Abstract
Purpose of the Review
This paper will review the evidence for mitochondrial dysfunction in critical illness, describe the mechanisms which lead to multiple organ failure, and detail the implications of this pathophysiologic process on nutritional therapy.
Recent Findings
Mitochondria are particularly sensitive to increased oxidative stress in critical illness. The functional and structural abnormalities which occur in this organelle contribute further to the excessive production of reactive oxygen species and the reduction in generation of adenosine triphosphate (ATP). To reduce metabolic demand, mitochondrial dysfunction develops (a process likened to hibernation), which helps sustain the life of the cell at a cost of organ system failure. Aggressive feeding in the early phases of critical illness might inappropriately increase demand at a time when ATP production is limited, further jeopardizing cell survival and potentiating the processes leading to multiple organ failure.
Summary
Several potential therapies exist which would promote mitochondrial function in the intensive care setting through support of autophagy, antioxidant defense systems, and the biogenesis and recovery of the organelle itself. Nutritional therapy should supplement micronutrients required in the mitochondrial metabolic pathways and provide reduced delivery of macronutrients through slower advancement of feeding in the early phases of critical illness. A better understanding of mitochondrial dysfunction in the critically ill patient should lead to more innovative therapies in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increasing evidence suggests that the acute insult of critical illness leads to early mitochondrial dysfunction [1••, 2••]. Greater degrees of oxidative stress exacerbate the demand for production of energy in the form of adenosine triphosphate (ATP). Both functional and structural abnormalities in mitochondria cause excessive production of reactive oxygen species (ROS) and leakage of mitochondrial products such as mitochondrial deoxyribonucleic acid (DNA), which serve as alarmins or danger-associated molecular patterns (DAMPs) exacerbating immune dysregulation and the systemic inflammatory response syndrome (SIRS). A process involving the mitochondria, akin to hibernation, occurs as an appropriate cell-adaptive response to preserve the life of the cell [1••, 2••]. However, the cell-adaptive response leads to an inappropriate organ-maladaptive response which emerges clinically as multiple organ failure [1••, 2••]. The insulin resistance incurred with increased oxidative stress and the direct deterioration of mitochondria, the site for fatty acid metabolism, lead to futile substrate cycling and have important implications for the delivery of nutritional therapy in critical illness [3].
The etiology of multiple organ failure in critical illness is variable, with the most common cause being attributed to fluid volume loss, shock, vasodilation, capillary leak, and ischemia/reperfusion [3, 4]. However, additional evidence points to the breakdown of gut-barrier defense systems, bacterial translocation, and the creation of toxic lymph [4, 5]. Immune dysregulation emerging clinically as gut sepsis may be a significant contributor to organ failure, as is the emergence of a virulent pathobiome (dysbiosis) from the commensal microbiome [4, 5]. Less appreciated, however, is the role of mitochondrial dysfunction in contributing to clinical organ failure in the intensive care unit (ICU) [4]. A large amount of data are now implicating that the mitochondria are the principal source of ROS leading to the induction of autophagy. A possible explanation for this surprising evidence is that nutrient deprivation leads to a sudden energetic stress that increases demand for ATP and causes overburdening of mitochondria to cope with adverse metabolic and pathologic conditions. This in turn results in electron leakage and increased ROS production [6].
This paper will discuss the traditional role of the mitochondrial organelle, the impact of mitochondrial dysfunction on the patient with a critical illness, and the implications of mitochondrial hibernation in the development of multiple organ failure. Most importantly, this report will detail how mitochondrial failure impacts the progression of nutritional therapy through the phases of critical illness.
Normal Mitochondrial Function
As a tiny organelle, the mitochondrion has a considerable responsibility to power all aspects of cellular function [1••]. Any work required of the cell, such as the maintenance of ion potential gradients, the active transport of particles across a membrane, or the synthesis and configuration of new proteins, is accomplished through the generation of high energy phosphate bonds in the form of ATP. In this sense, the mitochondria represent the “battery” or the energy powerhouse of the cell, generating ATP to meet the demands of cellular metabolism [2••].
Mitochondria have other roles as well, in addition to ATP production [1••]. Mitochondria are responsible for heat generation and thermal regulation. Mitochondria regulate the concentration of intracellular calcium, preventing excess build up in the cytosol. Further, mitochondria are responsible for the production of ROS and nitric oxide which are critical for cell signaling, vascular tone, and oxygen-sensing systems. They are also involved in the function of certain hormones. Mitochondria are the site of production for cortisol, the site of action for thyroid hormone and estrogen, and may serve as a site of biosynthesis for heme and iron-sulfur clusters [1••].
