Abstract
Preclinical detection of Alzheimer’s disease is critical to determining at-risk individuals in order to improve patient and caregiver planning for their futures, and to identify individuals likely to benefit from treatment as advances in therapeutics develop over time. Identification of olfactory dysfunction at the preclinical and early stages of the disease is a potentially useful method to accomplish these goals. We first review basic olfactory circuitry. We then evaluate the evidence of pathophysiological change in the olfactory processing pathways during aging and Alzheimer’s disease in both human and animal models. We also review olfactory behavioral studies during these processes in both types of models. In doing so, we suggest hypotheses about the localization and mechanisms of olfactory dysfunction and identify important avenues for future work.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The predicted exponential rise in the aging population and Alzheimer’s disease (AD) prevalence has emboldened efforts to develop better therapies. It is therefore critical to elucidate the pathological mechanism in AD itself, as well as its relationship to aging, its biggest demographic risk factor. In addition, it has become imperative to develop strategies for pre-clinical detection, given the possibility that future therapies may be used to prevent rather than reverse the disease. Evidence of age-dependent decline in olfactory ability, and anatomical involvement of olfactory pathways and olfactory behavioral deficits early in AD have led to the idea that this sensory system may provide an interesting avenue by which to tackle these issues. Additionally, olfactory dysfunction is also implicated in Parkinson’s disease, reviewed recently [1], suggesting that olfactory pathways may be particularly vulnerable to neurodegenerative conditions. Here, we review the pathophysiological changes in olfactory circuits as well as deficits in olfactory-related behaviors during normal aging and AD, both in humans and in rodent models. In this manner, we provide a background by which to better understand the localization and pathological basis of olfactory deficits found in these interrelated processes, and to cite critical areas for future research.
Brief Overview of the Olfactory System
The olfactory pathway is summarized in Fig. 1 (reproduced with permission from Wolters Kluwer Health). For further detail see extensive reviews (e.g. [2]). Odors enter the nasal cavity, dissolve in the olfactory mucosa, and interact with olfactory receptor neurons (ORNs) in the epithelium via transmembrane G-protein coupled olfactory receptor (OR) proteins. Each ORN expresses one OR type, and ORNs expressing the like ORs project to the same glomeruli on the olfactory bulb (OB). Glomeruli are spherical structures in which ORN axons synapse onto apical dendrites of OB output neurons, which are mitral and tufted cells. This is modulated by periglomerular (PG) interneuron subtypes expressing GABA, dopamine, and other neurotransmitters. In the external plexiform layer of the OB, output is further modulated by granule cell (GC) inhibition delivered to lateral dendrites. Deeper layers of the OB include the mitral cell layer, internal plexiform layer, and granule cell layer. In rodents, PG and GCs are replenished by adult neurogenesis in the subventricular zone (SVZ). OB principal neurons subsequently project to higher structures via the lateral olfactory tract that include the anterior olfactory nucleus (AON), olfactory tubercle, amygdala, piriform cortex (PC), and entorhinal cortex (EC). From here, information flows to neocortical regions including the orbital or orbitofrontal region of the prefrontal cortex (PFC) that occurs directly or via the mediodorsal thalamus. The EC provides the primary input to the hippocampus. There are also recurrent pathways between these regions, and even back to the OB. Modulation by noradrenergic, serotoninergic, and cholinergic input from locus coeruleus, raphe nuclei, and nucleus basalis, respectively, occurs across all levels of the system.
Brief Overview of Olfactory Behavioral Assessment
Olfaction is tested in many ways in both humans and laboratory animals. Odor identification tests assess the ability to correctly name an odor, describe its quality, or choose descriptive words from a list. Examples include the University of Pennsylvania Smell Identification Test and the Brief Smell Identification Test. Details regarding these and other aspects of human olfactory testing have been reviewed [3]. Odor threshold is the minimum concentration for reliable detection, and sensitivity is inversely proportional to this. Discrimination is the ability to differentiate two or more odors. Social odor memory, tested in rodents, represents reduced exploration of an unfamiliar rodent with repeated exposure. Similarly, habituation is decreased responsiveness to a non-social odor upon repeated presentations, and dishabituation represents responsiveness to a habituated odor as if it were again novel. In olfactory reversal testing of animals, positive and negative rewards for discriminating between two odors are switched to assess cognitive flexibility. Olfactory working memory can be tested if a task requires the subject to actively remember a particular sequence of odors.
Pathophysiological Changes in the Olfactory Areas with Normal Aging and Alzheimer’s Disease in Humans
Few studies have examined human olfactory pathology with respect to normal aging. During senescence, the ORN population declines as olfactory epithelium is gradually replaced with respiratory epithelium [4]. ORNs from older versus young, live donors show reduced response specificity rather than decreased thresholds [5]. In the olfactory bulb, there are reports of either no decline in glomerular number [6], or reduction in glomeruli and mitral cells with age [7]. At the network level, late components of the olfactory event-related potential show increased latency with age, suggesting that central structures are involved [8]. Similarly, functional magnetic resonance imaging (MRI) during olfactory stimulation, identification, and discrimination tests in young and old patients have revealed abnormal blood-oxygen-level dependent (BOLD) responses in the piriform cortex, frontal, and temporal lobes of older subjects [9–13]. Therefore, olfactory decline with normal aging in humans appears to localize to the olfactory epithelium and higher cortical areas.
In AD, the human OB shows minimal mature amyloid plaque [14–23]. Neurofibrillary tangle (NFT) burden in the OB is typically higher [14, 15, 17, 20, 22], though sometimes also negligible [16, 19, 21, 23]. With disease progression, there is consistently more tau than beta amyloid-related pathology in the OB, but the likelihood and extent both increase with Braak stage [24]. In a study of brain tissue derived from AD patients and controls, pathology was reported in the OB at Braak stage 0 and 1, including in some controls [25]. This suggests that OB involvement can occur at early stages. It is unclear if controls represented pre-clinical AD or normal aging, as such changes have not been reported in studies of pure senescence discussed above.
