Abstract
Purpose of Review
Weight gain and body fat redistribution are common side effects of many widely used drugs. We summarize recent literature on prevalence data and mechanisms associated with drug-induced body fat changes and mechanisms to prevent or treat metabolic side effects.
Recent Findings
The highest prevalence of metabolic complications is seen with antipsychotics and antiretroviral drugs used in the treatment of HIV and may, at least partly, be responsible for the increased risk for co-morbid diseases such as diabetes, steatosis of the liver, and cardiovascular disease. The pathogenetic mechanisms leading to weight gain from antipsychotics are increasingly known and help to unravel the complex interaction that exists between psychopathology and metabolic complications. Although the classic lipodystrophy mainly occurred with older HIV drugs, also with the newer HIV treatment, weight gain seems to be a major side effect.
Summary
Early detection of the metabolic consequences of drugs can lead to an early diagnosis of the complications and their treatment. Different medications, including the newer antidiabetics, are being studied in the therapy of drug-induced obesity. Future research should focus on identifying individuals at risk for metabolic side effects and on early markers to identify individuals with side effects so that timely treatment of metabolic complications can be initiated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Weight gain and body fat redistribution, including visceral fat accumulation, are common side effects of several widely used drugs, thereby contributing to the worldwide epidemic of overweight and obesity. Obesity and visceral fat accumulation are associated with increases in insulin resistance, dyslipidemia, metabolic syndrome, and the risk for type 2 diabetes (T2DM), non-alcoholic fatty liver disease (NAFLD), cardiovascular disease, cancer, and even increased mortality. These body weight and metabolic side effects warrant close monitoring and potentially additional therapies to minimize their health impact, thereby increasing medical costs and contributing to non-compliance, which risks worsening of the underlying condition.
Weight gain is consistently associated with many older agents for the treatment of diabetes and with neuropsychotropic medications, including atypical antipsychotics, antidepressants, and antiepileptic drugs [1].
The mechanisms behind these effects on body weight and fat distribution are often poorly understood, which hampers the identification of high-risk patients for prevention, development of lower risk-drugs, and possible treatments [2].
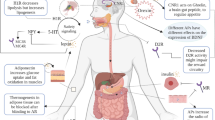
In this review, we focus on recent data on drugs affecting body weight, fat distribution, and glucometabolic outcomes, as well as the possible mechanisms contributing to these side effects (Fig. 1). Recent data, highlighting predictors that could identify those patients at risk for weight gain and metabolic complications, and options for prevention and therapy will be discussed.
Mechanisms of weight gain and metabolic disturbance. Schematic representation of the major pathways by which drugs affect body weight and metabolic disturbances. GCS, glucocorticosteroids; HPA, hypothalamic–pituitary–adrenal axis; TZD, thiazolidinediones; SAT, subcutaneous adipose tissue; IR, insulin resistance; GLUT, glucose transporter
Antidiabetic Drugs
Insulin, sulfonylurea (SU), and thiazolidinediones (TZD) may cause substantial weight gain when compared to placebo [1]. Metformin and dipeptidyl peptidase-4 (DPP-4) inhibitors are considered to be weight neutral, whereas sodium glucose cotransporter 2 inhibitors (SGLT2i) and glucagon-like peptide 1 (GLP-1) receptor analogues (GLP1RA) are associated with weight loss [3].
The mechanisms behind the weight gaining properties of insulin, SU, and TZD were reviewed comprehensively [2,3,4,5]. The weight-promoting properties of insulin are dose dependent and are more pronounced in injection regimens that include rapid-acting insulin compared to basal insulin only [6]. The addition of metformin to insulin therapy reduces the effects of insulin on body weight by decreasing energy intake [7].
Thiazolidinediones also cause a substantial time and dose-dependent weight gain ranging from 1.5 to 4 kg in the first year of treatment [1, 8]. However, TZD use is associated with almost uniquely subcutaneous fat accumulation, while amounts of visceral fat remain stable or even decrease. TZD’s improve hepatic steatosis and inflammation in patients with non-alcoholic steatohepatitis (NASH), although safety concerns including osteoporosis and bladder cancer with pioglitazone restricts its use [5, 9]. Newer PPAR modulators are currently being studied in diverse metabolic diseases including dyslipidemia, T2DM, and NASH [10, 11].
Newer drug classes, SGLT2 inhibitors, and GLP-1 RAs concomitantly target weight loss and glycemic control [12].
SGLT2i inhibits renal glucose reabsorption, promoting approximately 75 g of urinary glucose excretion with an associated caloric loss of approximately 300 kcal/day [13]. In patients with type 2 diabetes, SGLT2i produces a mean weight loss of 2 to 3 kg, irrespective of background therapy. In subjects with overweight or obesity and without diabetes, observed weight loss over 12 weeks was modest, resulting in approximately 1 to 1.5 kg of extra weight loss compared with placebo [14].
There is great interest as to why SGLT2i therapy is not associated with more pronounced decreases in weight considering the caloric loss accompanying the enhanced glucose excretion. Indeed, with an energy deficit of 2100 kcal/week, the expected weight loss over 24-week treatment with SGLT2i would be approximately 7 kg. In rodent models, chronic treatment with the SGLT2i resulted in a compensatory increase in caloric intake. Also in humans, modeling studies suggest that an increased energy intake may exceed adaptions in energy expenditure, contributing to difficulties with sustained weight loss. Chronic administration of SGLT2i causes a shift in fuel utilization from carbohydrate towards fatty substrates and loss of body fat, but no changes were seen in energy expenditure as measured by indirect calorimetry, implying that energy intake is increased to explain the observed discrepancies in weight loss [12].
All GLP-1 receptor agonists have the potential to decrease body mass index (BMI) by decreasing appetite and increasing satiety, by their interaction in brain areas involved in the homeostasis of food intake, energy expenditure, and energy balance [15]. There is a substantial difference in the weight losing effects among the different GLP-1 RA, with an average weight loss of 2–4 kg with exenatide, lixisenatide, liraglutide, and dulaglutide. Semaglutide, both in the weekly SC and in the daily oral formulations, is associated with higher weight losses [16]. Differences in uptake across the blood–brain barrier have been postulated as an explanation.
However, there is also markedly inter-individual variability regarding weight loss, with some subjects not losing any weight, while others losing up to 25 kg [12]. Most of this weight loss is maintained as long as the treatment is adhered to, while weight will regain when treatment with GLP-1 RA is interrupted. Gastrointestinal side effects are common with these classes of drugs. However, weight loss does not seem to correlate with the intensity of nausea and/or vomiting.
In phase 2 trials, several dual and tri-receptor agonist peptides, targeting GLP-1, glucagon, and glucose-dependent insulinotropic polypeptide (GIP) receptors, have been developed and show promising results in their effects on reductions of HbA1c and BMI as compared to placebo and GLP-1 RA monotherapy [17,18,19].
Psychotropic Medications
Obesity is two to three times more common among patients with psychiatric disorders than in the general population and individuals with overweight or obesity suffer more frequently from psychiatric illnesses than those with a normal weight. This interaction between obesity and psychiatric disease likely includes a clustering of adverse metabolic risk behaviors, such as unhealthy eating, insufficient physical activity, and substance abuse that accompanies many psychiatric conditions [20]. But psychiatric diseases also seem to share some pathophysiological brain pathways in common with those leading to weight gain and its metabolic complications, thereby creating a kind of vicious circle where each of the pathways influences the risk for the other [21]. Mounting evidence points to a critical role for chronic inflammatory processes including related alterations of brain functions and chronic stimulation of the hypothalamic–pituitary–adrenal (HPA) axis [21, 22].
Medical therapies for depression, mood disorders, and other psychiatric illnesses have been associated with sometimes very large increases in weight (Table 1). Epidemiologic data show a positive correlation between fat accumulation and the time exposed to psychotropic medication or the number of different psychotropic drugs used [23]. However, the variation in mean weight gain is large between the different drug classes and even within the same class. For most psychiatric treatments, no correlation is found with BMI increase and original diagnosis or severity of the underlying psychiatric condition, treatment outcome, BMI at the onset of the disease or treatment, age, or sex, which impedes prediction of those patients who will or will not have metabolic side effects [23]. What has been consistently shown is that early weight gain (in the first month after the start of treatment) is a strong predictor of the risk for long-term overweight and obesity [24]. Therefore, weight should be monitored before and shortly after starting a psychotropic drug and a 5% increase above baseline weight after the first month should prompt physicians to reconsider therapeutic options or to initiate weight-controlling strategies [24, 25].
