Abstract
Pomegranate (Punica granatum Linn), has been widely used in India’s ancient Ayurveda system of traditional medicine which is commonly portrayed as a constituent in remedies. The present study was aimed to investigate the anticancer activity of the aqueous extract of P. granatum fruits (PGET) against ehrlich-ascites-carcinoma (EAC)-bearing Swiss albino mice. The PGET were administered to EAC bearing mice at the doses of 100, 200 and 400 mg/kg body weight (BW) intraperitonially for 14 successive days and 24 h of last dose and 18 h of fasting, the mice were sacrificed and the anticancer effect of PGET was appraised by evaluating tumor volume, viable, nonviable tumor cell count, tumor weight, hematological, biochemical parameters and histopathological changes of EAC mice. PGET showed momentous decrease in tumor volume, viable cell count, tumor weight and elevated the life span of EAC bearing mice. Hematological profile such as RBC, hemoglobin and lymphocyte count reverted to normal level in PGET treated mice. The extract at 400 mg/kg BW showed a noteworthy reduction in the level of lipid peroxidation and considerably increased the levels of antioxidant enzymes in the liver and observed significant restoration of histopathological changes in experimental animals. Hence, the current study revealed that the PGET was efficient in inhibiting the tumor growth in ascitic models and that is comparable to 5-Fluorouracil. The anticancer properties of P. granatum could be due to the presence of the various phytoconstituents in it.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is a disgraceful disease and fighting this disease is of immense magnitude to public health. Over the past few decades, cancer has remained as the biggest cause of death global and the number of persons existing with cancer is gradually mounting. Cancer is the second foremost cause of mortality after heart disease in India and its numbers in India are lower than those noticed in western countries but are rising with increasing migration of rural population to the cities, augment in life expectancy and changes in lifestyles (Jahan et al. 2009). The escalating occurrence of cancer reported over the last a few years among metropolitan population in India has led to development of new anticancer drugs, drug combinations, and chemotherapy strategies by scientific and methodological investigation of massive pool of biological, synthetic and natural products (Mukherjee et al. 2001).
Excess oxidative stress and undermined antioxidant protection system are crucial aspects of the development and pathogenesis of cancers. Oxidative stress replicates inequity between the ability of biological systems and the systemic manifestation of reactive oxygen species (ROS) to restore the resultant damage or to willingly detoxify the reactive intermediate. Superoxide radicals make other kinds of cell destructive oxidizing agents and free radicals. The unfavorable action of the hydroxyl radical is the strongest bounded by free radicals. Hydroxyl radicals are mostly prone to commence the multistage carcinogenesis progression starting with cellular manifestation, DNA damage and degenerative cell growth and eventually resulting in carcinoma. Cellular antioxidant enzymes and the free radical scavengers typically guard the cell from toxic effects of the ROS. Antioxidants produced from a plant source illustrate additional attention as free radical protectors because they are protective against ROS-induced oxidative damage. Chemoprevention by nutritional components has been materialized as a cost-effective move towards handling incidences of cancers (Uddandrao et al. 2016). A miscellaneous collection of herbal medicines and medicinal plants have conventionally been used in India and also the corresponding therapies based on herbal medicines are the world’s most antique form of medicine and recent reports recommended that such therapies still benefit from enormous popularity, particularly in developing countries like India where the majority of the population does not have easy access to contemporary medicine (Uddandrao et al. 2017). Plants and its active compounds give useful sources for the development of drugs in the management of cancer. Edible plants contains rich amount of polyphenols and these plants are being well thought-out as sources of anticancer drugs (Ferguson et al. 2004). Experimental investigations established that many naturally occurring agents and plant extracts have publicized anticancer prospective in a variety of bioassay systems and animal models having consequence to human disease (Aziz et al. 2003).