The OB of patients with Alzheimer’s disease may show reduced volume compared to age-matched controls [16, 17]. All layers can be affected by NFTs and plaque, some noting a preponderance in the superficial layer [20, 21, 26]. Morphological changes have also been noted in the superficial strata. Specifically, there, the olfactory epithelium shows plaque [23, 26] and glomeruli may be hyperplastic [23] or reduced in area [18]. Processing may be further affected by an increase in dopaminergic interneurons [17] or loss of D2 dopamine receptors [21]. Mitral cell loss is evident in some studies but not others [16, 21–23]. These studies suggest that, similar to normal aging, the first layers of the peripheral olfactory system are particularly vulnerable to AD-related changes compared to the rest of the OB.
Compared to the OB, higher order regions display more extensive plaque and tangles [15]. Functional MRI and single-photon emission computerized tomography (SPECT) of AD patients have been useful to detail region-specific physiological implications, demonstrating abnormal BOLD responses and hypoperfusion, respectively, in the piriform cortex during various olfactory tests [27–29]. FDG-PET during an olfactory memory task in AD patients showed hypometabolism in the anterior medial temporal cortex [30]. Reduced odor identification, discrimination, and detection threshold in patients with early Alzheimer’s disease correlated with hypometabolism in a broad network involving parietal and frontal cortex, thalamus, and cerebellum [31]. In sum, these studies suggest that olfactory deficits in AD can correlate with dysfunction in higher cortical areas, in addition to or despite pathologic changes in the OB and epithelium.
Pathophysiological Changes in the Olfactory Areas in Aged Animals and Animal Models of Alzheimer’s Disease
Age-related changes in the olfactory system of rodents have been studied in animals greater than 2 years of age, at which point age-related cognitive impairment is most apparent. At this age, there is a decline in ORN density [32–34], but maintenance of individual ORN sensitivity [34]. Consequently, within the glomeruli, there is a reduction of synaptic interactions [33, 35]. In some mouse strains there is age-related proliferation of dopaminergic interneurons [36], despite decreased neurogenesis [37–39]. This could suggest age-related alteration in interneuron turnover. There are inconsistent findings regarding mitral cell stability and synapses with granule cells [35, 40, 41]. Overall, changes in the rodent appear similar to those in the human.
The aging piriform cortex shows a loss of AMPA receptors [42]. Research on the aging frontal and temporal lobe has been extensive and is beyond the scope of this review, though the prefrontal cortex and dentate gyrus have been implicated in age-related cognitive decline [43]. Few studies have approached these areas in relation to olfaction. Dysfunction of the cholinergic septohippocampal pathway leads to a deficit in social odor memory [44]. In-vivo neuronal recordings from the orbitofrontal cortex in older rats show abnormal rigidity of their responses to odors during reversal tasks [45]. The implication of rodent frontal and temporal lobe dysfunction with olfactory dysfunction is consistent with human studies.
There exist multiple mouse models of AD based on the overexpression of one or more amyloidogenic human genes, and these include the Tg2576, APP/PS1, and 5xFAD mice. The 3xTg mouse is unique because it also includes tau. We review features of these models, beginning peripherally and progressing centrally.
Dysmorphic ORN axon terminals can be seen in multiple models prior to the appearance of Aβ plaque in the epithelium [18], which does not form until 18 months in the Tg2576 mouse [46, 47]. Nonspecific immunoreactivity to Aβ can occur in the epithelium as early as 1–2 months, preceding insoluble plaque formation [48]. This suggests a role for ORN axonal dysfunction, via actions of soluble Aβ, in the activity-dependent pathophysiology of OB function. Indeed, glomerular area is reduced in the 5xFAD model prior to plaque appearance [18]. Additionally, mice in which APP is overexpressed only in ORNs demonstrate pre-plaque apoptosis in the epithelium, and disruption of axonal targeting and circuitry in the glomerulus [49, 50•, 51].
In contrast to the epithelium, the OB shows plaque formation as early as 3–4 months ([52, 53], [18, 47, 48], but see [54•]). Despite the earlier onset of amyloidogenesis in the glomerular layer, the granule cell layer is particularly susceptible to volume reduction and both soluble and insoluble Aβ [18, 46, 55, 56]. Interneuron markers are also reduced, with calretinin (CR) and somatostatin (SOM) affected prior to parvalbumin (PV) [56]. Regarding interneurons, neurogenesis has also been reported to be disrupted early, evidenced by hypoactivity, hyperactivity, or plaque in the SVZ ([18, 46, 57–59], but see [60]). A role for non-plaque related pathology is suggested by abnormal spontaneous electrical activity in the Tg2576 mouse at 3–4 months, prior to granule cell layer plaque deposition, which corresponds instead to abnormal odor-evoked activity at 6–7 months [55]. Lastly, the OB does not show tau immunoreactivity in the 3xTg mouse [54•].
The piriform cortex displays both signs of general amyloidogenesis, insoluble Aβ plaque, and also neurofibrillary tangles [48, 54•, 55, 61, 62]. In general, the progression of changes is temporally delayed when compared to the olfactory bulb and epithelium, beginning as early as 6–7 months, and layer III is particularly vulnerable [48, 55]. Similar to the OB, SOM- and CR-expression in interneurons decline first, followed by PV and calbindin (CB), prior to diffuse amyloidosis [63]. Also analogous to the OB, in the Tg2576 mouse there is aberrant spontaneous electrical activity in the piriform cortex at an early stage, followed by abnormal odor-evoked activity and hypoactivity at late stages that largely precedes insoluble plaque formation [64•].