Antidepressant Medications
Mean long-term weight changes of ≥ 5% of initial weight are seen with many antidepressant agents, irrespective of drug class. In contrast to older data where the highest obesogenic property was attributed to tricyclic agents, recent long-term prospective cohort data attribute most weight gain to mirtazapine, fluoxetine, citalopram, escitalopram, sertraline, paroxetine, trazodone, venlafaxine, and duloxetine. The magnitude of the weight increase significantly differs by class, and also within the same class of drugs, significant individual differences are reported. In most studies, no clear predisposing individual factors could be identified, although younger patients seem to be more prone to weight gain than those above 65 years [26].
Antidepressant drug–induced weight gain does not correlate with disease severity and also occurs when these drugs are used in other indications, such as neuropathic pain or anxiety [27].
Agomelatin, a melatonergic receptor agonist and postsynaptic serotonin antagonist, has been shown to be weight neutral [28].
Only bupropion, a norepinephrine and dopamine reuptake inhibitor and nicotinic antagonist, has systematically been associated with weight loss. Bupropion reduces appetite and food cravings [29]. In combination with naltrexone, it is approved as an anti-obesity drug in the USA and Europe [30]. Compared to placebo, bupropion/naltrexone combination therapy results in mean weight losses ranging between 2.5 and 5.2% of initial body weight [31].
Lithium
In randomized controlled trials, the majority of patients on lithium therapy for bipolar disorder gain more than 5% of initial body weight. Risk factors for weight gain are a high baseline weight, younger age, co-administration of antidepressants, and female sex. Weight gain also seems to be dose-dependent in some, but not all of the studies [32].
Antipsychotics
The number of individuals in the population receiving antipsychotic drugs is surprisingly high, most commonly for psychosis, although antipsychotic drugs are also widely used to treat other psychiatric conditions like bipolar disorders, attention deficit disorder, and dementia in the elderly [33, 34].
Typical or “conventional” neuroleptics (phenothiazines, butyrophenones, and thioxanthines) have been introduced since the 1950s and are effective antipsychotic drugs because they are dopamine D2 receptor antagonists, but they all have major neurological side effects [35]. Therefore, the atypical antipsychotics or second-generation antipsychotics (SGAP) are increasingly replacing the conventional neuroleptics. SGAPs are characterized by a combined activity on both the D2 and 5-HT2a receptors. Besides their antagonistic effects on these receptors, they possess diverse pharmacologic interactions with a number of other neurotransmitter receptors [36].
Up to 80% of patients taking antipsychotic medication experience weight gain that exceeds their ideal body weight by 20% or more [37]. The largest obesogenic effect is consistently associated with olanzapine and clozapine. Weight gain–promoting effects of the antipsychotics seem to be more pronounced in people with a normal body weight at baseline and more in women than in men. Weight gain is time- and dose-dependent and can be predicted by weight increases in the first weeks of treatment. Drug-naïve patients gain significantly more weight than patients exposed to antipsychotics in the past and studies in pediatric patients demonstrate greater absolute weight gain in this group than in adults. Patients who have greater treatment-emergent weight gain are more likely to benefit from treatment with antipsychotics [24, 38, 39].
Although an increased prevalence of metabolic syndrome has been reported in drug-naïve patients with diverse psychoses, there is a significant association with longer disease duration and with the intake of clozapine in particular [40, 41]. The development of glucose intolerance and diabetes in patients taking antipsychotics has also been reported in patients without significant weight changes [42]. In one 5-year study of clozapine-treated patients, 52% experienced one or more episodes of hyperglycemia and 30% were diagnosed as having type 2 diabetes [43]. SGAPs increase the synthesis of free fatty acids and triglycerides in the liver and induce hypertriglyceridemia, although newer SGAP appears to have fewer metabolic side effects [1, 2, 44, 45].
The induction of these metabolic alterations, dyslipidemia, metabolic syndrome, and type 2 diabetes, contribute to the higher incidence of cardiovascular disease in patients taking these drugs. People with psychosis have a 20% shorter life expectancy than the general population, mainly driven by an increase in cardiovascular disease [41, 46].
In view of the high cardiometabolic risk associated with antipsychotic drug use, the American Diabetes Association and American Psychiatric Association (ADA/APA) Consensus Development Conference recommends close monitoring of weight and metabolic and cardiovascular risk factors in all patients taking SGAPs [25].
Mechanisms of Psychotropic Induced Weight Changes and Metabolic Complications
For a detailed and comprehensive review on the different mechanisms explaining the metabolic side effects of SGAP, we refer to the recent work of Singh [47]. Different mechanisms explaining the weight gain have been studied, varying in importance between the different classes of psychotropic drugs. SGAP affects central and peripheral neurotransmitter function and acts on dopaminergic (DA), serotonergic (5-HT2), histaminergic (H1), adrenergic and muscarinic cholinergic (mACh), and cannabinoid (CB1) receptors, thereby influencing appetite and satiety, leading to hyperphagia, excess caloric consumption, and influencing energy homeostasis [36, 48]. The varying affinity of SGAP for these receptors and polymorphisms in the neuroreceptor genes partly explain the large differences in effects on weight by the different drugs [38].
Olanzapine and clozapine increase ghrelin release and the expression of the ghrelin receptor, further leading to decreased satiety and hyperphagia [49].
Psychosis itself is associated with altered levels of pro-inflammatory cytokines (e.g., IL-1, IL-6, and TNF-α). Alterations in the gut microbioma are proposed as another mechanism linking psychosis and obesity. Indeed, alterations in the gut microbioma influence the adaptive immune response and inflammation, which are implicated not only in the pathogenesis of psychosis but also in that of obesity and its metabolic complications [50]. Moreover, chronic SGAP use also causes dysbiosis of the gut, leading to an increased Firmicutes:Bacteroides ratio which has been associated with metabolic side effects. In rats, olanzapine-induced weight gain can be reversed by specific antibiotic therapy [51].
SGAPs are also associated with reduced adiponectin concentrations, possibly further adding to the pre-existing pro-inflammatory state and increasing insulin resistance. Moreover, central disruption of inflammatory cytokines and downregulation of neuroinflammation by SGAPs are associated with disturbances in anorexigenic pathways and increases in food intake [52].
SGAPs promote lipogenesis and enhance antilipolytic effects of insulin, thereby favoring lipid accumulation and adipocyte enlargement and inducing insulin resistance [40, 53, 54].
SGAPs are associated with a marked increase in insulin resistance in muscle, adipose tissue, and liver [48], possibly mediated by impaired GLUT-4 and GLUT-5 glucose transporter function [55]. In addition, a direct impairment of pancreatic β-cell function and decreased insulin secretion has been linked to the affinity of these drugs for the 5HT-1a and 5HT-2 serotonin receptors of the β-cells [48]. SGAPs decrease GLP-1 concentrations and as such increase glucagon synthesis and increase gluconeogenesis and glycogenolysis [42].
Therapy and Prevention of Antipsychotic Weight Gain
Many studies have evaluated pharmacological and non-pharmacological approaches to prevent or treat weight gain that accompanies SGAP treatment. Also, in SGAP-induced weight gain, diet and exercise represent the mainstay of weight loss treatment and positively impact metabolic complications and risk factors [56]. Benefits are thought to be the greatest when delivered as early as possible, before weight gain has occurred [57].
Switching to another antipsychotic drug with less potential for weight and cardiometabolic side effects has been endorsed by the ADA/APA consensus guidelines in those with more than 5% weight gain or worsening of their lipid or glycemia parameters [25, 58].
Reboxetine may reduce olanzapine-induced weight gain in schizophrenia patients. Weight and metabolic benefits have also been reported by switching to, or the addition of, topiramate, amantadine, fluvoxamine, and orlistat [59,60,61]. With metformin, an attenuation or reduction of weight gain and an amelioration of the metabolic side effects of SGAP therapy have been demonstrated, with greater benefits the earlier metformin was started [62]. In a rodent study, the addition of metformin and berberine prevented the loss of brown fat induced by olanzapine and was associated with favorable changes in the expression of several genes controlling energy expenditure [63].