Punica granatum Linn. (Punicaceae), regularly called pomegranate, has been used expansively as conventional medicine in several countries. P. granatum is a rich resource of bioactive compounds which are demonstrated to have antioxidant (Parmar and Kar 2008), anti-atherosclerotic (Aviram et al. 2004), and antibacterial (Braga et al. 2005) properties. Modern studies have made known that P. granatum is a potent anticancer agent (Motaa and Shaker 2011). Taking the above facts into consideration as well as the properties exhibited by the plant, an attempt has been made, in this study, to evaluate the potential anticancer activity of the pomegranate extract (PGET) against in vivo ehrlich ascites carcinoma (EAC) tumor model.
Materials and methods
Chemicals
5-Fluorouracil (5-FU) was obtained from Ranbaxy Laboratories, Ltd., India. All other chemicals used were of analytical grade.
Preparation of extract
A fresh P. granatum fruits were procured from the local market. It was authenticated by Dr. P. Ponnumurugan, Associate Professor and specimen (Voucher No: 1959/2017) was stored at the Herbarium of Botany, Department of Biotechnology, K.S. Rangasamy College of Technology, Tiruchengode, India. The air-dried powdered fruits (250 g) were extracted with water in soxhlet apparatus for 6 h. The extract was evaporated to dryness under reduced pressure to give solid residues. The residue was stored at 0–4 °C for subsequent experiments.
Phytochemicals analysis
Qualitative phytochemical analysis of plant extract powder was tested as follows: Tannins (200 mg extract in 10 mL distilled water, filtered). A 2 mL filtrate + 2 mL FeCl3, blue-black precipitate indicated the presence of Tannins. Alkaloids (200 mg extract in 10 mL methanol, filtered). A 2 mL filtrate + 1% HCl + steam, 1 mL filtrate + 6 drops of Wagner’s reagent. Browinsh-red precipitate indicated the presence of alkaloids. Carbohydrate (0.5 mL extract + 2 drops molish reagent, violet color ring persistence meant carbohydrate present). Glycosides (Keller-kiliani test: 2 mL filterate + 1 mL glacial acetic acid + FeCl3 + conc. H2SO4). Green–blue color indicated the presence of glycosides. Steroids (Liebermann Burchard reaction: (200 mg extract in 10 mL chloroform, filtered). Flavonoids (200 mg extract in 10 mL ethanol, filtered). A 2 mL filtrate + conc. HCl + magnesium ribbon. Pink-tomato red color indicated the presence of flavonoids.
Animals
Healthy Swiss albino mice (Body weight 20 ± 2 g, Age 5–7 weeks) were taken for the study. The animals were kept in polypropylene cages with sawdust bedding and maintained under standard laboratory conditions. Standard pellet diet (Hindustan Lever, Kolkata, India) and water were given ad libitum. Before the commencement of the experimentation, the mice were habituated for 7 days to the laboratory conditions. They were maintained under restrained temperature (20 ± 2 °C) and 12 h light/12 h dark rhythm. The protocol of this study was approved by the institutional ethical committee of Muthyammal College of Arts and Science, Rasipuram, Tamilnadu, India (Approval No: 1416/P0/a/11/CPCSEA).
Toxicity study
Healthy Swiss albino mice, starved overnight, were divided into seven groups (n = 4). Group I–VI animals were orally fed with extract in increasing dose levels of 0.5, 1.5, 2.0, 2.5, 3.0 and 4.0 g/kg BW, while group VII (untreated) served as control. The animals were observed continuously for 2 h, and then intermittently and after 24 h for 14 days. The animals were observed for behavioral, neurological and autonomic profiles. One-tenth and one-twentieth of the maximum safe dose of the extract tested for acute toxicity were selected for the in vivo experiment.
Tumor cells
EAC cells were acquired from Amala Cancer Research Centre, Trissur, and Kerala, India. The EAC cells were maintained by intraperitoneal inoculation of 2 × 107 cells/mouse. EAC cells aspirated from the peritoneal activity of mice were washed with saline and were given intraperitonially (IP) to develop ascitic tumor.