Few studies have studied hippocampal and frontal cortical changes in reference to olfactory system pathology. In the Tg2576 and APP/PS1 model, changes occur later in hippocampus and entorhinal cortex than in the OB and epithelium, typically matching the time course of changes in the piriform cortex, and with the frontal cortex involved similarly or later [48, 55]. A reverse or alternative time course is suggested in the double APP mutation mouse and the 5xFAD model [18, 52, 65]. At 18 months, amyloid and tau-related changes are found in the orbitofrontal and entorhinal cortices, as well as in the hippocampus in the 3xTg model [54•].
In summary, mouse models of AD have been useful to study the effects of amyloid plaque and soluble Aβ on olfactory brain circuits. However, there are several limitations. First, AD models develop deficits before age-related dysfunction commences. Secondly, the temporal progression of pathology does not correlate well with human AD. Lastly, there has been minimal work on the role of tau, despite more NFTs than plaque in the olfactory system in humans. Regarding this, the lack of OB tau immunoreactivity in the 3xTg mouse may diminish its utility.
Olfactory Behavioral Testing in Humans with Normal Aging and Alzheimer’s Disease
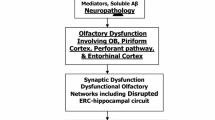
A summary of some key findings is provided in Table 1. We focus on recent studies with at least 50 total participants, and, if included, total longitudinal follow-up of no less than 1 year, typically with age matched controls. These primarily focus on Alzheimer’s disease and mild cognitive impairment (MCI) restricted to the memory domain, amnestic MCI, with odor detection identification as the primary test. Common to nearly all the studies is a correlation between baseline olfactory test scores and cognitive status, either quantitatively defined or qualitatively defined as MCI or dementia. Although this could be due to changes in the peripheral olfactory system detailed above, this has also been specifically associated with signs of functional and structural impairment related to the temporal lobe, including recall and hippocampal volume [73, 77, 83]. It should be noted that a general decline in odor identification with aging, especially above age 70, has long been documented [84]. Indeed, an inverse correlation with age and olfactory test scores is corroborated in some of these studies [73, 79]. However, contributions of true normal aging are difficult to ascertain in human subjects because it is unclear whether “controls” harbor preclinical Alzheimer-related changes and because the likelihood of such changes increases with age. This is evident with findings in the longitudinal studies that lower performance on odor identification or odor sensitivity tests predicts cognitive decline, specifically conversion from normal to MCI or from MCI to dementia [74–81, 82•]. Therefore, early olfactory impairment may signify early Alzheimer pathology and may be useful as part of a preclinical detection strategy. Olfactory identification deficits are associated with a 4–5 fold increased risk of conversion from MCI to AD, and appear to contribute unique variance in the prediction of conversion from MCI to AD, reinforcing the view that multiple biomarkers and clinical markers may need to be assessed to improve diagnostic and predictive accuracy [80].
Amyloid imaging with Pittsburgh compound B (PiB) revealed that the presence of amyloid did not distinguish olfactory identification scores within the amnestic MCI group, despite lower performance compared to control [69]. This may indicate that the neocortical amyloid plaque burden itself is not related to olfactory identification deficits, at least at this early stage, and the findings are consistent with the neuropathological findings of predominantly tau and not amyloid pathology in the olfactory bulb and other olfactory regions in the early stages of AD [22]. A neuropathological study also found that entorhinal cortex and CA1/subiculum tau burden predicted olfactory test scores better than plaque, though testing was done on average over 2 years prior to death [85]. Such findings would also further limit the utility of the predominantly amyloid-based mouse models.
Lastly, because other variants of MCI may signify neurodegenerative diseases other than Alzheimer’s disease or higher rates of conversion to dementia, MCI subtypes have also been examined. MCI can be amnestic, defined above, or non-amnestic, in which one cognitive domain other than memory is affected. Multidomain amnestic MCI involves memory and at least one other cognitive area, whereas multidomain non-amnestic MCI involves at least two domains other than memory. The results have been conflicting, either finding no difference in olfactory performance between MCI subtypes [71], distinct differences between them [73], or significant differences from controls only in multidomain amnestic MCI [72].
However, overall there is a clear increase in olfactory deficits from normal to MCI to AD [73]. In humans, these deficits occur initially in odor identification and progress to odor discrimination and odor sensitivity [67, 86]. This suggests that the early olfactory deficit in AD incorporates not only damage in the olfactory bulb but also in higher order olfactory pathways (e.g., piriform, hippocampal, entorhinal, orbitofrontal cortex) that subserve olfactory memory and interpretation of odors. More correlation with Braak stage is needed, though OB changes can indeed occur with early entorhinal and hippocampal changes [25].
Olfactory Behavioral Testing in Senescent Animals and Models of Alzheimer’s Disease
We first review the findings in studies of normal aging, in the progression of cognitive complexity of the task. Overall, behavioral deficits in animals with normal aging occur at older ages than the earliest deficits in mice with Alzheimer’s disease. As mentioned above, this should be interpreted in light of age-related pathology also occurring much later than Alzheimer changes. We first review studies in normal aging, and then studies with AD mice.
Odor sensitivity has been reported by some groups to decline with pure aging in rodents ([87, 88], but see [38, 89]). Odor discrimination, which involves processing in the OB and higher, is less consistently affected by age [38, 87, 88, 90, 91]. Odor habituation and dishabituation, which depend on piriform cortex function, were examined in one study of rats, which found intact habituation but impaired dishabituation at 22 months [92]. Olfactory-specific learning deficits have been reported in 2-year-old rodents [89, 93] and odor long-term memory, which may be cortical-dependent, was impaired in 2-year-old rats [93]. Performance on the olfactory reversal task, which is orbitofrontal cortex-dependent, declines with age as well [45]. Finally, social memory, which is highly olfactory-related in rodents, has been found to be impaired with senescence [44, 90]. Lesion studies implicated a role of hippocampal CA1 and the septohippocampal projection [44].