In patients who are obese or those with diabetes, GLP-1 RA demonstrates long-lasting weight loss and benefits on glucose metabolism [1, 3]. Growing evidence suggests that patients who are overweight and those with psychosis exhibit similar structural brain changes, cognitive deficits, and central neuropeptide alterations, suggesting an overlap between the pathophysiological pathways of these disorders [64]. GLP-1 RAs have been shown to provide neuroprotective effects in cerebral degenerative diseases such as Parkinson’s disease, Huntington’s chorea, and Alzheimer’s dementia [64]. Liraglutide, a once-daily injected GLP-1 RA, reverses SGAP-induced weight gain, impaired glucose tolerance, metabolic side effects, and behavioral depression [65].
Betahistine (an H1 agonist), recently studied in monotherapy or in combination therapy with metformin, protects against SGAP-induced weight gain and hyperlipidemia through modulations of SREBP-1- and PPAR-α-dependent pathways [66, 67].
Mifepristone (a glucocorticoid and progestin receptor antagonist) attenuated increases in weight and reduced the metabolic changes induced by risperidone and olanzapine, suggesting mechanistic involvement of the hypothalamic–pituitary–adrenal axis in the weight and cardiometabolic side effects of antipsychotic medications [68].
The orally effective selective protein kinase C-β (PKC- β) inhibitor ruboxistaurin, which is used in treatment of diabetes-associated retinopathy and macular edema, attenuates the effects on adipose tissue differentiation by clozapine in rodents. If this is shown to be relevant for humans, it could offer a new target for the prevention of antipsychotic-induced weight gain [69].
Because of the associations between inflammation, adiposity, and psychiatric disease, other therapeutic options being explored to improve psychiatric symptoms without adverse metabolic sequelae include COX-2 selective non- steroidal anti-inflammatory drugs, and monoclonal antibodies against anti-TNF-α and interleukin-6 [70, 71].
Anti-seizure Drugs
Many of the antiepileptic treatments are associated with weight change. Most prominent effects are recorded with valproate and carbamazepine, inducing weight gain in 71% and 43% of the patients, respectively. Pregabalin and gabapentin can also induce weight gain and are of particular importance since they are used more and more in the treatment of neuropathic pain, including in patients with diabetes. Weight neutral antiepileptic drugs include lamotrigine, levetiracetam, and phenytoin. Some others are associated with weight loss, including felbamate, topiramate, and zonisamide [72, 73].
Greater weight gain is associated with longer duration of treatment with valproate, although most of the weight gain is observed within the first year, women, post-pubertal adolescents, and those who are overweight before treatment begin seem to be most susceptible to weight gain with valproate than men [74].
Valproate adversely affects glucose and lipid metabolism that contributes to weight-independent worsening of insulin resistance, decreased insulin secretion, and an increased risk for type 2 diabetes [75,76,77]. Besides its diabetogenic effect, valproate also induces other features of the metabolic syndrome (e.g., dyslipidemia) as well as endothelial dysfunction. Up to 60% of the patients taking valproate develop non-alcoholic steatohepatitis, further contributing to insulin resistance, chronic inflammation, and increased risk for cardiovascular disease [78].
With the other anticonvulsants, weight gain can be considerable in susceptible patients [72]. However, in contrast to valproate, metabolic side effects accompanying use of carbamazepine, pregabalin, and gabapentin are thought to be secondary to the induced weight gain rather than weight-independent mechanisms [79].
Topiramate and zonisamide have been shown to decrease body weight, even when studied in populations with obesity and overweight without any seizure history. Adding topiramate to another antiepileptic, antipsychotic, or antidepressant drug or changing anticonvulsant therapy for topiramate or zonisamide can help preventing or treating weight gain that accompanies psychiatric or anticonvulsant therapy [61, 80]. In the USA, the combination therapy topiramate/phentermine is approved as an anti-obesity drug [81].
β-Blockers
The obesogenic propensity of β-blockers has been known for years [82]. Their use is associated with a slight weight gain of 1 to 4 kg compared to controls, mostly occurring in the first few months of treatment [82]. Several large trials have linked β-receptor antagonists to dysglycemia and new-onset diabetes, even without significant weight gain [83]. Non-vasodilating beta-blockers (atenolol, metoprolol, and propranolol) in particular are associated with a worsening of glycemic and lipid parameters. In contrast, vasodilating beta-blockers (nebivolol, labetolol, and carvedilol) have more favorable metabolic effects [84].
Corticosteroids
Weight gain and a substantial increase in the risk for type 2 diabetes are generally known side effects of long-term treatment with glucocorticosteroids (GC) [85]. Self-reported data in patients using chronic corticoid therapy show weight gain in up to 70% of all patients [86]. The GC-induced weight gain can be massive, with over 10 kg increases in approximately 20% of patients in their first year of treatment, is dose-dependent, and significantly increases with intakes equivalent to 5 mg prednisone per day. Where older data suggested that inhaled and topical GCs were metabolically safe, recent data demonstrate the association of higher doses of inhaled GC with BMI increases almost twice higher in children with asthma, when compared to those children that were treated with GC-free treatments [87]. A significant positive association between exposure to topical CSs and new-onset T2DM was found in two large adult population-based cohort studies [88].
Glucocorticoids may induce an increase in food intake and dietary preference for high-caloric, high-fat “comfort foods” through changes in the activity of AMP-activated protein kinase in the hypothalamus [89,90,91]. GC decreases thermogenesis and uncoupling protein 1 (UCP-1) expression in brown adipose tissue, thereby influencing metabolic rate [92]. Chronic glucocorticoid therapy or a state of chronic hyperactivation of the hypothalamic–pituitary–adrenal (HPA) axis is associated with activation of the endocannabinoid (eCB) system, which is a potent regulator of food intake and decreases energy expenditure [93].
Glucocorticoids also affect body fat distribution by increasing visceral fat mass, thereby increasing insulin resistance and the risk for impaired glucose tolerance, diabetes, and cardiovascular disease [94]. Although the mechanisms are not completely understood, GC acutely stimulates lipolysis through the activation of hormone-sensitive lipase and an increased catecholamine responsiveness. When this disproportionately affects fat stores on extremities, it can lead to loss of these depots, or lipodystrophy, the risk of which has been reported to be higher among females and younger patients and increases with a higher baseline body mass index [95]. During chronic administration, GC promotes adipocyte hypertrophy by increasing synthesis and storage of lipids and enhances adipose tissue hyperplasia by increasing differentiation of preadipocytes to mature adipocytes. Visceral adipose tissue has a higher glucocorticoid receptor density as compared with other fat depots, which might favor enhanced expansion of visceral adipose tissue [94]. These differential effects on visceral and subcutaneous fat may be mediated by differential regulation of key metabolic genes including lipoprotein lipase, 11-beta-hydroxysteroid-dehydrogenase-1 (11β-HSD-1), and UCP-1 [94].
Glucocorticoid-induced overexpression of 11β-HSD-1 in adipose tissue leads to an increase of plasma triglycerides and cholesterol levels, while 11β-HSD-1 overexpression in liver promotes insulin resistance, hepatic steatosis, and increased lipid synthesis [96, 97].
In the liver, glucocorticoids act through peripheral stimulation of the cannabinoid-1 receptor (CB1R), inducing hepatic lipogenesis, steatosis, and dyslipidemia. By enhancing CB1R in adipose tissue, glucocorticoids induce insulin resistance (IR) and obesity. Blocking the peripheral CB1R attenuates all aspects of metabolic dysregulation by glucocorticoids, leading the path to potential therapeutic option by selective peripheral CB1R blockers [93]. These properties make specific 11β-HSD-1 inhibitors or peripheral CB1R blockers promising candidate drugs to reverse or prevent glucocorticoid-induced side effects.