The process of glycolysis alone, wherein glucose is metabolized to pyruvate and ultimately to lactate, is a relatively weak process for energy production yielding only two ATP per molecule of glucose (Fig. 1) [7••]. The buildup of lactic acidosis in critical illness may reflect mitochondrial dysfunction and the inability to utilize the Krebs cycle and the electron transport chain (ETC) [7••]. In contrast, entry into the Krebs cycle and passage through the proton gradient of the ETC is an energy-rich process yielding 32 ATP [7••].
Effect of critical illness on mitochondrial function. Reprinted from [7••], with permission from Elsevier
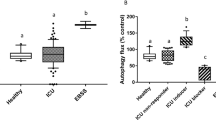
Mitochondria play an essential role in its auto-regulation. Because damaged or surplus mitochondria can worsen the morbidity of critical illness, two processes of fusion/fission and mitophagy help remove damaged mitochondria [1••]. The mitochondrial auto-regulatory systems are important as the functional and structural abnormalities of mitochondria in critical illness serve to exacerbate the clinical sequelae of the increased oxidative stress. Damaged mitochondria produce excessive ROS and leak mitochondrial DNA, which worsens the SIRS response. One of the two auto-regulatory processes which have evolved to contain such damage is the combination of fusion and fission (Fig. 2) [1••]. In this situation, two damaged mitochondria fuse, and the defects are contained in the new product. With subsequent fission, this combined mitochondria splits into two new mitochondria, somehow concentrating all of the defects into one new mitochondrion, with the other being normal without defects. The other process is mitophagy, which specifically represents the autophagy of the mitochondrial organelle (Fig. 2) [1••]. In this latter process, a double-layer membrane encircles the damaged mitochondria forming an autosome, which then fuses with the lysosome containing proteolytic enzymes capable of breaking down this large organelle structure. The same system can remove the damaged endoplasmic reticulum which would otherwise worsen an unfolded protein response (another critical driver of oxidative stress) (Fig. 3) [1••, 8].
b, c Self-regulation mechanisms of the mitochondria. Reprinted from [7••], with permission from Elsevier
Effect of oxidative stress on function of organelles (mitochondria and endoplasmic reticulum): the unfolded protein response. From [8]. Reprinted with permission from The American Association for the Advancement of Science
An essential function lies in the responsibility of the mitochondrial organelle to determine the fate of the cell in the setting of increasing oxidative stress, serving as the trigger for cell death pathways (Fig. 4) [1••, 9]. In an uncompensated stress state, an increasing tissue oxygen deficit is created, and the capability for ATP production by the mitochondria to meet the demands of metabolism becomes overwhelmed. If ATP levels fall below a certain critical point, cell death pathways are initiated [1••]. Early in critical illness with lesser degrees of disease severity, autophagy (also known as programmed cell death type-1) of damaged endoplasmic reticulum and mitochondria may be sufficient to sustain function and preserve the life of the cell. However, at some point with increasing stress, the cell is marked for death [9]. A quieter more efficient process for cell death is apoptosis (also known as programmed cell death type-2). This process leads to shrinkage and condensation of the organelle and a subsequent silent means of removal with minimal inflammatory upregulation. Apoptosis requires sufficient mitochondrial function to resist the accumulation of intracellular calcium and cause the release of cytochrome C into the cytosol of the cell. With greater degrees of oxidative stress, these processes fail, and necrosis (also called non-programmed cell death) results with disintegration of the cell and the generation of an associated inflammatory response (Fig. 4) [9].