Regarding animal models of Alzheimer’s disease, a summary of some key studies is provided in Table 2. Overall, such studies have demonstrated behavioral deficits roughly correlating with temporal progression of pathology. Despite epithelial changes, general olfactory sensitivity is typically not affected ([46, 54•, 94, 95], but see [48, 98]). This may be because animals are tested prior to age-dependent decline in epithelial function. Nonetheless, the AD mouse model findings are consistent with human data showing early odor identification deficits in AD while odor sensitivity remains largely preserved [67]. Because each type of mouse displays slightly different Alzheimer pathology, we briefly review each model in the context of pathology reviewed more extensively above.
The 5xFAD mouse shows decreased performance on frontal-dependent tasks as early as 4 months, corresponding to early neocortical plaque burden in this model [18, 65]. The 3xTg mouse, with minimal OB changes but extensive amyloid and tau pathology in the limbic system and cortex, demonstrates impaired social transmission of odor above 12 months [54•]. The APP/PS1 model provides conflicting results. Some have found impairment in odor sensitivity at 3–4 months, spatial memory at 9–10 months, and odor habituation above 12 months, which roughly correlate with the progression of pathology from the epithelium and OB to the hippocampus and piriform cortex [48, 98]. However, others have found intact odor sensitivity, odor discrimination, and spatial memory, but learning impairments [94, 95]. The Tg2576 model demonstrates deficits in atypical odor habituation at 3 months, short-term odor memory at 6.5–8 months, odor discrimination at 6–7 months, short-term odor habituation at 12–16 months, and social odor memory at 23 months [46, 55, 64•, 96, 97]. This also maps to the progression of pathology from early non-fibrillar Ab in superficial layers of the OB to later onset pathology in higher cortical regions [55]. There is also a report of an early deficit in olfactory working memory at 4 months, despite unclear evidence of classical neocortical pathology at this age [96].
Conclusions
In summary, examination of structural and physiological decline in the olfactory pathways during normal aging has revealed some commonality between human and mouse models. This is critical because it may indicate brain regions or specific circuits that may be additionally susceptible to the Alzheimer’s disease process. The olfactory sensory neurons within the epithelium, and therefore glomerular level processing, appear to be particularly affected by aging. This may explain changes in odor sensitivity in advanced age. In addition, there is pathophysiological evidence in both species that the piriform cortex, frontal cortex, and temporal lobe regions are also predisposed to dysfunction during senescence. Further work is needed to elucidate the precise mechanisms by which aging induces altered olfactory processing within these regions, notably the oribitofrontal region of prefrontal cortex and the dentate gyrus of the hippocampus. Additionally, little is known regarding age-dependent perturbation of the piriform cortex and other primary olfactory cortices, for example at the level of circuit anatomy and physiology in the mouse and fMRI in human.
In AD, human and animal behavioral studies provide evidence that olfactory dysfunction can occur early in the disease process, even at a preclinical stage. In mice, a temporal sequence of olfactory deficits can sometimes correlate with disease spread. However, this spread does not correlate well to the human Braak stages. Human studies have shown progression from odor identification deficits to odor discrimination and odor sensitivity deficits as the disease process in AD leads to clinical manifestations, as well as strong predictive utility for conversion from normal to MCI and from MCI to AD. The relationship between olfactory deficits and another predictive risk factor, the ε4 allele of the apolipoprotein E gene (APO-ε4), is unclear and warrants further research. Some studies find that APO-ε4 carriers perform worse on olfactory identification at baseline [75, 99, 100], whereas another study did not [101]. APO-ε4 status appears to have an additive effect to olfactory dysfunction alone for predicting cognitive decline [75], though it has also been associated with low scores independent of dementia conversion [100]. This may suggest an additional role for apolipoprotein E in normal olfactory processing. Additionally, though there is evidence for preclinical utility in sporadic AD, in a small clinical sample of family members of AD patients with the autosomal dominant presenelin-1 mutation, smell testing was not predictive for conversion to dementia [102]. Further validation is needed with larger populations, but this could suggest that olfactory involvement can be different in AD variants. This may also partly explain inconsistencies within mouse models that utilize different human amyloidogenic genes.
Furthermore, regional brain specificity, neurophysiology, and correlation to Braak stage are not as well defined in human AD studies. Further exploration is needed regarding the role of various kinds of pathology and which brain region most contributes to deficits at these different stages. At an early stage, the behavioral effects of olfactory bulb and sensory neuron dysfunction, given early Alzheimer changes and age-related susceptibility in both species, seem plausible from animal studies but are not clear from human work. However, a major discrepancy is that tau pathology in the OB, prominent in the human, has not been demonstrated in the mouse models. In humans, evidence currently supports a role for the piriform cortex and medial temporal lobe, apart from the OB. More studies coupling amyloid imaging with olfactory testing at the preclinical stage, with longitudinal followup, would be helpful to more clearly define the role of amyloid plaque in perturbing neocortical odor processing. Additionally, other forms of pathology, especially tau, soluble Aβ, and subtle circuit perturbations, have been studied to various extents in animal work but their contribution to olfactory behavioral deficits in the human remain elusive, though this may change as tau PET ligands are developed. Further experiments regarding these issues are needed in the mouse model while technical hurdles to examine them in human need to be addressed.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Doty RL. Olfactory dysfunction in Parkinson disease. Nat Rev Neurol. 2012;8:329–39.
Shepherd GM, et al. Olfactory Bulb. In: Shepherd GM, editors. Synaptic Organization of the Brain. Oxford, New York, 2004. pp. 165-216.