Antiretroviral Therapy
Shortly after the introduction of antiretroviral therapy (ART) for the treatment of human immunodeficiency virus (HIV) disease, it became clear that patients on these medications often had disorders of fat storage and/or wasting (lipodystrophy). The term “HIV-associated lipodystrophy syndrome” was introduced to describe a typical loss of subcutaneous fat in the limbs and face (lipoatrophy) and central or truncal fat accumulation (lipohypertrophy). This typical form of lipodystrophy is merely attributed to the first-generation ART, stavudine (d4T), and zidovudine (AZT), in particular when used together with first-generation protease inhibitors (PIs). However, it still is important because these drugs are still in use in some (lower resource) countries. Moreover, these drugs have long-lasting side effects on body fat distribution and metabolic parameters, even years after the use of d4T and AZT has been interrupted [98]. In HIV-infected patients treated with the first-generation ART, the prevalence of lipodystrophy ranges from 10 to 80%, depending on the studied population, duration of ART treatment, and duration of HIV infection [99].
Adopting modern ART regimens has largely reduced the incidence of lipodystrophy and metabolic side effects have markedly evolved, shifting more to weight gain and fat accumulation.
Weight gain after initiation of ART is more difficult to define, since it is often difficult to distinguish between genuine weight gain by ART and the weight gain which is seen by improving infectious parameters and HIV itself. During the first decades of HIV, weight gain was associated with an improvement of the clinical status of the patient, by reversal of the catabolic state and wasting induced by the disease. However, multiple more recent studies also demonstrate that weight gain after starting anti-HIV treatment is most pronounced in patients with a low baseline weight and in those individuals with a high viral load and low white blood cell count, indicating the most advanced HIV status. In these patients, weight gain may therefore represent effective treatment of the condition. Yet, weight gain is frequent in patients treated with ART, even when therapy is started at a very early stage, as indicated by current guidelines. This is suggestive of weight gain by the treatment itself [100].
On the other hand, the prevalence of overweight and obesity in HIV patients reflects the trend in weight gain seen in uninfected patients and the prevalence of overweight and obesity are not higher in treated people with HIV than in the general Western population. Keeping this in mind, it could be that the obesogenic environment interacts with anti-HIV treatment in the documented increases overweight and obesity [101].
Since their development, PIs were associated with weight gain. However, a recent systematic review evaluating lipodystrophy risk with newer PIs showed neutral effects on peripheral lipoatrophy and conflicting effects on central lipohypertrophy [102]. Recent data report weight gain with the newest antiretrovirals such as integrase inhibitors, entry inhibitors, and more modern PIs and NNRTIs [100].
Where lipoatrophy is typical for HIV-infected patients and NRTI treatment, the metabolic consequences of the abdominal fat accumulation seen with other anti-HIV drugs are not different from those seen in the classical metabolic syndrome. In HIV-positive patients, however, the metabolic changes can also be seen without the typical abdominal fat accumulation. Metabolic consequences such as insulin resistance and dyslipidemia associated with ART seem to be more severe than can be expected from the perceived weight changes [103].
Since HIV is becoming a chronic disease with more and more patients surviving to longevity, these metabolic side effects pose a threat to a significant increase of the risk for diabetes, cardiovascular, and liver disease (e.g., steatosis and cirrhosis) in this population [104]. In the non-HIV-infected population, weight gain and obesity are well-documented risk factors for diabetes and CVD, and to premature mortality [105]. However, although overweight and obesity during HIV treatment definitely increases the risk of developing diabetes and CVD, a higher BMI has also been associated with more effective virological suppression, higher CD4+ counts, slower disease progression, and decreased mortality [100, 106, 107].
Mechanisms of HIV-Induced Lipodystrophy and Weight Gain
Lipodystrophy is considered to be multifactorial, resulting from the complex interaction of host characteristics, HIV-related effects, and antiretroviral drug-specific factors. While lipodystrophy is clearly linked to antiretroviral therapy, disturbances in adipose tissue gene expression are present in treatment-naïve patients with HIV, indicating that HIV-1 infection itself likely creates alterations in adipose tissue that are worsened by antiretroviral therapy [108, 109].
Adipose tissue serves as a reservoir for HIV virus, altering the adipose tissue environment and causing adipose tissue inflammation, dysfunction, and hypertrophy [108]. Therefore, it appears that HIV-1 infection itself initiates a first wave of alterations in adipose tissue that is amplified by ART.
The older NRTIs, stavudine and zidovudine in particular, inhibit mitochondrial DNA polymerase-γ within adipocytes causing mtDNA depletion and mitochondrial dysfunction and oxidative stress in subcutaneous adipose tissue (SAT). Together with a genetic predisposition (mt haplogroups) and mitochondrial dysfunction secondary to HIV itself, they inhibit adipogenesis and adipocyte differentiation, subsequently promoting apoptosis, adipose tissue inflammation, and fibrosis [110, 111].
Increases in proinflammatory cytokines inhibit adipocyte differentiation and increase apoptosis and lipolysis, finally inducing dyslipidemia and insulin resistance. In addition, increased fat tissue fibrosis and lipohypertrophy are associated with ectopic fat accumulation in liver, muscle, and heart, further increasing cardiometabolic complications [112, 113].
PIs are more closely associated with lipo-accumulation, especially increases in VAT, and interfere with adipocyte maturation and differentiation [114]. Older PIs (indinavir, lopinavir, and ritonavir in particular) induce hyperlipidemia and liver steatosis by excess fatty acid synthesis and altering the expression of several transcriptase genes (e.g., SREBP-1, PPAR γ,…) [113].
They are associated with abnormalities in glucose tolerance and their use is associated with a threefold increase in the risk of diabetes compared to other treatment options. PIs increase insulin resistance by interfering with the GLUT-4 glucose transporter and decrease insulin secretion through direct effects on β-cell function. Newer PIs like darunavir and atazanavir and integrase inhibitors (INSTIs) have only limited effects on glucose metabolism [113, 115].
Therapy of HIV-Associated Lipodystrophy
In patients with visceral fat accumulation and obesity, treatment is not different from that of the non-HIV infected population.
The use of the older NRTIs, stavudine, or zidovudine has decreased dramatically since the last decennium, thereby reducing the incidence of lipoatrophy. However, patients that have taken these drugs in the past can suffer from the complications of lipodystrophy years after withdrawal of these drugs. Switching from stavudine to other NRTIs has been shown to improve mitochondrial indices, reduce fat apoptosis, and decrease some adipose tissue markers of inflammation [113, 116]. A switch from NRTI and NNRTI to protease inhibitors showed no weight changes whereas a switch to newer integrase inhibitors may cause even greater weight gain [117].
Pituitary growth hormone (GH) secretion is decreased in HIV patients. The FDA approved tesamorelin, a recombinant human GH-releasing hormone, for the treatment of excess abdominal fat in HIV-infected patients. Tesamorelin decreases VAT by 15 to 18%, with a significant improvement of triglyceride and cholesterol levels. Despite this reduction in VAT, a small but statistically significant increase in HbA1c is seen in subjects receiving tesamorelin. Therefore, monitoring of IGF-1 and glycemic parameters is warranted [118].
Metformin is associated with small reductions of VAT in patients with glucose intolerance and abdominal obesity. However, in patients with lipoatrophy, metformin should be used with caution, since a further decrease of subcutaneous fat can be induced.
Doleglutavir increases plasma metformin concentration and total daily dose of metformin therefore should not exceed 1000 mg in patients treated with doleglutavir [99].
Glucose-intolerant patients on antiretroviral therapy display an altered GLP-1 response to oral glucose and GLP-1 RA may ameliorate the metabolic side effects associated with antiretroviral therapy [119, 120].
For patients with morbid obesity and/or major obesity-related diseases, bariatric surgery can be considered. An average of 20% reduction of initial BMI improved body composition and metabolic status was observed in patients after bariatric surgery, similarly to non-HIV patients with obesity. However, ART treatment should be monitored to control HIV infection and some ART doses should be adjusted following this degree of weight loss [121].
Lipodystrophy-associated changes in adipokine concentration could be the basis of future therapeutic options. Leptin and adiponectin decreases have been demonstrated in patients with lipodystrophy. Treatment with recombinant human leptin increases adiponectin levels and could potentially add a positive contribution in the treatment of insulin resistance, dyslipidemia, and hepatic steatosis in patients with HIV and lipodystrophy [122].
