Abstract
In this study, the synthesis and characterization of a novel nano-porous superabsorbent hydrogel with high water swelling capacity is described. A nano-porous hydrogel was prepared by employing (2-dimethylaminoethyl) methacrylate (PDMAEMA) as a pH sensitive monomer and sodium alginate (SA) as a water soluble polysaccharide under γ-ray irradiation. The polymerization reaction was performed at room temperature in the absence of chemically toxic crosslinking agent and initiators. The interactive parameters including biopolymer backbone concentration, monomer concentration and γ-irradiation dose were selected as major factors in the synthesis of superabsorbent and three levels for each factor were applied to obtain the highest water swelling according to the central composite design (CCD) method. According to the results of nine different tests which were derived by CCD method, the optimum conditions were determined. The results showed that the hydrogel prepared at concentration of 1.5 g SA, 2.1 mol/L PDMAEMA and at a radiation dose of 5 kGy displayed the highest swelling capacity. In continuation, the effect of salt, pH, and particle size on the swelling behavior of the obtained samples was investigated. We found that the swelling of the optimized sample first increased and then dropped with increases in pH from 2 to 12 and the maximum water absorbency was observed at pH 7. Finally, different techniques such as Fourier transform infrared spectroscopy (FTIR), thermogravimetric analysis (TGA) and scanning electron microscope (SEM) were applied for the characterization of optimized nano-porous hydrogel.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Superabsorbent hydrogels are crosslinked, three-dimensional networks of hydrophilic polymers which can absorb and retain large amounts of aqueous fluids even under pressure. These systems swell in water up to an equilibrium state and retain their original shape [1–3]. As a result of important research during the last two decades, hydrogels have been recognized as a well-established class of polymers with widespread applications in biomedicine, bioengineering, pharmaceutical, veterinary, food industry, agriculture and related fields [4–6]. They have been used as controlled release systems for drugs and carriers for water and pesticides in agricultural field [7, 8]. The successful use of hydrogels in these applications greatly relies on their physical and chemical properties which mainly depend on the degree of crosslinking, the chemical composition of the polymer, and the interaction between the network and surrounding liquids [9–11].
In recent years, the preparation of hydrogels by γ-radiation is being used increasingly around the world [12–16]. It carries various advantages such as easy process control, possibility of forming combined hydrogels and sterilization in one step, and no necessity to add any initiators and crosslinkers that are possibly harmful and often difficult to remove [17, 18]. In the meantime, the degree of crosslinking, which significantly determines the extent of swelling in gels, can be controlled by varying the total γ-radiation doses. With regard to these arguments, the γ-radiation method is very practical to produce hydrogels for medical applications [19, 20].
Sodium alginate (SA) is a well-known natural polysaccharide with negative charge extracted from brown seaweed or produced by bacteria, and so it is abundant, renewable, non-toxic, water-soluble, biodegradable and biocompatible. It is composed of (1-4)-b-d-mannuronic acid and (1-4)-a-l-guluronic acid units in the form of homopolymeric and heteropolymeric sequences (Fig. 1) [21, 22]. SA is widely used in various applications such as chelating and thickening agents, emulsifiers, stabilizers, encapsulation, swelling and suspending agents, or used to form gels, films, and membranes [23]. To prepare new polymeric materials with special properties and expanding the range of SA utilization, considerable works have been devoted to the synthesis of various SA-based hydrogels using vinyl monomers such as methyl acrylate, acrylamide, and acrylonitrile [24–26]. However, the interaction of hydrophilic poly((2-dimethylamino) ethyl methacrylate) (PDMAEMA) and SA has not been studied up to now.