Programmed cell death pathways for apoptosis and necrosis. Reprinted from [9], with permission from Elsevier
Impact of Critical Illness
The insult of critical illness has a direct impact on mitochondrial function (Fig. 1) [4, 7••]. Hypoxemia, high levels of reactive oxygen species, reactive nitrogen species, and gaseous molecules of carbon monoxide and hydrogen sulfide produced in critical illness disrupt the ETC [2••, 7••]. The effect is to reduce ATP production and, as a result, accumulation of the byproduct adenosine diphosphate (ADP). Glycolysis is increased leading to greater accumulation of lactate. Hyperglycemia, stemming from insulin resistance, causes an increase in the formation of dicarbonyl compounds which adds a toxic effect, further inhibiting the function of the ETC (Fig. 1) [7••]. Under normal conditions, the formation of nitric oxide, reactive oxygen species, and reactive nitrogen species is necessary to fight infection, regulate vascular tone, and promote cell signaling. The process is contained through detoxification by the glutathione antioxidant defense system (Fig. 5) [7••]. In critical illness, however, the inflammation and hypoxia lead to excessive stimulation of inducible nitric oxide synthetase (iNOS), and nitric oxide formation causing over-production of superoxide radicals. Both manganese and copper/zinc superoxide dismutase enzymes are all increased, causing the glutathione antioxidant systems to be overwhelmed, leading to excessive formation of hydroxyl radicals (Fig. 5) [7••].
a Effect of critical illness on glutathione antioxidant defense systems. Reprinted from [7••], with permission from Elsevier
In the face of these changes, an odd process evolves, that of uncoupling of the Krebs cycle and the ETC [2••]. The mitochondria switch to uncoupled fat metabolism, similar to that seen in brown fat and non-shivering thermogenesis. The uncoupling refers to inhibition or slowing of specific steps in the Krebs cycle and the ETC, causing inefficient metabolism and reduced phosphorylation. The uncoupling of the proton gradient in the ETC is lost as heat, causing clinical pyrexia [2••]. The loss of the proton gradient varies by organ, with the heart losing only 15%, while the skeletal muscle and liver lose as much as 50% [1••, 2••]. The uncoupling may be protective, however, as the proportion of respiration coupled to ATP production is reduced by 55–89% [2••]. The reduced mitochondrial membrane potential leads to decreased production or generation of reactive oxygen species.
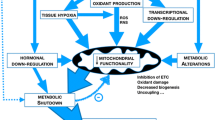
In critical illness, a variety of factors contribute to the compromise of mitochondrial oxidative phosphorylation (Fig. 7) [2••]. Such factors include excessive inflammatory mediators, direct oxygen stress, reduced gene expression for the production of mitochondrial proteins, the uncoupling of fat metabolism, increasing tissue oxygen deficit, and alteration in hormone functions (thyroid) [2••]. Excess thyroid hormone increases ATP production by mitochondria, but reduces efficiency through an uncoupling effect (Fig. 6). The sick euthyroid syndrome seen in critical illness may lead to reduced but more efficient respiration [3]. An interesting trade-off appears to then occur. Cell-adaptive changes occur leading to “hibernation” of the mitochondria to reduce metabolic demand and preserve the life of the cell [10••]. Such changes occur, however, at the cost of organ-maladaptive processes which lead to functional compromise of various organ systems in the clinical sequelae of multiple organ failure [10••].
Impact of critical illness on mitochondrial function. Reprinted from [2••], with permission (https://creativecommons.org/licenses/by/4.0/)
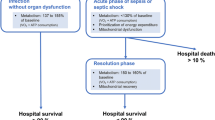
Physiologic studies in animal models of sepsis (later confirmed in clinical trials) have shown that the multiple organ failure incurred is associated with a minimal degree of histologic abnormalities, inflammation, or structural damage to the organs themselves (Fig. 7) [11]. With the resolution of sepsis, organ function often recovers rapidly and completely in a relatively short period of time. This metabolic bioenergetic shutdown of the mitochondria has been likened to hibernation or aestivation (the summer equivalent of hibernation, seen in desert rodents and reptiles in hot arid climates) [10••]. In this process, cells suppress some of the energy-dependent activities to favor those which are essential to cell survival. The hibernation or aestivation of the mitochondria occurs to reduce metabolic demand and prolong the life of the cell. Numerous clinical examples have been demonstrated [10••]. In the kidney, 98% of energy is required for complete filtration of the plasma. With acute kidney injury, reducing the percent of plasma that is filtered preserves energy to sustain the organ. In heart disease, high levels of drugs are required to maintain cardiac output and vascular tone, yet little cell death occurs and myocytes have been shown actually to hibernate after an acute myocardial infarction [10••]. In the liver, reduced synthesis of protein and biliary secretion help preserve energy for this organ. In the lungs, “hypoxic conformance” occurs because of the reduced function of ATP-ase-dependent pumps, presenting clinically as pulmonary edema [10••].