Conclusion
Many commonly used drugs, antipsychotics, antidepressants, and medications used in the treatment of HIV in particular have metabolic side effects such as weight gain and increase the risk of diabetes and cardiovascular disease. Although the frequency of metabolic complications appears to be lower for more recently developed products, it remains important to continue to pay attention to these metabolic complications, especially in view of the high frequency of obesity in the general population.
Where possible, the use of metabolically safe medications should be preferred over drugs with known side effects. It remains important to monitor patients treated with these drugs closely, to take preventative measures to avoid additional metabolic complications timely and to initiate weight-losing treatments when needed.
Additional research is needed in the search for efficient and early methods or biomarkers to identify those patients at the highest risk. Finally, it is important to set up thorough and large-scale studies to study the most efficient treatment of the metabolic complications in this target group.
References
Domecq JP, Prutsky G, Leppin A, Sonbol MB, Altayar O, Undavalli C, et al. Clinical review: drugs commonly associated with weight change: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100(2):363–70. https://doi.org/10.1210/jc.2014-3421.
Verhaegen AA, Van Gaal LF. Drug-induced obesity and its metabolic consequences: a review with a focus on mechanisms and possible therapeutic options. J Endocrinol Investig. 2017;40(11):1165–74. https://doi.org/10.1007/s40618-017-0719-6.
Van Gaal L, Scheen A. Weight management in type 2 diabetes: current and emerging approaches to treatment. Diabetes Care. 2015;38(6):1161–72. https://doi.org/10.2337/dc14-1630.
Russell-Jones D, Khan R. Insulin-associated weight gain in diabetes--causes, effects and coping strategies. Diabetes Obes Metab. 2007;9(6):799–812. https://doi.org/10.1111/j.1463-1326.2006.00686.x.
Aleman-Gonzalez-Duhart D, Tamay-Cach F, Alvarez-Almazan S, Mendieta-Wejebe JE. Current advances in the biochemical and physiological aspects of the treatment of type 2 diabetes mellitus with thiazolidinediones. PPAR Res. 2016;2016:7614270–10. https://doi.org/10.1155/2016/7614270.
Pontiroli AE, Miele L, Morabito A. Increase of body weight during the first year of intensive insulin treatment in type 2 diabetes: systematic review and meta-analysis. Diabetes Obes Metab. 2011;13(11):1008–19. https://doi.org/10.1111/j.1463-1326.2011.01433.x.
Makimattila S, Nikkila K, Yki-Jarvinen H. Causes of weight gain during insulin therapy with and without metformin in patients with type II diabetes mellitus. Diabetologia. 1999;42(4):406–12. https://doi.org/10.1007/s001250051172.
Medici V, McClave SA, Miller KR. Common medications which lead to unintended alterations in weight gain or organ lipotoxicity. Curr Gastroenterol Rep. 2016;18(1):2. https://doi.org/10.1007/s11894-015-0479-4.
Mills EP, Brown KPD, Smith JD, Vang PW, Trotta K. Treating nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus: a review of efficacy and safety. Ther Adv Endocrinol Metab. 2018;9(1):15–28. https://doi.org/10.1177/2042018817741852.
Gross B, Pawlak M, Lefebvre P, Staels B. PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nat Rev Endocrinol. 2017;13(1):36–49. https://doi.org/10.1038/nrendo.2016.135.
Cheng HS, Tan WR, Low ZS, Marvalim C, Lee JYH, Tan NS. Exploration and development of PPAR modulators in health and disease: an update of clinical evidence. Int J Mol Sci. 2019;20(20):5055. https://doi.org/10.3390/ijms20205055.
Brown E, Wilding JPH, Barber TM, Alam U, Cuthbertson DJ. Weight loss variability with SGLT2 inhibitors and GLP-1 receptor agonists in type 2 diabetes mellitus and obesity: mechanistic possibilities. Obes Rev. 2019;20(6):816–28. https://doi.org/10.1111/obr.12841.
Rajeev SP, Cuthbertson DJ, Wilding JP. Energy balance and metabolic changes with sodium-glucose co-transporter 2 inhibition. Diabetes Obes Metab. 2016;18(2):125–34. https://doi.org/10.1111/dom.12578.
Pereira MJ, Eriksson JW. Emerging role of SGLT-2 inhibitors for the treatment of obesity. Drugs. 2019;79(3):219–30. https://doi.org/10.1007/s40265-019-1057-0.
Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–705. https://doi.org/10.1016/s0140-6736(06)69705-5.
Nauck MA, Meier JJ. Management of endocrine disease: are all GLP-1 agonist equal in the treatment of type 2 diabetes? Eur J Endocrinol. 2019;181:R211–34. https://doi.org/10.1530/eje-19-0566.
Capozzi ME, DiMarchi RD, Tschop MH, Finan B, Campbell JE. Targeting the Incretin/glucagon system with triagonists to treat diabetes. Endocr Rev. 2018;39(5):719–38. https://doi.org/10.1210/er.2018-00117.
Bastin M, Andreelli F. Dual GIP-GLP1-receptor agonists in the treatment of type 2 diabetes: a short review on emerging data and therapeutic potential. Diabetes Metab Syndr Obes. 2019;12:1973–85. https://doi.org/10.2147/dmso.S191438.
Frias JP, Nauck MA, Van J, Benson C, Bray R, Cui X, et al. Efficacy and tolerability of tirzepatide, a dual glucose-dependent insulinotropic peptide and glucagon-like peptide-1 receptor agonist in patients with type 2 diabetes: a 12-week, randomized, double-blind, placebo-controlled study to evaluate different dose-escalation regimens. Diabetes Obes Metab. 2020. https://doi.org/10.1111/dom.13979.
Compton MT, Daumit GL, Druss BG. Cigarette smoking and overweight/obesity among individuals with serious mental illnesses: a preventive perspective. Harv Rev Psychiatry. 2006;14(4):212–22. https://doi.org/10.1080/10673220600889256.
Milaneschi Y, Simmons WK, van Rossum EFC, Penninx BW. Depression and obesity: evidence of shared biological mechanisms. Mol Psychiatry. 2018;24:18–33. https://doi.org/10.1038/s41380-018-0017-5.
Menard C, Pfau ML, Hodes GE, Russo SJ. Immune and neuroendocrine mechanisms of stress vulnerability and resilience. Neuropsychopharmacology. 2017;42(1):62–80. https://doi.org/10.1038/npp.2016.90.
Zimmermann U, Kraus T, Himmerich H, Schuld A, Pollmacher T. Epidemiology, implications and mechanisms underlying drug-induced weight gain in psychiatric patients. J Psychiatr Res. 2003;37(3):193–220.
Vandenberghe F, Gholam-Rezaee M, Saigi-Morgui N, Delacretaz A, Choong E, Solida-Tozzi A, et al. Importance of early weight changes to predict long-term weight gain during psychotropic drug treatment. J Clin Psychiatry. 2015;76(11):e1417–23. https://doi.org/10.4088/JCP.14m09358.
American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596–601. https://doi.org/10.2337/diacare.27.2.596.
Alonso-Pedrero L, Bes-Rastrollo M, Marti A. Effects of antidepressant and antipsychotic use on weight gain: a systematic review. Obes Rev. 2019;20(12):1680–90. https://doi.org/10.1111/obr.12934.
Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta-analysis. J Clin Psychiatry. 2010;71(10):1259–72. https://doi.org/10.4088/JCP.09r05346blu.
Laux G, Barthel B, Hajak G, Lemke M, Volz HP. Pooled analysis of four non-interventional studies: effectiveness and tolerability of the antidepressant agomelatine in daily practice. Adv Ther. 2017;34(4):895–914. https://doi.org/10.1007/s12325-017-0485-z.
Gadde KM, Xiong GL. Bupropion for weight reduction. Expert Rev Neurother. 2007;7(1):17–24. https://doi.org/10.1586/14737175.7.1.17.
Billes SK, Sinnayah P, Cowley MA. Naltrexone/bupropion for obesity: an investigational combination pharmacotherapy for weight loss. Pharmacol Res. 2014;84:1–11. https://doi.org/10.1016/j.phrs.2014.04.004.