PDMAEMA is a water-soluble polymer containing tertiary amino groups, which can be protonated in acidic solutions [27, 28]. Thus, PDMAEMA is often used to prepare pH-sensitive materials, which can be used as controlled drug delivery systems and gene transfer agents [29]. The main problem of these hydrogels is their low swelling capacity in water. Although several groups of PDMAEMA-based hydrogels have been synthesized, their swelling ratios are reported to be almost low for water-based applications. For example, Uzun et al. synthesized PDMAEMA hydrogels by γ-irradiation of a ternary systems composed of dimethylaminoethyl methacrylate/water/ethylene glycol dimethacrylate at varying compositions and irradiations. The highest water absorption was obtained around 34 g/g [30]. In another study, radiation synthesis and swelling behavior of PDMAEMA and poly(N,N-dimethylaminoethyl methacrylate-co–N-vinyl 2-pyrrolidone) (P(DMAEMA-co-VP)) hydrogels were investigated and the maximum water swelling at their optimum conditions was achieved under 30 g/g [31]. These swellings were almost lower than traditional synthetic hydrogels like crosslinked copolymers of acrylic acid and acrylamide (~1,000 g/g). In the present study, the γ-irradiation was used to synthesize a superabsorbent hydrogel based on PDMAEMA and SA (SA-g-PDMAEMA) to obtain a PDMAEMA-based hydrogel with high water absorbency.
Experimental
Materials
Sodium alginate (SA) (Mw 270,000), obtained from Acros (Germany), was washed with acetone to remove any adhering impurities before use and was then dried under reduced pressure. (2-Dimethylaminoethyl) methacrylate monomer (DMAEMA) (Merck) was used without any purification. Sodium chloride (NaCl), lithium chloride (LiCl), barium chloride (BaCl2), magnesium chloride (MgCl2), calcium chloride (CaCl2) and aluminum chloride (AlCl3) were purchased from Sigma-Aldrich. All other chemicals were also of analytical grade. De-ionized water was used for hydrogel preparation and swelling measurements.
Instrumental analysis
FTIR spectra of samples in the form of KBr pellets were recorded using an ABB Bomem MB-100 FTIR spectrometer. Thermogravimetric analysis (TGA) was carried out on a 2050 thermogravimetric analyzer at a heating rate of 20 °C/min under N2 atmosphere. A scanning electron microscope (SEM) (Philips, XL30, 25 kV) was used to observe the morphology of the samples. Irradiation was carried out using γ-rays from 60Co source, in a Gammacell-220 (PX100, Russian) with a dose rate of 0.3 Gy/Sat room temperature.
Central composite design
SA-g-PDMAEMA hydrogel with different mixture ratios was obtained by varying the masses of SA, DMAEMA, and radiation dose. In this paper, central composite design (CCD) method was used to analyze the effectiveness of SA, DMAEMA, and radiation dose on swelling of the SA-g-PDMAEMA hydrogel, and then the best overall performance ratio was selected. SA, DMAEMA, and radiation dose were determined as three experimental factors of central composite tests and each factor was constituted of three levels (Table 1). It was assumed that none of the factors interacted with each other. The test program is given in Table 2.
Preparation of SA-g-PDMAEMA hydrogel
A required amount of SA (0.5–1.5 g) was dissolved in water (30 mL) in three-neck reactor equipped with a mechanical stirrer. DMAEMA (2.1–3.5 mol/L (3.55–5.92 mL)) was added to the reactor while stirring (200 rpm). The reactor was placed in a thermostated water bath pre-set at the desired temperature (50 °C) for 20 min. The cold mixture was removed into a 50-mL glassy tube. To prevent penetration of air to the samples during irradiation, the mixture was heat sealed by a vacuum machine. The tube was then irradiated under γ-rays source, according to the desired total doses (5, 12 and 20 kGy). Scissors were used to cut the prepared hydrogels into small pieces. The products were then immersed in an excess of de-ionized water and then for dewatering, ethanol (200 mL) was added to the gelled product for 24 h, after that the product was filtered, washed with fresh ethanol and dried in an oven at 50 °C for 10 h to constant weight. Next, the resulting powdered superabsorbent hydrogel was stored away from moisture, light and heat for further experiments.
Gel fraction of SA-g-PDMAEMA hydrogel
Gel content of the SA-g-PDMAEMA hydrogels was measured by extraction in de-ionized water for 48 h at room temperature until they reached constant weight. The gel content was defined as Eq. 1; where G d, the dried gel weight after extraction, and G i the initial weight of the SA-g-PDMAEMA hydrogel.