Comparison of histologic specimens in multiple organ failure between septic animals and controls. Reprinted from [11], with permission from The American Physiological Society
In critical illness, a diverse number of cells are shown to respond to the stress of hypoxia and the release of inflammatory cytokines [10••]. Mitochondria in these cells appear to be particularly sensitive to such stress. Functional and subsequent structural abnormalities in the mitochondria are seen [1••, 4]. The leaking mitochondria spill mitochondrial DNA into the systemic circulation and they act as DAMPs [1••]. DAMPS are similar to pathogen-associated molecular patterns (PAMPs), which are active bacterial pathogens that trigger danger signals and activate toll-like receptors all over the body [2••, 10••, 12]. Activation of these mitochondrial-dependent signals triggers cellular responses, and the level of DAMPs in the systemic circulation is linked to multiple organ failure and reduced survival [2••, 13].
The functional compromise is seen as an immediate response to oxidative stress. Subsequent to that, morphologic changes occur with the fragmentation of the mitochondria and ultimate loss of mitochondrial volume [10••]. In an experimental animal model of sepsis, the skeletal muscle of the control rodents was shown to have orderly columns of actin-myosin sheaths with numerous mitochondria in between [14]. Following sepsis, the mitochondria of the skeletal muscle in study animals demonstrated noticeable morphologic change becoming clumpy and misshapen and markedly reduced in number. The changes appeared to be more severe in non-survivors compared to survivors [14].
The impact of oxidative stress on mitochondrial dysfunction follows a well-described sequence or progression of events (Fig. 8) [15]. The initial insult of critical illness with its release of inflammatory mediators and early circulatory hypoxia promotes mitochondrial inhibition in the early phases. Hormonal changes interact at this point, and specific therapeutic agents such as sedatives, antibiotics, and inotropes may exacerbate mitochondrial inhibition [15]. Propofol may be the best example of an inhibitory sedative. Propofol is a known mitochondrial toxin which inhibits key enzymes in the mitochondrial transport chain and impairs fatty acid oxidation and ATP generation [16]. Many antibiotics work by binding to bacterial cell walls or organelles. The teleological origins of mitochondria suggest that they are essentially “enslaved” bacteria. Thus, antibiotic targeting of mitochondria or endoplasmic reticulum of healthy human cells may contribute to the mechanism of their mitochondrial inhibition and potential injury [4, 17]. The inhibition leads to bioenergetic shutdown or hibernation, resulting in biochemical and functional abnormalities of multiple organ failure [2••]. The next step in the sequence of events is critical—the point at which biogenesis of the mitochondria recovers resulting in the repair and/or regeneration of new mitochondria [15]. Adequate energy supply to meet metabolic demand is restored, and the resolution of organ failure occurs. While this sequence of events plays out over the initial phases of critical illness, few clinical signs, symptoms, or monitors tell the clinician at which point mitochondrial dysfunction resolves and the biogenesis/regeneration occurs (Fig. 8) [15].
Sequence of events of mitochondrial function through critical illness. Reprinted from [15], with permission
Potential Therapies for Mitochondrial Dysfunction
Several potential therapies exist to address mitochondrial dysfunction in critical illness with the theoretical possibility of speeding recovery. One strategy is to stimulate mitochondrial biogenesis through a variety of possible agents. Resveratrol and PPAR-gamma agonists (such as rosiglitazone) increase PPAR-gamma Costimulator-1a, which is a known stimulant of mitochondrial biogenesis [1••]. Biogenesis may be stimulated via induction of heme oxygenase by low-dose carbon monoxide. Recombinant human transcription factor A for the mitochondrion (TFAM) is another agent with potential for biogenesis stimulation [1••]. Another strategy involves boosting antioxidant defense systems [7••]. Direct mitochondrial protection may be provided by such antioxidant agents as melatonin, mitoQ, or mitoE [1••]. Specific vitamins such as vitamin A, vitamin C, and vitamin E serve as direct antioxidants, while trace elements such as copper, zinc, and selenium may serve as enzyme cofactors in the antioxidant defense system [18••]. Alpha-lipoic acid has also been hypothesized to be a mitochondrial protective agent via reduction in oxidative stress [19]. Certain activators of autophagy exist which theoretically should help reduce the unfolded protein response and help in the clean-up of dysfunctional damaged mitochondria [7••]. Two such agents, rapamycin and anthracycline, are known stimulators of mitophagy and autophagy, but have unacceptable side effects. Rapamycin is associated with immune suppression, and anthracycline is associated with cardiotoxicity. 4-Phenylbutyric acid (4-PBA) is a direct inducer of the unfolded protein response and is an agent used typically in the treatment of urea cycle abnormalities [7••]. Whether this would be appropriate for use to speed recovery from critical illness is not clear.