Singh AK, Singh R. Pharmacotherapy in obesity: a systematic review and meta-analysis of randomized controlled trials of anti-obesity drugs. Expert Rev Clin Pharmacol. 2020;13(1):53–64. https://doi.org/10.1080/17512433.2020.1698291.
Gitlin M. Lithium side effects and toxicity: prevalence and management strategies. Int J Bipolar Disord. 2016;4(1):27. https://doi.org/10.1186/s40345-016-0068-y.
Sohn M, Moga DC, Blumenschein K, Talbert J. National trends in off-label use of atypical antipsychotics in children and adolescents in the United States. Medicine. 2016;95(23):e3784. https://doi.org/10.1097/md.0000000000003784.
Verdoux H, Tournier M, Begaud B. Antipsychotic prescribing trends: a review of pharmaco-epidemiological studies. Acta Psychiatr Scand. 2010;121(1):4–10. https://doi.org/10.1111/j.1600-0447.2009.01425.x.
Seeman P, Lee T, Chau-Wong M, Wong K. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature. 1976;261(5562):717–9.
Allison DB, Casey DE. Antipsychotic-induced weight gain: a review of the literature. J Clin Psychiatry. 2001;62(Suppl 7):22–31.
Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999;156(11):1686–96. https://doi.org/10.1176/ajp.156.11.1686.
Kaar SJ, Natesan S, McCutcheon R, Howes OD. Antipsychotics: mechanisms underlying clinical response and side-effects and novel treatment approaches based on pathophysiology. Neuropharmacology. 2020;172:107704. https://doi.org/10.1016/j.neuropharm.2019.107704.
Raben AT, Marshe VS, Chintoh A, Gorbovskaya I, Muller DJ, Hahn MK. The complex relationship between antipsychotic-induced weight gain and therapeutic benefits: a systematic review and implications for treatment. Front Neurosci. 2017;11:741. https://doi.org/10.3389/fnins.2017.00741.
Mitchell AJ, Vancampfort D, Sweers K, van Winkel R, Yu W, De Hert M. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders--a systematic review and meta-analysis. Schizophr Bull. 2013;39(2):306–18. https://doi.org/10.1093/schbul/sbr148.
De Hert M, Detraux J, van Winkel R, Yu W, Correll CU. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2011;8(2):114–26. https://doi.org/10.1038/nrendo.2011.156.
Teff KL, Rickels MR, Grudziak J, Fuller C, Nguyen HL, Rickels K. Antipsychotic-induced insulin resistance and postprandial hormonal dysregulation independent of weight gain or psychiatric disease. Diabetes. 2013;62(9):3232–40. https://doi.org/10.2337/db13-0430.
Henderson DC, Cagliero E, Gray C, Nasrallah RA, Hayden DL, Schoenfeld DA, et al. Clozapine, diabetes mellitus, weight gain, and lipid abnormalities: a five-year naturalistic study. Am J Psychiatry. 2000;157(6):975–81. https://doi.org/10.1176/appi.ajp.157.6.975.
Balf G, Stewart TD, Whitehead R, Baker RA. Metabolic adverse events in patients with mental illness treated with antipsychotics: a primary care perspective. Prim Care Companion J Clin Psychiatry. 2008;10(1):15–24.
Orsolini L, Tomasetti C, Valchera A, Vecchiotti R, Matarazzo I, Vellante F, et al. An update of safety of clinically used atypical antipsychotics. Expert Opin Drug Saf. 2016;15(10):1329–47. https://doi.org/10.1080/14740338.2016.1201475.
Daumit GL, Goff DC, Meyer JM, Davis VG, Nasrallah HA, McEvoy JP, et al. Antipsychotic effects on estimated 10-year coronary heart disease risk in the CATIE schizophrenia study. Schizophr Res. 2008;105(1–3):175–87. https://doi.org/10.1016/j.schres.2008.07.006.
Singh R, Bansal Y, Medhi B, Kuhad A. Antipsychotics-induced metabolic alterations: recounting the mechanistic insights, therapeutic targets and pharmacological alternatives. Eur J Pharmacol. 2019;844:231–40. https://doi.org/10.1016/j.ejphar.2018.12.003.
Ballon JS, Pajvani U, Freyberg Z, Leibel RL, Lieberman JA. Molecular pathophysiology of metabolic effects of antipsychotic medications. Trends Endocrinol Metab. 2014;25(11):593–600. https://doi.org/10.1016/j.tem.2014.07.004.
van der Zwaal EM, Merkestein M, Lam YK, Brans MA, Luijendijk MC, Bok LI, et al. The acute effects of olanzapine on ghrelin secretion, CCK sensitivity, meal size, locomotor activity and body temperature. Int J Obes. 2012;36(2):254–61. https://doi.org/10.1038/ijo.2011.97.
Nemani K, Hosseini Ghomi R, McCormick B, Fan X. Schizophrenia and the gut-brain axis. Prog Neuro-Psychopharmacol Biol Psychiatry. 2015;56:155–60. https://doi.org/10.1016/j.pnpbp.2014.08.018.
Skonieczna-Zydecka K, Loniewski I, Misera A, Stachowska E, Maciejewska D, Marlicz W, et al. Second-generation antipsychotics and metabolism alterations: a systematic review of the role of the gut microbiome. Psychopharmacology. 2019;236(5):1491–512. https://doi.org/10.1007/s00213-018-5102-6.
Fonseka TM, Muller DJ, Kennedy SH. Inflammatory cytokines and antipsychotic-induced weight gain: review and clinical implications. Mol Neuropsychiatry. 2016;2(1):1–14. https://doi.org/10.1159/000441521.
Vestri HS, Maianu L, Moellering DR, Garvey WT. Atypical antipsychotic drugs directly impair insulin action in adipocytes: effects on glucose transport, lipogenesis, and antilipolysis. Neuropsychopharmacology. 2007;32(4):765–72. https://doi.org/10.1038/sj.npp.1301142.
Holt RI, Peveler RC. Association between antipsychotic drugs and diabetes. Diabetes Obes Metab. 2006;8(2):125–35. https://doi.org/10.1111/j.1463-1326.2005.00495.x.
Dwyer DS, Donohoe D. Induction of hyperglycemia in mice with atypical antipsychotic drugs that inhibit glucose uptake. Pharmacol Biochem Behav. 2003;75(2):255–60.
Gurusamy J, Gandhi S, Damodharan D, Ganesan V, Palaniappan M. Exercise, diet and educational interventions for metabolic syndrome in persons with schizophrenia: a systematic review. Asian J Psychiatr. 2018;36:73–85. https://doi.org/10.1016/j.ajp.2018.06.018.
Alvarez-Jimenez M, Hetrick SE, Gonzalez-Blanch C, Gleeson JF, McGorry PD. Non-pharmacological management of antipsychotic-induced weight gain: systematic review and meta-analysis of randomised controlled trials. Br J Psychiatry J Ment Sci. 2008;193(2):101–7. https://doi.org/10.1192/bjp.bp.107.042853.
Mukundan A, Faulkner G, Cohn T, Remington G. Antipsychotic switching for people with schizophrenia who have neuroleptic-induced weight or metabolic problems. Cochrane Database Syst Rev. 2010;(12):Cd006629. https://doi.org/10.1002/14651858.CD006629.pub2.
Gierisch JM, Nieuwsma JA, Bradford DW, Wilder CM, Mann-Wrobel MC, McBroom AJ et al. AHRQ comparative effectiveness reviews. Interventions To Improve Cardiovascular Risk Factors in People With Serious Mental Illness. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013.
Mizuno Y, Suzuki T, Nakagawa A, Yoshida K, Mimura M, Fleischhacker WW, et al. Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2014;40(6):1385–403. https://doi.org/10.1093/schbul/sbu030.
Maayan L, Vakhrusheva J, Correll CU. Effectiveness of medications used to attenuate antipsychotic-related weight gain and metabolic abnormalities: a systematic review and meta-analysis. Neuropsychopharmacology. 2010;35(7):1520–30. https://doi.org/10.1038/npp.2010.21.
Hendrick V, Dasher R, Gitlin M, Parsi M. Minimizing weight gain for patients taking antipsychotic medications: the potential role for early use of metformin. Ann Clin Psychiatry. 2017;29(2):120–4.