Experimental design
Healthy mice were separated into six groups each comprising a minimum of six mice. EAC cells (2 × 106 cells/mouse) were injected IP, to each mouse of each group except normal saline group. This was taken as Day 0. Extract treatment was continued for subsequent 9 days starting from Day 1. On 10th day, 24 h after the last dose four mice were sacrificed from each group and the rest were kept for the life span study of the tumor hosts. At the end of the experimental period, the mice were fasted overnight, anaesthetized using low doses of phenobarbitone and sacrificed by cervical decapitation. Blood was collected by intracardially to evaluate the hematological and biochemical parameters. Liver tissue was collected from the animals for the evaluation of in vivo antioxidant status.
The groups and the design of the experiment were as follows.
-
Group I: 2% Tween-80 (5 mL/kg BW, IP)
-
Group II: EAC (2 × 106 cells/mouse) + 2% Tween-80 (5 mL/kg BW, IP)
-
Group III: EAC (2 × 106 cells/mouse) + PGET (100 mg/kg BW, IP)
-
Group IV: EAC (2 × 106 cells/mouse) + PGET (200 mg/kg BW, IP)
-
Group V: EAC (2 × 106 cells/mouse) + PGET (400 mg/kg BW, IP)
-
Group VI: EAC (2 × 106 cells/mouse) + 5-FU (20 mg/kg BW, IP)
The antitumor efficacy of PGET was compared with the standard which served as the sixth group (5-FU, 20 mg/kg/day IP). Antitumor activity of PGET was assessed by observation of changes with respect to the following parameters.
Tumor growth response
The effect of PGET on tumor growth and host’s survival time were examined by studying the following parameters such as tumor volume, packed cell volume, tumor cell count, viable tumor cell count, nonviable tumor cell count, median survival time (MST) and increase in life span (ILS).
Hematological studies
Red blood cells (RBC), white blood cells (WBC) counts and estimation of hemoglobin was done by standard procedures from the blood obtained intracardially.
Hemoglobin estimation
0.1 mL of heparinized blood was taken in Sahli’s Hemoglobinometer and diluted with 0.1 N HCl until the color matched with standard. The reading was then taken from the graduated cylinder and expressed as g/100 mL of blood.
Biochemical parameters
After the collection of blood samples, the mice were sacrificed. Then the liver was excised, rinsed in ice cold normal saline followed by ice-cold 10% KCl solution, blotted, dried and weighed. A 10% w/v homogenate was prepared in ice-cold KCl solution and centrifuged at 2000 rpm for 10 min at 4 °C. The supernatant thus obtained were used for the estimation of thio-barbituric acid substances [TBARS] (Fraga et al. 1988), glutathione [GSH] (Beutler and Kelly 1963), superoxide dismutase [SOD] (Kakkar et al. 1984), catalase [CAT] (Aebi 1984) and glutathione peroxidase [GPx] was analyzed by Paglia and valentine method (Paglia and Valentine 1967).
Histopathological examination
A piece of liver samples were fixed in 10% formalin for histopathological examination. The thin sections were cut and then stained by haematoxylin and eosin and observed under light microscope.
Statistical analysis
All the results were expressed as the mean ± SD for six animals in each group. All the grouped data were statistically evaluated with SPSS\10.0 software. Hypothesis testing methods included one way analysis of variance (ANOVA) followed by least significant difference (LSD) test, significance level at and p < 0.05, 0.01, 0.001 were considered to indicate statistical significance.
Results
Toxicity study and preliminary phytochemical analysis
In acute toxicity study, PGET did not show any toxic effect up to the dose of 4 g/kg BW, accordingly 100, 200 and 400 mg/kg BW were taken as doses of PGET for the experiment. Preliminary phytochemical analysis of PGET showed the presence of flavonoids, saponins, glycosides, terpenoids, amino acids, alkaloids, carbohydrates, phenolic compounds and proteins. Qualitative analysis of the extract confirmed the presence of ellagic acid, a potent antioxidant.