Doty RL. Office procedures for quantitative assessment of olfactory function. Am J Rhinol. 2007;21:460–73.
Paik SI, Lehman MN, Seiden AM, Duncan HJ, Smith DV. Human olfactory biopsy. The influence of age and receptor distribution. Arch Otolaryngol Head Neck Surg. 1992;118:731–8.
Rawson NE, Gomez G, Cowart BJ, Kriete A, Pribitkin E, Restrepo D. Age-associated loss of selectivity in human olfactory sensory neurons. Neurobiol Aging. 2012;33:1913–9.
Maresh A, Rodriguez Gil D, Whitman MC, Greer CA. Principles of glomerular organization in the human olfactory bulb–implications for odor processing. PLoS One. 2008;3:e2640.
Meisami E, Mikhail L, Baim D, Bhatnagar KP. Human olfactory bulb: aging of glomeruli and mitral cells and a search for the accessory olfactory bulb. Ann N Y Acad Sci. 1998;855:708–15.
Morgan CD, Geisler MW, Covington JW, Polich J, Murphy C. Olfactory P3 in young and older adults. Psychophysiology. 1999;36:281–7.
Yousem DM, Maldjian JA, Hummel T, Alsop DC, Geckle RJ, Kraut MA, et al. The effect of age on odor-stimulated functional MR imaging. AJNR Am J Neuroradiol. 1999;20:600–8.
Suzuki Y, Critchley HD, Suckling J, Fukuda R, Williams SC, Andrew C, et al. Functional magnetic resonance imaging of odor identification: the effect of aging. J Gerontol A Biol Sci Med Sci. 2001;56:M756–760.
Kareken DA, Mosnik DM, Doty RL, Dzemidzic M, Hutchins GD. Functional anatomy of human odor sensation, discrimination, and identification in health and aging. Neuropsychology. 2003;17:482–95.
Cerf-Ducastel B, Murphy C. FMRI brain activation in response to odors is reduced in primary olfactory areas of elderly subjects. Brain Res. 2003;986:39–53.
Wang J, Eslinger PJ, Smith MB, Yang QX. Functional magnetic resonance imaging study of human olfaction and normal aging. J Gerontol A Biol Sci Med Sci. 2005;60:510–4.
Mann DM, Tucker CM, Yates PO. Alzheimer's disease: an olfactory connection? Mech Ageing Dev. 1988;42:1–15.
Reyes PF, Deems DA, Suarez MG. Olfactory-related changes in Alzheimer's disease: a quantitative neuropathologic study. Brain Res Bull. 1993;32:1–5.
ter Laak HJ, Renkawek K, van Workum FP. The olfactory bulb in Alzheimer disease: a morphologic study of neuron loss, tangles, and senile plaques in relation to olfaction. Alzheimer Dis Assoc Disord. 1994;8:38–48.
Mundinano IC, Caballero MC, Ordonez C, Hernandez M, DiCaudo C, Marcilla I, et al. Increased dopaminergic cells and protein aggregates in the olfactory bulb of patients with neurodegenerative disorders. Acta Neuropathol. 2011;122:61–74.
Cai Y, Xue ZQ, Zhang XM, Li MB, Wang H, Luo XG, et al. An age-related axon terminal pathology around the first olfactory relay that involves amyloidogenic protein overexpression without plaque formation. Neuroscience. 2012;215:160–73.
Esiri MM, Wilcock GK. The olfactory bulbs in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1984;47:56–60.
Ohm TG, Braak H. Olfactory bulb changes in Alzheimer's disease. Acta Neuropathol. 1987;73:365–9.
Loopuijt LD, Sebens JB. Loss of dopamine receptors in the olfactory bulb of patients with Alzheimer's disease. Brain Res. 1990;529:239–44.
Hyman BT, Arriagada PV, Van Hoesen GW. Pathologic changes in the olfactory system in aging and Alzheimer's disease. Ann N Y Acad Sci. 1991;640:14–9.
Struble RG, Clark HB. Olfactory bulb lesions in Alzheimer's disease. Neurobiol Aging. 1992;13:469–73.
Attems J, Lintner F, Jellinger KA. Olfactory involvement in aging and Alzheimer's disease: an autopsy study. J Alzheimers Dis. 2005;7:149–57. discussion 173-180.
Kovacs T, Cairns NJ, Lantos PL. Olfactory centres in Alzheimer's disease: olfactory bulb is involved in early Braak's stages. Neuroreport. 2001;12:285–8.
Kovacs T, Cairns NJ, Lantos PL. beta-amyloid deposition and neurofibrillary tangle formation in the olfactory bulb in ageing and Alzheimer's disease. Neuropathol Appl Neurobiol. 1999;25:481–91.
Li W, Howard JD, Gottfried JA. Disruption of odour quality coding in piriform cortex mediates olfactory deficits in Alzheimer's disease. Brain. 2010;133:2714–26.
Wang J, Eslinger PJ, Doty RL, Zimmerman EK, Grunfeld R, Sun X, et al. Olfactory deficit detected by fMRI in early Alzheimer's disease. Brain Res. 2010;1357:184–94.
Kareken DA, Doty RL, Moberg PJ, Mosnik D, Chen SH, Farlow MR, et al. Olfactory-evoked regional cerebral blood flow in Alzheimer's disease. Neuropsychology. 2001;15:18–29.
Buchsbaum MS, Kesslak JP, Lynch G, Chui H, Wu J, Sicotte N, et al. Temporal and hippocampal metabolic rate during an olfactory memory task assessed by positron emission tomography in patients with dementia of the Alzheimer type and controls. Preliminary studies. Arch Gen Psychiatry. 1991;48:840–7.
Forster S, Vaitl A, Teipel SJ, Yakushev I, Mustafa M, la Fougere C, et al. Functional representation of olfactory impairment in early Alzheimer's disease. J Alzheimers Dis. 2010;22:581–91.