Hu Y, Young AJ, Ehli EA, Nowotny D, Davies PS, Droke EA, et al. Metformin and berberine prevent olanzapine-induced weight gain in rats. PLoS One. 2014;9(3):e93310. https://doi.org/10.1371/journal.pone.0093310.
Ebdrup BH, Knop FK, Ishoy PL, Rostrup E, Fagerlund B, Lublin H, et al. Glucagon-like peptide-1 analogs against antipsychotic-induced weight gain: potential physiological benefits. BMC Med. 2012;10:92. https://doi.org/10.1186/1741-7015-10-92.
Siskind D, Hahn M, Correll CU, Fink-Jensen A, Russell AW, Bak N, et al. Glucagon-like peptide-1 receptor agonists for antipsychotic-associated cardio-metabolic risk factors: a systematic review and individual participant data meta-analysis. Diabetes Obes Metab. 2019;21(2):293–302. https://doi.org/10.1111/dom.13522.
Lian J, Huang XF, Pai N, Deng C. Ameliorating antipsychotic-induced weight gain by betahistine: mechanisms and clinical implications. Pharmacol Res. 2016;106:51–63. https://doi.org/10.1016/j.phrs.2016.02.011.
Kang D, Jing Z, Li R, Hei G, Shao T, Li L, et al. Effect of Betahistine and metformin on antipsychotic-induced weight gain: an analysis of two clinical trials. Front Psych. 2018;9:620. https://doi.org/10.3389/fpsyt.2018.00620.
Gross C, Blasey CM, Roe RL, Belanoff JK. Mifepristone reduces weight gain and improves metabolic abnormalities associated with risperidone treatment in normal men. Obesity (Silver Spring). 2010;18(12):2295–300. https://doi.org/10.1038/oby.2010.51.
Rimessi A, Pavan C, Ioannidi E, Nigro F, Morganti C, Brugnoli A, et al. Protein kinase C beta: a new target therapy to prevent the long-term atypical antipsychotic-induced weight gain. Neuropsychopharmacology. 2017;42(7):1491–501. https://doi.org/10.1038/npp.2017.20.
Capuron L, Lasselin J, Castanon N. Role of adiposity-driven inflammation in depressive morbidity. Neuropsychopharmacology. 2017;42(1):115–28. https://doi.org/10.1038/npp.2016.123.
Muller N. COX-2 inhibitors as antidepressants and antipsychotics: clinical evidence. Curr Opin Investig Drugs. 2010;11(1):31–42.
Ben-Menachem E. Weight issues for people with epilepsy--a review. Epilepsia. 2007;48(Suppl 9):42–5. https://doi.org/10.1111/j.1528-1167.2007.01402.x.
Grootens KP, Meijer A, Hartong EG, Doornbos B, Bakker PR, Al Hadithy A, et al. Weight changes associated with antiepileptic mood stabilizers in the treatment of bipolar disorder. Eur J Clin Pharmacol. 2018;74(11):1485–9. https://doi.org/10.1007/s00228-018-2517-2.
Verrotti A, D'Egidio C, Mohn A, Coppola G, Chiarelli F. Weight gain following treatment with valproic acid: pathogenetic mechanisms and clinical implications. Obes Rev. 2011;12(5):e32–43. https://doi.org/10.1111/j.1467-789X.2010.00800.x.
Belcastro V, D'Egidio C, Striano P, Verrotti A. Metabolic and endocrine effects of valproic acid chronic treatment. Epilepsy Res. 2013;107(1–2):1–8. https://doi.org/10.1016/j.eplepsyres.2013.08.016.
Aycicek A, Iscan A. The effects of carbamazepine, valproic acid and phenobarbital on the oxidative and antioxidative balance in epileptic children. Eur Neurol. 2007;57(2):65–9. https://doi.org/10.1159/000098053.
Wong HY, Chu TS, Lai JC, Fung KP, Fok TF, Fujii T, et al. Sodium valproate inhibits glucose transport and exacerbates Glut1-deficiency in vitro. J Cell Biochem. 2005;96(4):775–85. https://doi.org/10.1002/jcb.20555.
Farinelli E, Giampaoli D, Cenciarini A, Cercado E, Verrotti A. Valproic acid and nonalcoholic fatty liver disease: a possible association? World J Hepatol. 2015;7(9):1251–7. https://doi.org/10.4254/wjh.v7.i9.1251.
Swann AC. Major system toxicities and side effects of anticonvulsants. J Clin Psychiatry. 2001;62(Suppl 14):16–21.
Antel J, Hebebrand J. Weight-reducing side effects of the antiepileptic agents topiramate and zonisamide. Handb Exp Pharmacol. 2012;209:433–66. https://doi.org/10.1007/978-3-642-24716-3_20.
Smith SM, Meyer M, Trinkley KE. Phentermine/topiramate for the treatment of obesity. Ann Pharmacother. 2013;47(3):340–9. https://doi.org/10.1345/aph.1R501.
Sharma AM, Pischon T, Hardt S, Kunz I, Luft FC. Hypothesis: beta-adrenergic receptor blockers and weight gain: a systematic analysis. Hypertension. 2001;37(2):250–4.
Elliott WJ, Meyer PM. Incident diabetes in clinical trials of antihypertensive drugs: a network meta-analysis. Lancet. 2007;369(9557):201–7. https://doi.org/10.1016/s0140-6736(07)60108-1.
Fonseca VA. Effects of beta-blockers on glucose and lipid metabolism. Curr Med Res Opin. 2010;26(3):615–29. https://doi.org/10.1185/03007990903533681.
Rice JB, White AG, Scarpati LM, Wan G, Nelson WW. Long-term systemic corticosteroid exposure: a systematic literature review. Clin Ther. 2017;39(11):2216–29. https://doi.org/10.1016/j.clinthera.2017.09.011.
Curtis JR, Westfall AO, Allison J, Bijlsma JW, Freeman A, George V, et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum. 2006;55(3):420–6. https://doi.org/10.1002/art.21984.
Han J, Nguyen J, Kim Y, Geng B, Romanowski G, Alejandro L, et al. Effect of inhaled corticosteroid use on weight (BMI) in pediatric patients with moderate-severe asthma. J Asthma. 2019;56(3):263–9. https://doi.org/10.1080/02770903.2018.1455853.
Andersen YMF, Egeberg A, Ban L, Gran S, Williams HC, Francis NA, et al. Association between topical corticosteroid use and type 2 diabetes in two European population-based adult cohorts. Diabetes Care. 2019;42(6):1095–103. https://doi.org/10.2337/dc18-2158.
Christ-Crain M, Kola B, Lolli F, Fekete C, Seboek D, Wittmann G, et al. AMP-activated protein kinase mediates glucocorticoid-induced metabolic changes: a novel mechanism in Cushing's syndrome. FASEB J. 2008;22(6):1672–83. https://doi.org/10.1096/fj.07-094144.
Larsen PJ, Jessop DS, Chowdrey HS, Lightman SL, Mikkelsen JD. Chronic administration of glucocorticoids directly upregulates prepro-neuropeptide Y and Y1-receptor mRNA levels in the arcuate nucleus of the rat. J Neuroendocrinol. 1994;6(2):153–9.
Harfstrand A, Cintra A, Fuxe K, Aronsson M, Wikstrom AC, Okret S, et al. Regional differences in glucocorticoid receptor immunoreactivity among neuropeptide Y immunoreactive neurons of the rat brain. Acta Physiol Scand. 1989;135(1):3–9. https://doi.org/10.1111/j.1748-1716.1989.tb08544.x.
Soumano K, Desbiens S, Rabelo R, Bakopanos E, Camirand A, Silva JE. Glucocorticoids inhibit the transcriptional response of the uncoupling protein-1 gene to adrenergic stimulation in a brown adipose cell line. Mol Cell Endocrinol. 2000;165(1–2):7–15.
Bowles NP, Karatsoreos IN, Li X, Vemuri VK, Wood JA, Li Z, et al. A peripheral endocannabinoid mechanism contributes to glucocorticoid-mediated metabolic syndrome. Proc Natl Acad Sci U S A. 2015;112(1):285–90. https://doi.org/10.1073/pnas.1421420112.