Effect of PGET on tumor growth response
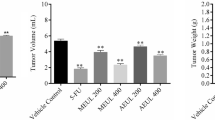
Table 1 showed the levels of tumor volume, tumor weight, MST, %ILS, viable tumor cell count and non-viable cell count in control and experimental animals. There was a significant (p < 0.01) reduction in tumor volume, tumor weight and viable tumor cell count with a significant elevation in MST, %ILS and non-viable cell count in the treated groups, compared with EAC control mice. Administration of PGET tended to bring all the parameters in tumor growth response study towards near normal levels.
Effect of PGET on hematological parameters
Table 2 summarized the level of RBC, WBC and hemoglobin levels in control and experimental animals. A significant reduction in the level of RBC and hemoglobin and a concomitant increase in the level of WBC were observed in EAC bearing mice. Administration of PGET significantly reduced WBC count in all the groups of treatment with respect to that of EAC control group. RBC count and hemoglobin content, which were decreased after EAC inoculation, were found to be significantly restored to the normal levels in animals treated with PGET as well as standard drug 5-FU.
Effect of PGET on biochemical parameters
Figures 1 and 2 depicted the level of TBARS, GSH, SOD, CAT and GPx in the liver of control and experimental groups of mice. A significant reduction in the level of GSH, SOD, CAT and GPx and a concomitant increase in the level of TBARS were observed in EAC bearing mice. Treatment with PGET and 5-FU showed a significant increase in activities of SOD, CAT and GPx and decrease of TBARS in liver of treated animals.
Histopathological analysis
The group of normal mice administrated PGET showed normal histological architecture (Fig. 3a). With respect to Ehrliched mice, microscopal examination of liver revealed diminishing in pathological structure, to a great degree, towards normal intact histological structure as shown in Fig. 3b. Interestingly, treatment with PGET and 5-FU reduced most of the pathological alterations induced by EAC cells in mice (Fig. 3c–f).
Effect of PGET on histopathological changes in control and experimental mice. a Liver showing normal histological architecture in normal control mice. b Liver showing congestion, c disrupted cords and anisokaryosis in hepatocytes in EAC control. c–e Liver showing moderate damage to hepatocytes with severe sinusoidal Congestion indicating the reversal effect of PGET (400 mg/kg). f Liver showing minimal cellular damage and moderate sinusoidal congestion indicating the reversal effect of 5-FU. H&E × 100
Discussion
Transplantable tumor cells such as EAC are quickly growing cancer cells with violent behavior (Segura et al. 2000). Since ascites fluid constitutes a direct nutritional source for tumor cells, it is mandatory for tumor growth (Shimizu et al. 2004). So, a hasty raise in ascites fluid with tumor growth would be a mean to meet the nutritional prerequisite of tumor cells (Rajeshwar et al. 2005). This proposition was apparent in the current study, since inoculation of EAC cells into mice caused considerable increase in the mice body weight and reduced MST, ILS. This may be due to superior mitosis which could be accredited to the decreased rate of natural death mechanism that occurs in the tumor (Badr et al. 2011). However with the administration of PGET the percent increase in tumor cell volume, and number of viable tumor cells were found to be drastically less when compared to the EAC control. Hence, this might be due to the direct cytotoxic effect, striking the tumor growth and increased the life span of EAC-bearing mice. The percentage of ILS at the 400 mg/kg BW dose of the extract indicates its powerful anticancer nature. In acute toxicity studies, the administration of PGET at the dose of 100, 200 and 400 mg/kg BW for 14 days did not display any unfavorable effects which may be due to its composite nature where the presence of phytoconstituents could counteract its toxicity.