Meisami E. A proposed relationship between increases in the number of olfactory receptor neurons, convergence ratio and sensitivity in the developing rat. Brain Res Dev Brain Res. 1989;46:9–19.
Hinds JW, McNelly NA. Aging in the rat olfactory system: correlation of changes in the olfactory epithelium and olfactory bulb. J Comp Neurol. 1981;203:441–53.
Lee AC, Tian H, Grosmaitre X, Ma M. Expression patterns of odorant receptors and response properties of olfactory sensory neurons in aged mice. Chem Senses. 2009;34:695–703.
Richard MB, Taylor SR, Greer CA. Age-induced disruption of selective olfactory bulb synaptic circuits. Proc Natl Acad Sci U S A. 2010;107:15613–8.
Mirich JM, Williams NC, Berlau DJ, Brunjes PC. Comparative study of aging in the mouse olfactory bulb. J Comp Neurol. 2002;454:361–72.
Tropepe V, Craig CG, Morshead CM, van der Kooy D. Transforming growth factor-alpha null and senescent mice show decreased neural progenitor cell proliferation in the forebrain subependyma. J Neurosci. 1997;17:7850–9.
Enwere E, Shingo T, Gregg C, Fujikawa H, Ohta S, Weiss S. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J Neurosci. 2004;24:8354–65.
Mobley AS, Bryant AK, Richard MB, Brann JH, Firestein SJ, Greer CA. Age-dependent regional changes in the rostral migratory stream. Neurobiol Aging. 2013;34:1873–81.
Hinds JW, McNelly NA. Aging in the rat olfactory bulb: quantitative changes in mitral cell organelles and somato-dendritic synapses. J Comp Neurol. 1979;184:811–20.
Forbes WB. Aging-related morphological changes in the main olfactory bulb of the Fischer 344 rat. Neurobiol Aging. 1984;5:93–9.
Gocel J, Larson J. Evidence for loss of synaptic AMPA receptors in anterior piriform cortex of aged mice. Front Aging Neurosci. 2013;5:39.
Samson RD, Barnes CA. Impact of aging brain circuits on cognition. Eur J Neurosci. 2013;37:1903–15.
Terranova JP, Perio A, Worms P, Le Fur G, Soubrie P. Social olfactory recognition in rodents: deterioration with age, cerebral ischaemia and septal lesion. Behav Pharmacol. 1994;5:90–8.
Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding changes in orbitofrontal cortex in reversal-impaired aged rats. J Neurophysiol. 2006;95:1509–17.
Guerin D, Sacquet J, Mandairon N, Jourdan F, Didier A. Early locus coeruleus degeneration and olfactory dysfunctions in Tg2576 mice. Neurobiol Aging. 2009;30:272–83.
Kameshima N, Nanjou T, Fukuhara T, Yanagisawa D, Tooyama I. Correlation of Abeta deposition in the nasal cavity with the formation of senile plaques in the brain of a transgenic mouse model of Alzheimer's disease. Neurosci Lett. 2012;513:166–9.
Wu N, Rao X, Gao Y, Wang J, Xu F. Amyloid-beta deposition and olfactory dysfunction in an Alzheimer's disease model. J Alzheimers Dis. 2013;37:699–712.
Cheng N, Cai H, Belluscio L. In vivo olfactory model of APP-induced neurodegeneration reveals a reversible cell-autonomous function. J Neurosci. 2011;31:13699–704.
Cao L, Schrank BR, Rodriguez S, Benz EG, Moulia TW, Rickenbacher GT, et al. Abeta alters the connectivity of olfactory neurons in the absence of amyloid plaques in vivo. Nat Commun. 2012;3:1009. By using a mouse model in which human APP is expressed only in olfactory receptor neurons, the authors demonstrate disruption of the peripheral olfactory circuits in the absence of Aβ plaque.
Cheng N, Bai L, Steuer E, Belluscio L. Olfactory functions scale with circuit restoration in a rapidly reversible Alzheimer's disease model. J Neurosci. 2013;33:12208–17.
Rockenstein E, Mallory M, Mante M, Sisk A, Masliaha E. Early formation of mature amyloid-beta protein deposits in a mutant APP transgenic model depends on levels of Abeta (1-42). J Neurosci Res. 2001;66:573–82.
Lehman EJ, Kulnane LS, Lamb BT. Alterations in beta-amyloid production and deposition in brain regions of two transgenic models. Neurobiol Aging. 2003;24:645–53.
Cassano T, Romano A, Macheda T, Colangeli R, Cimmino CS, Petrella A, et al. Olfactory memory is impaired in a triple transgenic model of Alzheimer disease. Behav Brain Res. 2011;224:408–12. Most animal models of Alzheimer’s disease utilize amyloidogenic genes. Here, odor-based memory testing was performed in a transgenic mouse expressing both Aβ and tau pathology. Severe deficits were found, but pathology was limited to higher order olfactory centers and not the olfactory bulb.
Wesson DW, Levy E, Nixon RA, Wilson DA. Olfactory dysfunction correlates with amyloid-beta burden in an Alzheimer's disease mouse model. J Neurosci. 2010;30:505–14.
Saiz-Sanchez D, De La Rosa-Prieto C, Ubeda-Banon I, Martinez-Marcos A. Interneurons and beta-amyloid in the olfactory bulb, anterior olfactory nucleus and olfactory tubercle in APPxPS1 transgenic mice model of Alzheimer's disease. Anat Rec (Hoboken). 2013;296:1413–23.
Haughey NJ, Liu D, Nath A, Borchard AC, Mattson MP. Disruption of neurogenesis in the subventricular zone of adult mice, and in human cortical neuronal precursor cells in culture, by amyloid beta-peptide: implications for the pathogenesis of Alzheimer's disease. Neuromol Med. 2002;1:125–35.