Fardet L, Feve B. Systemic glucocorticoid therapy: a review of its metabolic and cardiovascular adverse events. Drugs. 2014;74(15):1731–45. https://doi.org/10.1007/s40265-014-0282-9.
Fardet L, Cabane J, Lebbe C, Morel P, Flahault A. Incidence and risk factors for corticosteroid-induced lipodystrophy: a prospective study. J Am Acad Dermatol. 2007;57(4):604–9. https://doi.org/10.1016/j.jaad.2007.04.018.
Dube S, Slama MQ, Basu A, Rizza RA, Basu R. Glucocorticoid excess increases hepatic 11beta-HSD-1 activity in humans: implications in steroid-induced diabetes. J Clin Endocrinol Metab. 2015;100(11):4155–62. https://doi.org/10.1210/jc.2015-2673.
Wolf G. Glucocorticoids in adipocytes stimulate visceral obesity. Nutr Rev. 2002;60(5 Pt 1):148–51.
Arrive E, Viard JP, Salanave B, Dollfus C, Matheron S, Reliquet V, et al. Metabolic risk factors in young adults infected with HIV since childhood compared with the general population. PLoS One. 2018;13(11):e0206745. https://doi.org/10.1371/journal.pone.0206745.
Mirza FS, Luthra P, Chirch L. Endocrinological aspects of HIV infection. J Endocrinol Investig. 2018;41(8):881–99. https://doi.org/10.1007/s40618-017-0812-x.
Kumar S, Samaras K. The impact of weight gain during HIV treatment on risk of pre-diabetes, diabetes mellitus, cardiovascular disease, and mortality. Front Endocrinol (Lausanne). 2018;9:705. https://doi.org/10.3389/fendo.2018.00705.
Koethe JR, Jenkins CA, Lau B, Shepherd BE, Justice AC, Tate JP, et al. Rising obesity prevalence and weight gain among adults starting antiretroviral therapy in the United States and Canada. AIDS Res Hum Retrovir. 2016;32(1):50–8. https://doi.org/10.1089/aid.2015.0147.
Guaraldi G, Stentarelli C, Zona S, Santoro A. HIV-associated lipodystrophy: impact of antiretroviral therapy. Drugs. 2013;73(13):1431–50. https://doi.org/10.1007/s40265-013-0108-1.
Feeney ER, Mallon PW. HIV and HAART-associated dyslipidemia. Open Cardiovasc Med J. 2011;5:49–63. https://doi.org/10.2174/1874192401105010049.
Nansseu JR, Bigna JJ, Kaze AD, Noubiap JJ. Incidence and risk factors for prediabetes and diabetes mellitus among HIV-infected Adults on Antiretroviral Therapy: A Systematic Review and Meta-analysis. Epidimiology. 2018;29(3):431–41. https://doi.org/10.1097/ede.0000000000000815.
Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444(7121):875–80. https://doi.org/10.1038/nature05487.
Sharma A, Hoover DR, Shi Q, Gustafson D, Plankey MW, Hershow RC, et al. Relationship between body mass index and mortality in HIV-infected HAART users in the women’s interagency HIV study. PLoS One. 2015;10(12):e0143740. https://doi.org/10.1371/journal.pone.0143740.
Koethe JR, Jenkins CA, Shepherd BE, Stinnette SE, Sterling TR. An optimal body mass index range associated with improved immune reconstitution among HIV-infected adults initiating antiretroviral therapy. Clin Infect Dis. 2011;53(9):952–60. https://doi.org/10.1093/cid/cir606.
Erlandson KM, Lake JE. Fat matters: understanding the role of adipose tissue in health in HIV infection. Curr HIV/AIDS Rep. 2016;13(1):20–30. https://doi.org/10.1007/s11904-016-0298-8.
Giralt M, Domingo P, Guallar JP, Rodriguez de la Concepcion ML, Alegre M, Domingo JC, et al. HIV-1 infection alters gene expression in adipose tissue, which contributes to HIV- 1/HAART-associated lipodystrophy. Antivir Ther. 2006;11(6):729–40.
Hulgan T, Haubrich R, Riddler SA, Tebas P, Ritchie MD, McComsey GA, et al. European mitochondrial DNA haplogroups and metabolic changes during antiretroviral therapy in AIDS Clinical Trials Group Study A5142. AIDS (London, England). 2011;25(1):37–47. https://doi.org/10.1097/QAD.0b013e32833f9d02.
Koczor CA, Lewis W. Nucleoside reverse transcriptase inhibitor toxicity and mitochondrial DNA. Expert Opin Drug Metab Toxicol. 2010;6(12):1493–504. https://doi.org/10.1517/17425255.2010.526602.
Caron-Debarle M, Lagathu C, Boccara F, Vigouroux C, Capeau J. HIV-associated lipodystrophy: from fat injury to premature aging. Trends Mol Med. 2010;16(5):218–29. https://doi.org/10.1016/j.molmed.2010.03.002.
Giralt M, Domingo P, Villarroya F. Adipose tissue biology and HIV-infection. Best Pract Res Clin Endocrinol Metab. 2011;25(3):487–99. https://doi.org/10.1016/j.beem.2010.12.001.
Flint OP, Noor MA, Hruz PW, Hylemon PB, Yarasheski K, Kotler DP, et al. The role of protease inhibitors in the pathogenesis of HIV-associated lipodystrophy: cellular mechanisms and clinical implications. Toxicol Pathol. 2009;37(1):65–77. https://doi.org/10.1177/0192623308327119.
Hruz PW. Molecular mechanisms for insulin resistance in treated HIV-infection. Best Pract Res Clin Endocrinol Metab. 2011;25(3):459–68. https://doi.org/10.1016/j.beem.2010.10.017.
Martin A, Smith DE, Carr A, Ringland C, Amin J, Emery S, et al. Reversibility of lipoatrophy in HIV-infected patients 2 years after switching from a thymidine analogue to abacavir: the MITOX extension study. AIDS. 2004;18(7):1029–36.
Norwood J, Turner M, Bofill C, Rebeiro P, Shepherd B, Bebawy S, et al. Brief report: weight gain in persons with HIV switched from efavirenz-based to integrase strand transfer inhibitor-based regimens. J Acquir Immune Defic Syndr. 2017;76(5):527–31. https://doi.org/10.1097/qai.0000000000001525.
Rochira V, Guaraldi G. Growth hormone deficiency and human immunodeficiency virus. Best Pract Res Clin Endocrinol Metab. 2017;31(1):91–111. https://doi.org/10.1016/j.beem.2017.02.006.
Culha MG, Inkaya AC, Yildirim E, Unal S, Serefoglu EC. Glucagon like peptide-1 receptor agonists may ameliorate the metabolic adverse effect associated with antiretroviral therapy. Med Hypotheses. 2016;94:151–3. https://doi.org/10.1016/j.mehy.2016.07.016.
Andersen O, Haugaard SB, Holst JJ, Deacon CF, Iversen J, Andersen UB, et al. Enhanced glucagon-like peptide-1 (GLP-1) response to oral glucose in glucose-intolerant HIV-infected patients on antiretroviral therapy. HIV Med. 2005;6(2):91–8. https://doi.org/10.1111/j.1468-1293.2005.00270.x.
Amouyal C, Buyse M, Lucas-Martini L, Hirt D, Genser L, Torcivia A, et al. Sleeve gastrectomy in morbidly obese HIV patients: focus on anti-retroviral treatment absorption after surgery. Obes Surg. 2018;28(9):2886–93. https://doi.org/10.1007/s11695-018-3308-7.
Polyzos SA, Perakakis N, Mantzoros CS. Fatty liver in lipodystrophy: a review with a focus on therapeutic perspectives of adiponectin and/or leptin replacement. Metab Clin Exp. 2019;96:66–82. https://doi.org/10.1016/j.metabol.2019.05.001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors report no conflict of interest relevant to this article.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Metabolism
Rights and permissions
About this article
Cite this article
Verhaegen, A.A., Van Gaal, L.F. Drugs Affecting Body Weight, Body Fat Distribution, and Metabolic Function—Mechanisms and Possible Therapeutic or Preventive Measures: an Update. Curr Obes Rep 10, 1–13 (2021). https://doi.org/10.1007/s13679-020-00419-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13679-020-00419-5