The accumulation of ascites fluid in the peritoneal cavity could have been due to (a) reduced lymphatic recovery system which is associated with the obstruction of the lymphatic system by tumor cells (b) angiogenesis, which was detected in the ascites tumor bearing peritoneal wall (c) the hyper permeability of micro vessels in the peritoneal cavity (Badr et al. 2011). Administration of PGET to the tumor bearing mice causes an increase in life span accompanied by a reduction in WBC count. The persistence of life span, vanishing of leukemic cells from the blood and diminution of solid tumor volume is a consistent decisive factor for judging the effectiveness of anticancer drugs (Man et al. 2010). This is in line with the present study. The anticancer effects of PGET have been attributed to ellagic acid, a metabolite of the ellagitanins, and an abundant class of phytochemicals in pomegranate (Nair et al. 2011).
The common properties of cancer chemotherapeutic agents are myelo suppression and anemia (Hogland 1982) and these are the general factors that have been recurrently observed in ascites carcinoma (Maseki et al. 1981). Anemia encountered in ascites carcinoma mainly due to iron insufficiency, either by hemolytic or myelopathic conditions which finally lead to condensed RBC number (Gupta et al. 2004). Pal et al. (1993) have documented that the growth of EAC in mice was accompanied by a diminution in hemoglobin and RBC levels. In EAC bearing tumor control animals, lofty total WBC count and reduced hemoglobin content was observed. Moreover, PGET showed a defensive effect on hematopoietic system by reversal of total WBC cells and hemoglobin content in EAC bearing mice towards the value of normal group animals. This demonstrates that PGET have power over protective action on the hematopoietic system.
Flavonoids and phenolics exhibit a wide range of pharmacological and biological properties and usually scavenge the free radicals and play a vital role in deterrence and treatment of cancer. It is well recognized that Pomegranates is one of the rich resource of these phenolics and flavonoids which have antioxidant nature. It is well known that the redox state of the cell control its growth behavior (Pahl and Baeuerle 1994). The association between the growth of malignant cells and endogenous antioxidant systems is an aspect observed in several studies. A turn downed level of endogenous antioxidant system was observed in cancer patients (Casado et al. 1995) and in experimental carcinoma cell lines (Yellin et al. 1994). Numerous reports suggest that EAC induces oxidative stress in mice (Gupta et al. 2004; Haldar et al. 2010). Over production of reactive oxygen species (ROS) is endorsed to oxidative stress resulting in lipid peroxidation (LPO) and consequently increased malondialdehyde (MDA) and other TBARS levels (Brahmanaidu et al. 2016; Uddandrao et al. 2018a, b). Neilson et al. (1997) reported that TBARS, the end product of LPO increased in carcinomatous tissue than in non-diseased organs. Our results are in line with the previous finding. In the present study, the TBARS levels in the EAC control liver tissues were higher than normal liver tissues. An increased level of TBARS in EAC control mice indicated enhanced LPO leading to tissue damage and failure of the endogenous antioxidant defense mechanisms to avoid over production of ROS. Treatment with PGET improved hepatic LPO as discovered by increase of the MDA level. This disguised induction of ROS generation by pomegranate in tumor bearing mice, revealing its pro-oxidant effect.
GSH is a key endogenous non-enzymatic antioxidant which counterbalance free radical mediated damage. It is an effective inhibitor of the neoplastic process. It is well recognized that GSH is concerned in the fortification of normal cell function and structure by maintaining the redox homeostasis, extinguishing of free radicals and participating in detoxification reactions (Brahmanaidu et al. 2017). Depleted glutathione content was reported in human cancer cell lines (Yellin et al. 1994) and also in tumor bearing animals (Haldar et al. 2010). The reduced GSH may be due to diminution in its synthesis or to its dilapidation by oxidative stress in tumor bearing animals (Sharma et al. 1993). PGET treatment notably lowered the reduced hepatic glutathione content in tumor bearing mice. The results exhibited that the tumor proliferating activity of PGET was accompanied with improved cellular nonenzymatic antioxidant defense system.