In a thorough review of potential nutritional strategies for mitochondrial dysfunction in critical illness, Wesselink and Van Zanten emphasized the myriad points in the glutathione antioxidant defense system where essential vitamins and trace elements act as cofactors, promoters or inhibitors (Fig. 9) [20]. Similarly, many of these same vitamins and trace elements, in addition to carnitine, cofactor Q10, and creatine phosphokinase, influence steps throughout the ETC (Fig. 10) [18••]. Both deficiencies and specific toxicities can inhibit steps throughout these mitochondrial processes [18••].
Role of micronutrients in the glutathione antioxidant defense system. Reprinted from [20], with permission from John Wiley and Sons, Inc.
Role of micronutrients in the function of the electron transport chain. Reprinted from [18••], with permission from Elsevier
While a better understanding of the function of these vitamin and trace element cofactors through the mitochondrial metabolic pathways may provide innovative strategies in the future [18••], clinicians at present must deal with the decision of whether or not to empirically provide supplemental micronutrient therapy. Berger makes a convincing argument that, while micronutrient levels may be within the normal range on admission to the ICU, the impact of injury and acute critical illness with a rising degree of oxidative stress may lead to depletion of the antioxidant vitamins and trace elements [21]. Giving empiric micronutrients at physiologic doses in the range of their Recommended Daily Allowance (RDA) is one straightforward strategy. Giving empiric micronutrients at supraphysiologic doses, especially selenium, was shown in early studies to portend a benefit in clinical outcome, but later studies failed to show the same benefit [22, 23]. Still another strategy is to check baseline micronutrient levels upon admission to the ICU and treat only those deficiencies which are identified. Studies in the literature, however, appear to contradict each other, as do the various societal guidelines, such that no clear global consensus has been reached. At the present time, guidelines from the 2016 American Society of Parenteral and Enteral Nutrition and the Society of Critical Care Medicine (ASPEN/SCCM) recommend empiric therapy with a multivitamin-trace element cocktail at near physiologic doses (based on the RDA of each) [5]. Guidelines in critical care from the European Society for Clinical Nutrition and Metabolism (ESPEN) recommend measuring only vitamin D levels and repleting if deficient, while providing empiric therapy with a cocktail of trace elements and vitamins at physiologic doses [24]. The Canadian Clinical Practice Guidelines at present (as of 2015) do not recommend empiric therapy with a micronutrient cocktail, a stance that represents a reversal of their recommendations from 2013 [25, 26].
The implications for nutritional therapy in the face of mitochondrial dysfunction are difficult to determine. Futile substrate cycling would be expected with the insulin resistance seen in critical illness and the fact that fatty acid oxidation occurs only within the mitochondria. Clinical studies comparing elite athletes, outpatients with diabetes mellitus, and critically ill patients in the ICU show varying degrees of failure of fatty acid oxidation with delays in its recovery over time (Fig. 11) [27]. In a series of experiments described by Wischmeyer, elite athletes who have been shown to have increased numbers of mitochondria in the skeletal muscle were tested on exercise cycles [27]. As the work effort was increased toward exhaustion, fat metabolism was maintained to a very high point before completely failing and rendering the athlete totally dependent on carbohydrate metabolism. In comparison, a diabetic outpatient was exercised on a similar device and showed that a single upgrade in work effort led to a rapid decline in fatty acid utilization. Such patients have been shown to have a significantly fewer number of mitochondria in their skeletal muscle [27]. In an ICU patient, there was complete failure of fatty acid metabolism throughout their hospitalization in the ICU, and the patient was entirely dependent on carbohydrate metabolism throughout. Surprisingly, 2 months following discharge from the hospital, virtually no capacity for fatty acid oxidation had recovered. It was not until 6 months post-discharge that increasing intermittent training efforts on an exercise cycle showed evidence of recovery of fatty acid oxidation (Fig. 11) [27].