Swelling measurements
To measure the swelling of samples in different media, the gel samples were added to a tea bag (i.e., a 100-mesh nylon screen) with average mesh between 40 and 60 (250–350 μm) containing an accurately weighed powdered sample (0.1 ± 0.0001 g) and were immersed in distilled water or desired salt solution (LiCl, MgCl2, AlCl3, CaCl2, NaCl and BaCl2) (0.1 mol/L, 100 mL) for 3 h at room temperature until the gel reached the equilibrium state of swelling. The mass of the gel was determined after removing the water from the surface of the swollen gels. The equilibrated swelling (ES) was defined as Eq. 2; where G s designated as the weight of the swollen gels and G i as the dried gel weight.
Swelling at various pH media
SA-g-PDMAEMA hydrogel (0.1 ± 0.0001 g) was immersed in 100 mL of acidic to basic pH media prepared by diluting NaOH or HCl solutions to achieve pH 2.0–12.0. Then ES in pH solution was calculated according to Eq. 2. The pH values were determined with a pH meter (Metrohm/620, accuracy ±0.1).
Swelling of different hydrogel particle sizes
To prepare hydrogel with different sizes, the obtained hydrogels were separated by various meshes (250–550 μm particle size) and their swellings were survived according to the above sections.
Results and discussion
Synthesis mechanism
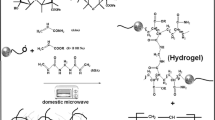
Scheme 1 shows the possible mechanism for synthesis of SA-g-PDMAEMA hydrogel.
In principle, during the γ-irradiation of reaction solution including DMAEMA and SA in water, the latter can absorb most of the γ-rays energy. Furthermore, water can increase the mobility of the polymer chains and consequently allows macro-radicals recombine to give polymer networks. On the other hand, the initiation step of polymerization reaction takes place by an indirect reaction and the products of water radiolysis can produce macro-radicals.
Hydroxyl radicals from water radiolysis can add to the double bond of DMAEMA monomer to form free radicals which can then react with SA. The increasing of irradiation dose can also result in cleavages of monomer double bonds. From the radiation chemistry of alcohols and carbohydrates in aqueous solutions, one can suggest that the attack of OH radicals on SA backbone would lead to the breakage of C–H bonds. The SA radicals from the later step can add to the DMAEMA molecules (not a radical), leading to the growth of a branched chain. The final step of the reaction includes the crosslinking of the polymer branches for the formation of the polymer networks. Some side reactions such as scissors polymerization by γ-rays are not shown in this mechanism [14].
Swelling of SA-g-PDMAEMA hydrogel
Table 3 presents the conditions for having the SA-g-PDMAEMA hydrogel with the highest water swelling. It can be seen that combination of 2.1 mol/L DMAEMA (3.55 mL), 5 kGy γ-irradiation, and 1.5 g SA produces the maximum swelling. This high swelling can be referred to its surface porosity (nano-porous structure). In the following, the optimized hydrogel sample was used for further characterization tests.
Since the SA-g-PDMAEMA hydrogel is potentially a kind of ionic hydrogel, the surrounding environment including its pH, ionic strength, etc., greatly affects its swelling capacity. Figure 2 shows the effect of NaCl solution concentration on the swelling behavior of the hydrogels. From the figure, the swelling ratios of the hydrogel decreased rapidly as the NaCl concentration increased. This well-known phenomenon commonly observed in the swelling of ionizable hydrogels [11] was often attributed to the charge screening effect of the additional cations.
To investigate the effect of salt type on the swelling, different cations such as NaCl, LiCl, BaCl2, CaCl2, MgCl2, and AlCl3 with same concentrations were chosen. As shown in Fig. 3, the swelling capacity of ionic hydrogels in saline solutions falls with increasing the charge of the salt cations. The reason can also be attributed to the higher ionic strength of these saline solutions. To achieve a comparative measure of sensitivity of the hydrogels in comparison with aqueous fluid, dimensionless swelling factor, f, was calculated according to Eq. 3 and results are shown in Table 4. The lowest values of f factor for NaCl and LiCl solutions show low charge screening effect in these media [8].