The lowered level of cellular oxidative damage is connected with multiple non-enzymatic and enzymatic antioxidant defense systems present in cells. Enzymatic antioxidants such as CAT, GPx and SOD synergistically scavenge ROS and prevent LPO (Uddandrao et al. 2016). SOD, the natural cellular antioxidant enzyme, plays a crucial role in oxygen defense metabolism by intercepting and reducing superoxide to hydrogen peroxide (H2O2) (Brahmanaidu et al. 2017). CAT, a haem containing enzyme, is acknowledged to be implicated in detoxification of high H2O2 concentrations and protects the tissues from highly reactive hydroxyl radicals. GPx, selenium containing tetrameric glycoprotein, present in noteworthy concentrations, detoxifies H2O2 into water and molecular oxygen through the oxidation of reduced glutathione (Ewis and Abdel-Rahman 1995; Uddandrao et al. 2018a, b). SOD and CAT are involved in the clearance of superoxide and H2O2 radicals respectively. GPx has been exposed to be an imperative adaptive response to condition of increased peroxidative stress. During carcinogenesis, enzymatic antioxidants level was found to be inhibited. SOD, CAT and GPx activities were reported to be lower as a result of tumor growth (Casado et al. 1995). In the present study, decreased hepatic SOD, CAT and GPx activities were found in tumor bearing mice. This is in line with Haldar et al. (2010) who reported the turn downed level of antioxidant enzymes in tumor bearing mice. Administration of PGET enhanced the SOD, CAT and GPx activities extensively in tumor bearing mice. This indicates the prospective of PGET as an inhibitor of tumor provoked intracellular oxidative stress. The antioxidant principles found in edible plant foods showed cytotoxicity towards tumor cells and antitumor activity in experimental animals (Ruby et al. 1995).
In the current study, the histopathological examinations of the liver of EAC mice showed vascular congestion and mononuclear cellular infiltration of the hepatocytes, as well as areas of intertubular haemorrhage and mononuclear cellular infiltration. Interestingly, treatment with PGET and 5-FU reduced most of the pathological alterations induced by EAC cells in mice since it contained antioxidant compounds which were considered to be cytotoxic towards tumor cells (Ruby et al. 1995).
Conclusion
In conclusion, from the present examination the PGET showed an astonishing anti cancer effect on Swiss albino mice bearing EAC. The anticancer properties of P. granatum is most likely because of high content and synergistic activity of specific constituents present in the extract such as gallocatechins, delphinidin, cyanidin, gallic acid, ellagic acid, pelargonidin and sitosterol in it. However, the exact molecular mechanism by which PGET mediates its antitumor activity is not known. Further we will be investigated to identify the active component responsible for anti tumor activity and to unveil the molecular mechanism behind its therapeutic action.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Aviram M, Rosenblat M, Gaitini D, Nitecki S, Hoffman A, Dornfeld L (2004) Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation. Clin Nutr 23:423–433
Aziz MH, Kumae R, Ahmad N (2003) Cancer chemoprevention by resveratrol: in vitro and in vivo studies and the underlying mechanisms (review). Int J Oncol 23:17–28
Badr OT, Edress MM, Abdallah AM (2011) Anti tumour effect of egyptian propolis on ehrlich ascites carcinoma. Vet Ital 47:341–350
Beutler E, Kelly BM (1963) The effect of sodium nitrate on RBC glutathione. Experientia 19:96–97