Demars M, Hu YS, Gadadhar A, Lazarov O. Impaired neurogenesis is an early event in the etiology of familial Alzheimer's disease in transgenic mice. J Neurosci Res. 2010;88:2103–17.
Sotthibundhu A, Li QX, Thangnipon W, Coulson EJ. Abeta(1-42) stimulates adult SVZ neurogenesis through the p75 neurotrophin receptor. Neurobiol Aging. 2009;30:1975–85.
Zhang C, McNeil E, Dressler L, Siman R. Long-lasting impairment in hippocampal neurogenesis associated with amyloid deposition in a knock-in mouse model of familial Alzheimer's disease. Exp Neurol. 2007;204:77–87.
Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G, et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293:1487–91.
Kim TK, Lee JE, Park SK, Lee KW, Seo JS, Im JY, et al. Analysis of differential plaque depositions in the brains of Tg2576 and Tg-APPswe/PS1dE9 transgenic mouse models of Alzheimer disease. Exp Mol Med. 2012;44:492–502.
Saiz-Sanchez D, Ubeda-Banon I, De la Rosa-Prieto C, Martinez-Marcos A. Differential expression of interneuron populations and correlation with amyloid-beta deposition in the olfactory cortex of an AbetaPP/PS1 transgenic mouse model of Alzheimer's disease. J Alzheimers Dis. 2012;31:113–29.
Wesson DW, Borkowski AH, Landreth GE, Nixon RA, Levy E, Wilson DA. Sensory network dysfunction, behavioral impairments, and their reversibility in an Alzheimer's beta-amyloidosis mouse model. J Neurosci. 2011;31:15962–71. The investigators used the Tg2576 Alzheimer mouse to demonstrate that aberrant electrical activity in the OB coincides with amyloid in that region, but prior to the behavioral deficits that coincide with piriform cortex dysfunction.
Girard SD, Baranger K, Gauthier C, Jacquet M, Bernard A, Escoffier G, et al. Evidence for early cognitive impairment related to frontal cortex in the 5XFAD mouse model of Alzheimer's disease. J Alzheimers Dis. 2013;33:781–96.
Eibenstein A, Fioretti AB, Simaskou MN, Sucapane P, Mearelli S, Mina C, et al. Olfactory screening test in mild cognitive impairment. Neurol Sci. 2005;26:156–60.
Djordjevic J, Jones-Gotman M, De Sousa K, Chertkow H. Olfaction in patients with mild cognitive impairment and Alzheimer's disease. Neurobiol Aging. 2008;29:693–706.
Laakso MP, Tervo S, Hanninen T, Vanhanen M, Hallikainen M, Soininen H. Olfactory identification in non-demented elderly population and in mild cognitive impairment: a comparison of performance in clinical odor identification versus Boston Naming Test. J Neural Transm. 2009;116:891–5.
Bahar-Fuchs A, Chetelat G, Villemagne VL, Moss S, Pike K, Masters CL, et al. Olfactory deficits and amyloid-beta burden in Alzheimer's disease, mild cognitive impairment, and healthy aging: a PiB PET study. J Alzheimers Dis. 2010;22:1081–7.
Velayudhan L, Pritchard M, Powell JF, Proitsi P, Lovestone S. Smell identification function as a severity and progression marker in Alzheimer's disease. Int Psychogeriatr. 2013;25:1157–66. This group investigated whether olfactory decline correlated with cognitive progression and illness severity in AD patients. AD patients with recent rapid cognitive progression were found to be associated with rapid olfactory decline at 3 months follow-up, whereas non-rapid cognitive progression was associated with slow olfactory decline. Rapid olfactory decline with higher olfactory dysfunction correlated with high illness severity.
Westervelt HJ, Bruce JM, Coon WG, Tremont G. Odor identification in mild cognitive impairment subtypes. J Clin Exp Neuropsychol. 2008;30:151–6.
Lehrner J, Pusswald G, Gleiss A, Auff E, Dal-Bianco P. Odor identification and self-reported olfactory functioning in patients with subtypes of mild cognitive impairment. Clin Neuropsychol. 2009;23:818–30.
Devanand DP, Tabert MH, Cuasay K, Manly JJ, Schupf N, Brickman AM, et al. Olfactory identification deficits and MCI in a multi-ethnic elderly community sample. Neurobiol Aging. 2010;31:1593–600.
Bacon AW, Bondi MW, Salmon DP, Murphy C. Very early changes in olfactory functioning due to Alzheimer's disease and the role of apolipoprotein E in olfaction. Ann N Y Acad Sci. 1998;855:723–31.
Graves AB, Bowen JD, Rajaram L, McCormick WC, McCurry SM, Schellenberg GD, et al. Impaired olfaction as a marker for cognitive decline: interaction with apolipoprotein E epsilon4 status. Neurology. 1999;53:1480–7.
Devanand DP, Michaels-Marston KS, Liu X, Pelton GH, Padilla M, Marder K, et al. Olfactory deficits in patients with mild cognitive impairment predict Alzheimer's disease at follow-up. Am J Psychiatry. 2000;157:1399–405.
Royall DR, Chiodo LK, Polk MS, Jaramillo CJ. Severe dysosmia is specifically associated with Alzheimer-like memory deficits in nondemented elderly retirees. Neuroepidemiology. 2002;21:68–73.
Tabert MH, Liu X, Doty RL, Serby M, Zamora D, Pelton GH, et al. A 10-item smell identification scale related to risk for Alzheimer's disease. Ann Neurol. 2005;58:155–60.
Wilson RS, Arnold SE, Schneider JA, Tang Y, Bennett DA. The relationship between cerebral Alzheimer's disease pathology and odour identification in old age. J Neurol Neurosurg Psychiatry. 2007;78:30–5.