Due to the presence of an amine group in the DMAEMA monomer, SA-g-PDMAEMA hydrogels could respond to pH changes. The swelling behavior of these hydrogels at different pH media was studied and the results are shown in Fig. 4. As seen, the swelling of the hydrogel increases to a maximum (222 g/g) with increasing pH and then decreases when pH was further raised to 12. This may be explained by the ionic strength of the medium. As the ionic strength of the medium increases, charges of the ionic groups are shielded by the counter ions and so prevent their repulsion efficiently. For instance, at above pH 7, the Na+ cations from NaOH shield the –COO− groups and prevent the perfect anion–anion repulsion.
The response of the hydrogels by repeatedly changing the pH in the medium from 2 to 7 was examined and it is given in Fig. 5. The curve in this figure shows that the SA-g-PDMAEMA hydrogel swells at pH 7 and then shrinks at pH 2, and the process can be repeated many times. This result demonstrates that the SA-g-PDMAEMA hydrogel has obvious pH-dependent swelling reversibility and it is suitable for tailoring a pulsatile (on/off swelling) drug-delivery system.
In continuation, the swelling capacity of SA-g-PDMAEMA hydrogel was examined by three ranges of hydrogel particle sizes (100–250, 250–400 and 400–550 μm). As seen in Fig. 6, the swelling capacity of SA-g-PDMAEMA hydrogel increased with decreasing the hydrogel particle size which can be attributed to the enhanced surface area of hydrogels.
Characterization
To confirm the hydrogel synthesis, different techniques such as FTIR, SEM and TGA were applied. FTIR spectra of PDMAEMA, SA, and SA-g-PDMAEMA are shown in Fig. 7. As one can see in Fig. 7, there are main groups of PDMAEMA (Fig. 7a) and SA (Fig. 7b) in Fig. 7c which prove the presence of SA and PDMAEMA in SA-g-PDMAEMA hydrogel. Investigation of SA spectrum (Fig. 7b) shows stretching vibration bands at 3,429, 1,616 and 1,033 cm−1, which can be attributed to hydroxyl, ester carbonyl groups and C–O groups, respectively. These peaks were kept in the spectrum of the SA-g-PDMAEMA hydrogel.
The TGA diagrams of the SA and SA-g-PDMAEMA hydrogel are shown in Fig. 8. These curves can highlight the differences between the SA and the SA-g-PDMAEMA hydrogel networks. The first step for loss of mass from T = 31–110 °C for SA and from T = 31–210 °C for SA-g-PDMAEMA hydrogel is mainly due to the loss of physically adsorbed water on the SA and synthesized hydrogel. The second step of mass loss is (from T = 210–280 °C) for SA-g-PDMAEMA, which may be attributed to the degradations, mainly caused by decarboxylation of the SA backbone and breaking of ester linkage of the PDMAEMA in the hydrogel. Comparing this range of decomposition with that of SA (T = 110–180 °C) for the observed changes can be attributed to the reticulation of the hydrogel. Besides, it was found in the first step of decomposition, from about T = 210–280 °C, SA-g-PDMAEMA hydrogel has higher thermal stability than SA because of its more compact network structure.
Water permeation and sensitivity of hydrogel are related to pores in hydrogel. These pores can be observed by SEM micrographs. Figure 9 shows the SEM images of the SA-g-PDMAEMA hydrogel and pure SA. These pictures verify that the obtained hydrogel has a nano-porous structure.
Conclusion
In this work, a superabsorbent hydrogel was synthesized by graft copolymerization of PDMAEMA and SA in aqueous medium using γ-irradiations as an initiator and crosslinking agent, simultaneously. Polymerization was confirmed by TGA and FTIR analyses. Besides, as shown in the SEM micrographs, the morphology was also changed greatly after the polymerization reaction. By the use of CCD method, it was possible to obtain the optimum conditions (SA 1.5 g, DMAEMA 0.021 mol (3.55 mL) and γ-ray 5 kGy) regarding the highest swelling capacity of the final product. The effects of pH and salt on the swelling capacity of the obtained hydrogel were evaluated. The obtained hydrogel showed responsive behavior in relation to pH and to the salt solution presenting good potential of application to develop intelligent soft materials with faster response ability in drug delivery system.