Comparison of metabolism of fatty acid between elite athletes, control outpatients with diabetes, and critically ill patients (at 2 months and 6 months post-discharge). Reprinted from [27], with permission
In the face of mitochondrial dysfunction in oxidative stress, potential treatment strategies are likely time sensitive through the phases of critical illness. The most important message may be that reduced macronutrient provision as delivered by a slow ramp-up in the rate of nutrient provision should be recommended to clinicians [28,29,30]. This is a dramatic shift from traditional societal guidelines. Parenteral protein, in particular, should be avoided, as this is one of the most significant inhibitors of mitophagy [31]. Glycemic control is essential to reduce flux through glycolysis and decrease the formation of dicarbonyl inhibitory compounds (Fig. 1) [7••]. Certain strategies have been utilized in specific disease states to decrease the metabolic rate, such as hypothermia after myocardial infarction or low-dose carbon monoxide or hydrogen sulfide in critical illness. Judicious use of antibiotics to avoid their inhibitory effect on mitochondria should be employed, and prescribing sedatives (especially propofol) which bind and inhibit mitochondria should be minimized [1••, 4]. In general, efforts should be designed to provide mitochondrial protection while stimulating mitochondrial biogenesis as the patient transitions through the acute phases of critical illness and on into the period of recovery.
Conclusions
Mitochondria are highly susceptible to oxidative stress, and compromised function in the initial phase of critical illness may contribute to multiple organ failure. This early phase in the ICU may not be the time to push aggressive nutritional support, as such strategies may increase metabolic demand at a time when ATP production is limited. While various theoretical strategies are described to reduce oxidative stress and support mitochondrial function, few studies exist in the literature showing outcome benefits to guide clinicians at the bedside. A greater understanding of the role of mitochondria in critical illness, the importance of strengthening antioxidant defense systems, and the ability to recognize biogenesis and recovery of mitochondrial function should lead to innovative nutritional therapies in the future.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
•• Singer M. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence. 2014;5(1):66–72 This excellent review describes the mechanism by which mitochondrial dysfunction leads to multiple organ failure in critical illness.
•• Singer M. Critical illness and flat batteries. Crit Care. 2017;21(Supp3):309–14 This short but classic review details the effects of mitochondrial dysfunction in critical illness.
Singer M, DeSantis V, Vitale D, Jeffcoate W. Multiorgan failure is an adaptive, endocrine-mediated metabolic response to overwhelming systemic inflammation. Lancet. 2004;364(9433):545–8.
Kozlov AV, Bahrami S, Calzia E, Dungel P, Gille L, Kuznetsov AV, et al. Mitochondrial dysfunction and biogenesis: do ICU patients die from mitochondrial failure? Ann Intensive Care. 2011;1(1):41.
McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, et al. Compher C; Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. 2016;40(2):159–211.
Filomeni G, De Zio D, Cecconi F. Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death Differ. 2015;22(3):377–88.
•• Thiessen SE, Van den Berghe G, Vanhorebeek I. Mitochondrial and endoplasmic reticulum dysfunction and related defense mechanisms in critical illness-induced multiple organ failure. Biochim Biophys Acta Mol basis Dis. 2017;1863(10 Pt B):2534–45 This is an excellent review showing the role of mitochondria in autophagy and multiple organ failure in critical illness.
Muoio DM, Newgard CB. Insulin resistance takes a trip through the ER. Science. 2004;306:425–6.
Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: bench to the bedside. Gastroenterol. 2007;132(3):1127–51.
•• Arulkumaran N, Deutschman DS, Pinsky MR, Zuckerbraun B, et al. Mitochondrial function in sepsis. Shock. 2016;45(3):271–81 This excellent report describes the “hibernation” of mitochondria in critical illness.
Brealey D, Karyampudi S, Jacques TS, Novelli M, et al. Mitochondrial dysfunction in a long-term rodent model of sepsis and organ failure. Am J Phys Regul Integr Comp Phys. 2004;286(3):R491–7.
McClave SA, Lowen CC, Martindale RG. The 2016 ESPEN Arvid Wretlind lecture: the gut in stress. Clin Nutr. 2018;37:19–36.
Mogensen KM, Lasky-Su J, Rogers AJ, Baron RM, Fredenburgh LE, Rawn J, et al. Metabolites associated with malnutrition in the intensive care unit are also associated with 28-day mortality. JPEN J Parenter Enteral Nutr. 2017;41(2):188–97.
Carré JE, Orban J-C, Re L, Felsmann K, Iffert W, Bauer M, et al. Survival in critical illness is associated with early activation of mitochondrial biogenesis. Am J Respir Crit Care Med. 2010;182:745–51.