Braga LC, Shupp JW, Cummings C, Jett M, Takahashi JA, Carmo LS (2005) Pomegranate extract inhibits Staphylococcus aureus growth and subsequent enterotoxin production. J Ethnopharmacol 96:335–339
Brahmanaidu P, Uddandrao VVS, Pothani S, Naik RR, Begum MS, Saravanan G et al (2016) Effects of S-allylcysteine on biomarkers of polyol pathway in experimental type II diabetes in rats. Can J Diab 40:442–448
Brahmanaidu P, Uddandrao VVS, Sasikumar V, Naik RR, Saravanan G et al (2017) Reversal of endothelial dysfunction in aorta of streptozotocinnicotinamide-induced type-2 diabetic rats by S-Allylcysteine. Mol Cell Biochem 432:25–32
Casado A, Torre R, Lopez-Fernandez M, Carrascosa D, Casado M, Ramirez M (1995) Superoxide dismutase and catalase blood levels in patients with malignant diseases. Cancer Lett 93:187–192
Ewis SA, Abdel-Rahman MS (1995) Effect of metformin on glutathione and magnesium in normal and streptozotocin-induced diabetic rats. J Appl Toxicol 15:387–390
Ferguson PJ, Kurowska E, Freeman DJ, Chambers AF, Koropatnick DJ (2004) A flavonoid fraction from cranberry extract inhibits proliferation of human tumor cell lines. J Nutr 134:1529–1535
Fraga CG, Leibouitz BE, Toppel AL (1988) Lipid peroxidation measured as TBARS in tissue slices: characterization and comparison with homogenates and microsomes. Free Radic Biol Med 4:155–161
Gupta M, Mazumder UK, Kumar RS, Sivakumar T, Vamsi MLM (2004) Antitumor activity and ntioxidant status of Caesalpinia bonducella against Ehrlich ascites carcinoma in Swiss albino mice. J Pharmacol Sci 94:177–184
Haldar PK, Kar B, Bala A, Bhattacharya S, Mazumder UK (2010) Antitumor activity of Sansevieria roxburghiana rhizome against Ehrlich ascites carcinoma in mice. Pharm Biol 48:1337–1343
Hogland HC (1982) Hematological complications of cancer chemotherapy. Semin Oncol 9:95–102
Jahan S, Chaudhary R, Goya PK (2009) Anticancer activity of an indian medicinal plant, alstonia scholaris, on skin carcinogenesis in mice. Integr Cancer Ther 8:273–279
Kakkar B, Das P, Viswanathan N (1984) Amodified spectrophotometric assay of SOD. Indian J Biochem Biophys 21:130–132
Man S, Gao W, Zhang Y, Huang L, Liu C (2010) Chemical study and medical applications of saponins as anticancer agents. Fitoterapia 81:703–714
Maseki M, Nishiagaki I, Hagishara M, Tamoda Y, Yagi K (1981) Lipid peroxidation levels and lipid content of serum lipoprotein fractions of pregnant subjects with or without pre-eclampsia. Clin Chim Acta 41:424–426
Motaa AA, Shaker S (2011) Anticancer and antioxidant activities of standardized whole fruit, pulp, and peel extracts of egyptian pomegranate. Open Conf Proc J 2:41–45
Mukherjee AK, Basu S, Sarkar N, Ghosh AC (2001) Advances in cancer therapy with plant based natural products. Curr Med Chem 8:1467–1486
Nair V, Dai Z, Maruf Khan P, Ciolino H (2011) Pomegranate extract induces cell cycle arrest and alters cellular phenotype of human pancreatic cancer cells. Anticancer Res 31:2699–2704
Neilson F, Mikkelson BB, Nielsen JB, Andersen HR, Grandjean P (1997) Plasma malondialdehyde as biomarker for oxidative stress, reference interval and effects of life-style factors. Clin Chem 47:1209–1214
Paglia D, Valentine W (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Pahl H, Baeuerle P (1994) Oxygen and the control of gene expression. Bio-Essays 16:497–502
Pal S, Ray MR, Maity P (1993) Tumor inhibition and hematopoietic stimulation in mice by a synthetic copper-ATP complex. Anticancer Drugs 4:505–510