References
Alcantara MTS, Brant AJC, Giannini DR, Pessoa JOCP, Andrade AB, Riella HG, Lugao AB (2012) Influence of dissolution processing of PVA blends on the characteristics of their hydrogels synthesized by radiation. Part I: gel fraction, swelling, and mechanical properties. Radiat Phys Chem 81:1465–1470
Zhou C, Li P, Qi X, Sharif ARM, Poon YF, Cao Y, Chang MW, Leong SSJ, Chan-Park MB (2011) A photopolymerized antimicrobial hydrogel coating derived from epsilon-poly-l-lysine. Biomaterials 32:2704–2712
Jha PK, Jha R, Gupta BL, Guha SK (2010) Effect of γ-dose rate and total dose interrelation on the polymeric hydrogel: a novel injectable male contraceptive. Radiat Phys Chem 79:663–671
Turturro SB, Guthrie MJ, Appel AA, Drapala PW, Brey EM, Pérez-Luna VH, Mieler WF, Kang-Mieler JJ (2011) The effects of cross-linked thermo-responsive PNIPAAm-based hydrogel injection on retinal function. Biomaterials 32:3620–3626
Raafat AI, Araby E, Lotfy S (2012) Enhancement of fibrinolytic enzyme production from Bacillus subtilis via immobilization process onto radiation synthesized starch/dimethylaminoethyl methacrylate hydrogel. Carbohydr Polym 87:1369–1374
Zuidema JM, Pap MM, Jaroch DB, Morrison FA, Gilbert RJ (2011) Fabrication and characterization of tunable polysaccharide hydrogel blends for neural repair. Acta Biomater 7:1634–1643
Yang C, Xu L, Zhou Y, Zhang X, Huang X, Wang M, Han Y, Zhai M, Wei S (2010) A green fabrication approach of gelatin/CM-chitosan hybrid hydrogel for wound healing. Carbohydr Polym 82:1297–1305
LoPresti C, Vetri V, Ricca M, Foderà V, Tripodo G, Spadaro G, Dispenza C (2011) Pulsatile protein release and protection using radiation-crosslinked polypeptide hydrogel delivery devices. React Funct Polym 71:155–167
Nizam El-Din HM, AbdAlla SG, El-Naggar AWM (2010) Swelling and drug release properties of acrylamide/carboxymethyl cellulose networks formed by gamma irradiation. Radiat Phys Chem 79:725–730
Khodja AN, Mahlous M, Tahtat D, Benamer S, Youcef SL, Chader H, Mouhoub L, Sedgelmaci M, Ammi N, Mansouri MB, Mameri S (2012) Evaluation of healing activity of PVA/chitosan hydrogels on deep second degree burn: pharmacological and toxicological tests. Burns (in press)
Ramseyer P, Micol LA, Engelhardt E, Osterheld M, Hubbell JA, Frey P (2010) In vivo study of an injectable poly(acrylonitrile)-based hydrogel paste as a bulking agent for the treatment of urinary incontinence. Biomaterials 31:4613–4619
Soler DM, Rodríguez Y, Correa H, Moreno A, Carrizales L (2012) Pilot scale-up and shelf stability of hydrogel wound dressings obtained by gamma radiation. Radiat Phys Chem 81:1249–1253
Park J, Kim H, Choi J, Gwon H, Shin Y, Lim Y, Khil MS, Nho Y (2012) Effects of annealing and the addition of PEG on the PVA based hydrogel by gamma ray. Radiat Phys Chem 81:857–860
Oliveira MJA, Parra DF, Amato VS, Lugão AB (2012) Hydrogel membranes of PVAI/clay by gamma radiation. Radiat Phys Chem (in press)
Bardajee GR, Pourjavadi A, Sheikh N, Amini-Fazl MS (2008) Grafting of acrylamide onto kappa-carrageenan via gamma-irradiation: optimization and swelling behavior. Radiat Phys Chem 77:131–137
Pourjavadi A, Barzegar S, Zeidabadi F (2007) Synthesis and properties of biodegradable hydrogels of kappa-carrageenan grafted acrylic acid-co-2-acrylamido-2-methylpropanesulfonic acid as candidates for drug delivery systems. React Funct Polym 67:644–654
