tablet was 100 mg. The recovery of oxytetracycline in five samples was determined to be 96.9–103.5 %. The results showed that this method was very practical and effective in sample analysis.
The reproducibility of fabricated electrode was investigated using oxytetracycline concentration of 10.0 μM. The peak current response of oxytetracycline was determined using four different electrodes, which were prepared under the same conditions. The relative standard deviation (RSD) of the peak current was 3.6 %. Thus, the reproducibility of fabricated sensor was considered excellent. The long-term stability of the composite electrode was investigated by measuring the responses to 10.0 μM oxytetracycline from day to day during storage in buffer solution (pH 2.0). Only a drop of 11.0 ± 0.5 % was observed in the current signal of oxytetracycline after the modified electrode was used for approximately 30 times during 15 days. This high stability was attributed to the excellent stability of the composite electrode. It is thus envisaged that, our designed method is very sensitive for the detection of oxytetracycline.
Conclusion
In this research, PoAP/MWCNTs nanosensor was fabricated through electro-polymerization of ortho-aminophenol monomer in the presence of MWCNTs. The optimum conditions were obtained for preparation of the modified electrode. The fabricated nanosensor showed an excellent recognition and sensitivity to oxytetracycline, with a low detection limit of 0.1 μM (signal/noise = 3). A linear relationship between the oxytetracycline concentration and the anodic peak current was obtained in the range of 0.2 μM–0.25 mM. The prepared sensor showed good stability and reproducibility. It has been successfully applied for detection of oxytetracycline hydrochloride in pharmaceutical formulations.
References
Wang HY, Park SM (2007) Polypyrrole-based optical probe for a hydrogen peroxide assay. Anal Chem 79:240–245
Pan X, Zhou S, Chen Ch, Kan J (2006) Preparation and properties of an uricase biosensor based on copolymer of o-aminophenol-aniline. Sens Actuators B Chem 113:329–334
Keyhanpour A, Seyed Mohaghegh SM, Jamshidi A (2012) Electropolymerization and characterization of polyaniline, poly(2-anilinoethanol) and poly(aniline-co-2-anilinoethanol). Iran Polym J 21:307–315
Li J, Shi L, An Y, Li Y, Chen X, Dong H (2006) Reverse micelles of star-block copolymer as nanoreactors for preparation of gold nanoparticles. Polymer 47:8480–8487
Liu L-P, Yin Zh-J, Yang Zh-Sh (2010) A l-cysteine sensor based on Pt nanoparticles/poly(o-aminophenol) film on glassy carbon electrode. Bioelectrochemistry 79:84–89
Li J, Zhao J, Wei X (2009) A sensitive and selective sensor for dopamine determination based on a molecularly imprinted electropolymer of o-aminophenol. Sens Actuators B Chem B 140:663–669
Chen Ch, Sun Ch, Gao Y (2009) Amperometric sensor for hydrogen peroxide based on poly(aniline-co-p-aminophenol). Electrochem Commun 11:450–453
Kan X, Zhou H, Li Ch, Zhu A, Xing Z, Zhao Zh (2012) Imprinted electrochemical sensor for dopamine recognition and determination based on a carbon nanotube/polypyrrole film. Electrochim Acta 63:69–75
Nabid MR, Sedghi R, Sharifi R, Abdi Oskooie H, Heravi MM (2013) Removal of toxic nitrate ions from drinking water using conducting polymer/MWCNTs nanocomposites. Iran Polym J 22:85–92
Pradhan AK, Swain SK (2013) Synthesis and characterization of poly(acrylonitrile-co-methylmethacrylate) nanocomposites reinforced by functionalized multiwalled carbon nanotubes. Iran Polym J 22:369–376
Benvidi A, Kakoolaki P, Zare HR, Vafazadeh R (2011) Electrocatalytic oxidation of hydrazine at a Co(II) complex multi-wall carbon nanotube modified carbon paste electrode. Electrochim Acta 56:2045–2050
Zamani MM, Fereidoon A, Sabet A (2012) Multi-walled carbon nanotube-filled polypropylene nanocomposites: high velocity impact response and mechanical properties. Iran Polym J 21:887–894
Schnappinger D, Hillen W (1996) Tetracyclines: antibiotic action, uptake, and resistance mechanisms. Arch Microbiol 165:359–369
Chopra I, Roberts M (2001) Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol R 65:232–260