Parmar HS, Kar A (2008) Medicinal values of fruit peels from Citrus sinensis, Punica granatum and Musa paradisiaca with respect to alterations in tissue lipid peroxidation and serum concentration of glucose, insulin and thyroid hormones. J Med Food 11:376–381
Rajeshwar Y, Gupta M, Mazumder UK (2005) Antitumor activity and in vivo antioxidant status of Mucuna pruriens (Fabaceae) seeds against Ehrlich ascites carcinoma in Swiss albino mice. Iranian J Pharmaco Ther 4:46–53
Ruby AJ, Kuttan G, Babu KD, Rajasekharan KN, Kuttan R (1995) Antitumor and antioxidant activity of natural curcuminoids. Cancer Lett 94:783–789
Segura JA, Barbero LG, Marquez J (2000) Ehrlich ascites tumor unbalances splenic cell population and reduces responsiveness of T cells to Staphylococcus aureus enterotoxin B stimulation. Immunol Lett 74:111–115
Sharma R, Singhal S, Srivastava S, Bajpai K, Frankel E, Awasthi S (1993) Glutathione and glutathione linked enzymes in human small lung cancer cell lines. Cancer Lett 75:111–119
Shimizu M, Azuma C, Taniguchi T, Murayama T (2004) Expression of cytosolic phospholipase A2a in murine C12 cells, a variant of L929 cells, induces arachidonic acid release in response to phorbol myristate acetate and Ca2++ ionophores, but not to tumor necrosis factor-a. J Pharmacol Sci 96:324–332
Uddandrao VVS, Brahmanaidu P, Meriga B, Saravanan G (2016) The potential role of S-allylcysteine as antioxidant against various disorders in animal models. Oxid Antioxid Med Sci 5:79–86
Uddandrao VVS, Brahmanaidu P, Saravanan G (2017) Therapeutical perspectives of S-allylcysteine: effect on diabetes and other disorders in animal models. Cardiovasc Hematol Agents Med Chem 15:71–77
Uddandrao VVS, Brahmanaidu P, Nivedha PR, Vadivukkarasi S, Saravanan G (2018a) Beneficial role of some natural products to attenuate the diabetic cardiomyopathy through Nrf2 pathway in cell culture and animal models. Cardiovasc Toxicol 18:199–205
Uddandrao VVS, Brahmanaidu P, Ravindarnaik R, Suresh P, Vadivukkarasi S, Saravanan S (2018b) Restorative potentiality of S-allylcysteine against diabetic nephropathy through attenuation of oxidative stress and inflammation in streptozotocin-nicotinamide induced diabetic rats. Eur J Nutr 1:1–2. https://doi.org/10.1007/s00394-018-1795-x
Yellin S, Davidson B, Pinto J, Sacks P, Qiao C, Schantz S (1994) Relationship of glutathione and glutathione-S-transferase to cisplatin sensitivity in human head and neck squamous carcinoma cell lines. Cancer Lett 85:223–232
Acknowledgement
The authors thank Muthyammal College of Arts and Science, Tamilnadu, India for providing facilities to do animal studies and also express heartfelt thanks to the management of K. S. Rangasamy College of Arts and Science, Tiruchengode, Tamilnadu for their support and encouragement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
The animal experiments were conducted according to the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India. The protocol of this study was approved by the institutional ethical committee of Muthyammal College of Arts and Science, Rasipuram, Tamilnadu, India (Approval No: 1416/P0/a/11/CPCSEA).
Conflict of interest
This manuscript described has not been published before; not under consideration for publication anywhere else; and has been approved by all co-authors.
Rights and permissions
About this article
Cite this article
Uddandrao, V.V.S., Parim, B., Nivedha, P.R. et al. Anticancer activity of pomegranate extract: effect on hematological and antioxidant profile against ehrlich-ascites-carcinoma in Swiss albino mice. Orient Pharm Exp Med 19, 243–250 (2019). https://doi.org/10.1007/s13596-018-0348-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13596-018-0348-4