Singer M, Glynne P. Treating critical illness: the importance of first doing no harm. PLoS Med. 2005;2(6):e167 Epub 2005 Jun 28.
Diedrich DA, Brown DR. Propofol infusion syndrome in the ICU. J Intensive Care Med. 2011;26(2):59–72.
Singh R, Sripada L, Singh R. Side effects of antibiotics during bacterial infection: mitochondria, the main target in host cell. Mitochondrion. 2014;16:50–4.
•• Wesselink E, Koekkoek WAC, Grefte S, Witkamp RF, et al. Feeding mitochondria: potential role of nutritional components to improve critical illness convalescence. Clin Nutr. 2019;38(3):982–95 This excellent review describes the potential targets for nutritional therapy in supporting mitochondrial function in critical illness.
Liu J. The effects and mechanisms of mitochondrial nutrient alpha-lipoic acid on improving age-associated mitochondrial and cognitive dysfunction: an overview. Neurochem Res. 2008;33(1):194–203.
Koekkoek W, van Zanten ARH. Antioxidant vitamins and trace elements in critical illness. J Parenter Enter Nutr. 2016;31(4):457–74.
Berger MM. Can oxidative damage be treated nutritionally? Clin Nutr. 2005;24(2):172–83.
Heyland D, Muscedere J, Wischmeyer PE, Cook D, Jones G, Albert M, et al. A randomized trial of glutamine and antioxidants in critically ill patients. N Engl J Med. 2013;368(16):1489–97.
Bloos F, Trips E, Nierhaus A, Briegel J, Heyland DK, Jaschinski U, et al. Effect of sodium selenite administration and procalcitonin-guided therapy on mortality in patients with severe sepsis or septic shock: a randomized clinical trial. JAMA Intern Med. 2016;176(9):1266–76.
Singer P, Blaser AR, Berger MM, Alhazzani W, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2018;38:48–79.
Heyland DK. criticalcarenutrition.com accessed Dec 2016.
Dhaliwal R, Cahill N, Lemieux M, Heyland DK. The Canadian critical care nutrition guidelines in 2013: an update on current recommendations and implementation strategies. Nutr Clin Pract. 2014;29(1):29–43.
Wischmeyer PE, San-Millan I. Winning the war against ICU-acquired weakness: new innovations in nutrition and exercise physiology. Crit Care. 2015;19(Suppl 3):S6.
Koekkoek W, van Setten C, Olthof L, Kars J, van Zanten ARH. Timing of PROTein INtake and clinical outcomes of adult critically ill patients on prolonged mechanical VENTilation: the PROTINVENT retrospective study. Clin Nutr. 2019;38(2):883–90.
Patel JJ, Martindale RG, McClave SA. Controversies surrounding critical care nutrition: an appraisal of permissive underfeeding, protein, and outcomes. JPEN J Parenter Enteral Nutr. 2018;42(3):508–15.
Wischmeyer PE. Nutrition therapy in sepsis. Crit Care Clin. 2018;34(1):107–25.
Schetz M, Casaer MP, Van den Berghe G. Does artificial nutrition improve outcome of critical illness? Crit Care. 2013;17(1):302–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Stephen A. McClave declares that he has no conflict of interest.
Paul E. Wischmeyer has received research funding from Baxter, Abbott Laboratories, Fresenius Kabi, the National Institutes of Health (NIH), and Nutricia; has received compensation from Baxter, Abbott Laboratories, and Nutricia for service as a consultant; and has received compensation from Abbott Laboratories and Nutricia for participating in Continuing Medical Education (CME) Speaking programs.
Keith R. Miller has received salary support from Nestlé Nutrition through its Nutritional Educational Fellowship.
Arthur R.H. van Zanten has received research funding from Nutricia, Cardinal Health, and Mermaid Pharmaceuticals GmbH; has received compensation from Baxter, Fresenius Kabi, and Nutricia for service as a consultant; and has received compensation from Baxter, Nestlé, Fresenius Kabi, B. Braun Medical, Inc., and Nutricia for participating in CME Speaking programs.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Gastroenterology, Critical Care, and Lifestyle Medicine
Rights and permissions
About this article
Cite this article
McClave, S.A., Wischmeyer, P.E., Miller, K.R. et al. Mitochondrial Dysfunction in Critical Illness: Implications for Nutritional Therapy. Curr Nutr Rep 8, 363–373 (2019). https://doi.org/10.1007/s13668-019-00296-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13668-019-00296-y