Wang Y, Liu W-H, Wang K-M, Shen G-L, Yu R-Q (1998) Fluorescence optical fiber sensor for tetracycline. Talanta 47:33–42
Debuf Y (1988) The veterinary formulary. Pharmaceutical Press, London (97)
De Wasch K, Okerman L, De Brabander H, Van Hoof J, De Backer P (1998) Detection of residues of tetracycline antibiotics in pork and chicken meat: correlation between results of screening and confirmatory tests. Analyst 123:2737–2741
Jeon M, Paeng IR (2008) Quantitative detection of tetracycline residues in honey by a simple sensitive immunoassay. Anal Chim Acta 626:180–185
Croubels SM, Vanoosthuyze KE, Van Peteghem CH (1997) Use of metal chelate affinity chromatography and membrane-based ion-exchange as clean-up procedure for trace residue analysis of tetracyclines in animal tissues and egg. J Chromatogr B 690:173–179
Pena ALS, Lino CM, Silveira IN (1999) Determination of oxytetracycline, tetracycline, and chlortetracycline in milk by liquid chromatography with postcolumn derivatization and fluorescence detection. J AOAC Int 82:55–60
Pellegrini GE, Carpico G, Coni E (2004) Electrochemical sensor for the detection and presumptive identification of quinolone and tetracycline residues in milk. Anal Chim Acta 520:13–18
Monser L, Darghouth F (2000) Rapid liquid chromatographic method for simultaneous determination of tetracyclines antibiotics and 6-epi-doxycycline in pharmaceutical products using porous graphitic carbon column. J Pharm Biomed Anal 23:353–362
Ng M, Linder SW (2003) HPLC separation of tetracycline analogues: comparison study of laser-based polarimetric detection with UV detection. J Chromatogr Sci 41:460–466
Kowalski P (2008) Capillary electrophoretic method for the simultaneous determination of tetracycline residues in fish samples. J Pharm Biomed Anal 47:487–493
Han S, Liu E, Li H (2006) Determination of tetracycline, chlortetracycline and oxytetracycline by flow injection with inhibitory chemiluminescence detection using copper(II) as a probe ion. Luminescence 21:106–111
Pastor-Navarro N, Morais S, Maquieira Á, Puchades R (2007) Synthesis of haptens and development of a sensitive immunoassay for tetracycline residues application to honey samples. Anal Chim Acta 594:211–218
Jeon M, Kim J, Paeng K-J, Park S-W (2008) Biotin–avidin mediated competitive enzyme-linked immunosorbent assay to detect residues of tetracyclines in milk. Microchem J 88:26–31
Virolainen NE, Pikkemaat MG, Elferink JWA, Karp MT (2008) Rapid detection of tetracyclines and their 4-epimer derivatives from poultry meat with bioluminescent biosensor. J Agric Food Chem 56:11065–11070
Ehsani A, Mahjani MG, Jafarian M, Naeemy A (2010) An electrochemical study of the synthesis and properties of multi-walled carbon nanotube/poly-ortho-aminophenol composites. Prog Org Coat 69:510–516
Ojani R, Raoof J-B, Safshekan S (2009) Poly(o-aminophenol) film prepared in the presence of sodium dodecyl sulfate: application for nickel ion dispersion and the electrocatalytic oxidation of methanol and ethylene glycol. Electrochim Acta 54:2190–2196
Shaolin M (2004) Electrochemical copolymerization of aniline and o-aminophenol. Synth Met 143:259–268
Yang Ch-Sh, Wen T-Ch (1994) Electrochemical copolymerization of aniline para-phenylenediamine on IrO2-coated titanium electrode. J Appl Electrochem 24:166–178
Bard AJ, Faulkner LR (2001) Elecrochemical methods: fundamentals and applications, chap. 6. Wiley, New York
Lota G, Fic K, Frackowiak E (2011) Carbon nanotubes and their composites in electrochemical applications. Energ Environ Sci 4:1592–1605
Tucceri RI (2003) Specularity change on a thin gold film surface coated with poly(o-aminophenol) during the polymer redox conversion. The pH effect on the redox sites distribution at the metal–polymer interface. J Electroanal Chem 543:61–71
Acknowledgments
The support of this research by Payame Noor University is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ajami, N., Bahrami Panah, N. & Danaee, I. Oxytetracycline nanosensor based on poly-ortho-aminophenol/multi-walled carbon nanotubes composite film. Iran Polym J 23, 121–126 (2014). https://doi.org/10.1007/s13726-013-0207-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-013-0207-6