Devanand DP, Liu X, Tabert MH, Pradhaban G, Cuasay K, Bell K, et al. Combining early markers strongly predicts conversion from mild cognitive impairment to Alzheimer's disease. Biol Psychiatry. 2008;64:871–9.
Schubert CR, Carmichael LL, Murphy C, Klein BE, Klein R, Cruickshanks KJ. Olfaction and the 5-year incidence of cognitive impairment in an epidemiological study of older adults. J Am Geriatr Soc. 2008;56:1517–21.
Conti MZ, Vicini-Chilovi B, Riva M, Zanetti M, Liberini P, Padovani A, et al. Odor identification deficit predicts clinical conversion from mild cognitive impairment to dementia due to Alzheimer's disease. Arch Clin Neuropsychol. 2013;28:391–9. This article assessed whether baseline olfactory deficit in MCI patients predicted conversion to dementia. At two years followup, 47 % of dysosmic MCI patients converted to dementia compared to 11 % of the normosmics, with an odds ratio for conversion of 5.1, independent of baseline MMSE.
Murphy C, Jernigan TL, Fennema-Notestine C. Left hippocampal volume loss in Alzheimer's disease is reflected in performance on odor identification: a structural MRI study. J Int Neuropsychol Soc. 2003;9:459–71.
Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, Rosenberg L. Smell identification ability: changes with age. Science. 1984;226:1441–3.
Wilson RS, Schneider JA, Arnold SE, Tang Y, Boyle PA, Bennett DA. Olfactory identification and incidence of mild cognitive impairment in older age. Arch Gen Psychiatry. 2007;64:802–8.
Bacon Moore AS, Paulsen JS, Murphy C. A test of odor fluency in patients with Alzheimer's and Huntington's disease. J Clin Exp Neuropsychol. 1999;21:341–51.
Kraemer S, Apfelbach R. Olfactory sensitivity, learning and cognition in young adult and aged male Wistar rats. Physiol Behav. 2004;81:435–42.
Patel RC, Larson J. Impaired olfactory discrimination learning and decreased olfactory sensitivity in aged C57Bl/6 mice. Neurobiol Aging. 2009;30:829–37.
LaSarge CL, Montgomery KS, Tucker C, Slaton GS, Griffith WH, Setlow B, et al. Deficits across multiple cognitive domains in a subset of aged Fischer 344 rats. Neurobiol Aging. 2007;28:928–36.
Prediger RD, De-Mello N, Takahashi RN. Pilocarpine improves olfactory discrimination and social recognition memory deficits in 24 month-old rats. Eur J Pharmacol. 2006;531:176–82.
Luu TT, Pirogovsky E, Gilbert PE. Age-related changes in contextual associative learning. Neurobiol Learn Mem. 2008;89:81–5.
Guan X, Dluzen DE. Age related changes of social memory/recognition in male Fischer 344 rats. Behav Brain Res. 1994;61:87–90.
Roman FS, Alescio-Lautier B, Soumireu-Mourat B. Age-related learning and memory deficits in odor-reward association in rats. Neurobiol Aging. 1996;17:31–40.
Phillips M, Boman E, Osterman H, Willhite D, Laska M. Olfactory and visuospatial learning and memory performance in two strains of Alzheimer's disease model mice–a longitudinal study. PLoS One. 2011;6:e19567.
Montgomery KS, Simmons RK, Edwards 3rd G, Nicolle MM, Gluck MA, Myers CE, et al. Novel age-dependent learning deficits in a mouse model of Alzheimer's disease: implications for translational research. Neurobiol Aging. 2011;32:1273–85.
Young JW, Sharkey J, Finlayson K. Progressive impairment in olfactory working memory in a mouse model of Mild Cognitive Impairment. Neurobiol Aging. 2009;30:1430–43.
Deacon RM, Koros E, Bornemann KD, Rawlins JN. Aged Tg2576 mice are impaired on social memory and open field habituation tests. Behav Brain Res. 2009;197:466–8.
Rey NL, Jardanhazi-Kurutz D, Terwel D, Kummer MP, Jourdan F, Didier A, et al. Locus coeruleus degeneration exacerbates olfactory deficits in APP/PS1 transgenic mice. Neurobiol Aging. 2012;33(426):e421–411.
Murphy C, Bacon AW, Bondi MW, Salmon DP. Apolipoprotein E status is associated with odor identification deficits in nondemented older persons. Ann N Y Acad Sci. 1998;855:744–50.
Olofsson JK, Nordin S, Wiens S, Hedner M, Nilsson LG, Larsson M. Odor identification impairment in carriers of ApoE-varepsilon4 is independent of clinical dementia. Neurobiol Aging. 2010;31:567–77.
Doty RL, Petersen I, Mensah N, Christensen K. Genetic and environmental influences on odor identification ability in the very old. Psychol Aging. 2011;26:864–71.
Nee LE, Lippa CF. Inherited Alzheimer's disease PS-1 olfactory function: a 10-year follow-up study. Am J Alzheimers Dis Other Demen. 2001;16:83–4.
Compliance with Ethics Guidelines
Conflict of Interest
Arjun V. Masurkar has received financial support through grants from the National Institute of Mental Health (T32) and the Charles and Lee Brown Fellowship.
D. P. Devanand has received financial support through grants from the National Institute on Aging and Eli Lilly (investigator-initiated research grant), and has served as an advisory board member for AbbVie.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Masurkar, A.V., Devanand, D.P. Olfactory Dysfunction in the Elderly: Basic Circuitry and Alterations with Normal Aging and Alzheimer’s Disease. Curr Geri Rep 3, 91–100 (2014). https://doi.org/10.1007/s13670-014-0080-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13670-014-0080-y