Choi J, Kim J, Srinivasan P, Kim J, Park H, Byun M, Lee J (2009) Comparison of gamma ray and electron beam irradiation on extraction yield, morphological and antioxidant properties of polysaccharides from tamarind seed. Radiat Phys Chem 78:605–609
Sokker HH, El-Sawy NM, Hassan MA, El-Anadouli BE (2011) Adsorption of crude oil from aqueous solution by hydrogel of chitosan based polyacrylamide prepared by radiation induced graft polymerization. J Hazard Mater 190:359–365
Jipa IM, Stroescu M, Stoica-Guzun A, Dobre T, Jinga S, Zaharescu T (2012) Effect of gamma irradiation on biopolymer composite films of poly(vinyl alcohol) and bacterial cellulose. Nucl Inst Method Phys Res Sect B 278:82–87
Wang D, Hill DJT, Rasoul F, Whittaker AK (2011) A study of the swelling and model protein release behaviours of radiation-formed poly(N-vinyl 2-pyrrolidone-co-acrylic acid) hydrogels. Radiat Phys Chem 80:207–212
Tan R, She Z, Wang M, Fang Z, Liu Y, Feng Q (2012) Thermo-sensitive alginate-based injectable hydrogel for tissue engineering. Carbohydr Polym 87:1515–1521
Wang Q, Xie X, Zhang X, Zhang J, Wang A (2010) Preparation and swelling properties of pH-sensitive composite hydrogel beads based on chitosan-g-poly(acrylic acid)/vermiculite and sodium alginate for diclofenac controlled release. Int J Biol Macromol 46:356–362
Hunt NC, Smith AM, Gbureck U, Shelton RM, Grover LM (2010) Encapsulation of fibroblasts causes accelerated alginate hydrogel degradation. Acta Biomater 6:3649–3656
Liu Y, Yang L, Li J, Shi Z (2005) Grafting of methyl methacrylate onto sodium alginate initiated by potassium ditelluratoargentate (III). J Appl Polym Sci 97:1688–1694
Zha L, Hu J, Wang C, Hu S, Elaissari A, Zhang Y (2002) Preparation and characterization of poly(N-isopropylacrylamide-co-dimethylaminoethyl methacrylate) microgel latexes. Colloid Polym Sci 280:1–6
Traitel T, Cohen Y, Kost J (2000) Characterization of glucose-sensitive insulin release systems in simulated in vivo conditions. Biomaterials 21:1679–1687
Basan H, Gümüsderelíoglu M, Orbey T (2002) Diclofenac sodium releasing pH-sensitive monolithic devices. Int J Pharm 245:191–198
Vande-Wetering P, Schuurmans-Nieuwenbroek NME, Van Steenbergen MJ, Crommelin DJA, Hennink WE (2000) Copolymers of 2-(dimethylamino) ethyl methacrylate with ethoxytriethylene glycol methacrylate or N-vinyl-pyrrolidone as gene transfer agents. J Control Release 64:193–203
Kurisawa M, Yokoyama M, Okano T (2000) Gene expression control by temperature with thermo-responsive polymeric gene carriers. J Control Release 69:127–137
Uzun C, Hassnisaber M, Sen M, Guven O (2003) Enhancement and control of cross-linking of dimethylaminoethyl methacrylate irradiated at low dose rate in the presence of ethylene glycol dimethacrylate. Nucl Inst Method Phys Res Sect B 208:242–246
Sen M, Sarı M (2005) Radiation synthesis and characterization of poly(N, N-dimethylaminoethyl methacrylate-co-N-vinyl2-pyrrolidone) hydrogels. Eur Polym J 41:1304–1314
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bardajee, G.R., Hooshyar, Z., Zehtabi, F. et al. A superabsorbent hydrogel network based on poly((2-dimethylaminoethyl) methacrylate) and sodium alginate obtained by γ-radiation: synthesis and characterization. Iran Polym J 21, 829–836 (2012). https://doi.org/10.1007/s13726-012-0089-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-012-0089-z