Abstract
Cisplatin (cis-diamminedichloroplatinum (II) (CDDP)) is a commonly used chemotherapeutic drug for the treatment of numerous forms of cancer, but it has marked adverse effects, namely nephrotoxicity, hepatotoxicity, ototoxicity, neurotoxicity, diarrhoea etc. CDDP-induced emesis and diarrhoea are also noticeable toxicities that may be due to intestinal injury. Zingerone; a phenolic alkanone, one of the active components of ginger, possesses multiple biological activities, such as antioxidant and anti-inflammatory properties. In the present study, we investigated the protective effect of zingerone against CDDP-induced jejunal toxicity. Animals were divided into five groups I-IV (n = 6). Group II, III and IV received single intraperitoneal administration of CDDP at 7.5 mg/kg body weight on day 14. Animals of group II and III received oral treatment of zingerone at doses of 25 and 50 mg/kg body weight respectively for 14 consecutive days. While groups I was given distilled water 5 ml/kg body weight for 14 days. All the animals were killed after 24 h of CDDP injection. Zingerone ameliorated CDDP-induced lipid peroxidation, increase in xanthine oxidase activity, glutathione depletion, decrease in antioxidant and phase-II detoxifying enzyme activities. Zingerone attenuated CDDP-induced nuclear factor (NF-κB) activation, enhanced levels of TNF-α and Nitrite. The results showed that zingerone had not only the antioxidant effect by suppression of ROS, but also anti-inflammatory effects by suppression of NF-κB activation. In addition, zingerone treatment suppressed gene activation of pro-inflammatory cytokine, TNF-α, which was up-regulated with CDDP administration through NF-κB activation. These experiments strongly indicate that zingerone treatment exercises a protective efficacy by suppressing both oxidative stress and inflammation through the modulation of key pro-inflammatory cytokine and transcription factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To improve the quality life of cancer patients, their treatment by the use of chemotherapeutic agents has opened a new future. Irrespective of this success, many anticancer drugs have shown punitive side effects in experimental systems and patients as well (Koc et al. 2005; Zicca et al. 2004). Cisplatin [cis-diamminedichloroplatinum (II) (CDDP) or cisplatinum] (Fig. 1) is a platinum (Pt) containing antineoplastic drug, which regardless of its associated side effects is commonly used for the treatment of many malignancies (Adenis et al. 2005; Wang et al. 2004; Saad et al. 2004). It has numerous intracellular effects that cause direct cytotoxicity with reactive oxygen species and activates mitogen-activated protein kinases, inducing apoptosis and stimulating inflammation (Rehman et al. 2013; Yao et al. 2007).
Effect of zingerone pre-treatment on CDDP induced increase in NFκB. Values are expressed as mean ± SEM. (n = 6). *** p < 0.001 shows significant difference in Group II (CDDP 7.5 mg/kg b.wt) when compared with Group I. # p < 0.001 shows significant difference in the Group III (CDDP 7.5 mg/kg b.wt + zingerone 25 mg/kg b.wt) when compared with Group II and ## p < 0.001 also shows significant difference in Group IV (CDDP 7.5 mg/kg b.wt + zingerone 50 mg/kg b.wt) as compared to Group II
The exact mechanism of CDDP toxicity although not fully understood, may possibly be through the formation of DNA adduct and the production of panoply of reactive oxygen species (ROS) e.g., superoxide anion (O2•−), hydrogen peroxide (H2O2), hydroxyl radical (•OH) etc. which in turn interact with DNA, lipids and proteins (Sun 1990). Although CDDP can act on the sulfhydryl (−SH) groups of cellular proteins (Basu and Krishnamurthy 2010), DNA is the main cellular target of CDDP that leads to DNA damage induced by ROS and Pt-DNA adduct formation, hampering not only the cell division or DNA synthesis both its repair mechanisms as well (Eastman 1985; Sherman et al. 1985).
Some reports have revealed not only the nonspecific nature of CDDP against tumours but also its cytotoxic nature to rapidly dividing normal cells viz., intestinal epithelial cells, through the production of ROS, which proposes a nidus for the development of oxidative stress (Vijayalakshmi et al. 2006). The upcoming nutraceuticals occuring naturally have been reported to increase the anticancer activities as well as reduce the severe side effects of antitumor drugs (Guerrero-Beltrán et al. 2010; Zhang et al. 2010; Longo et al. 2011). Hence the need of the hour is to look out for those natural compounds that can reduce the CDDP-induced toxicity and recover its chemotherapeutic efficacy.
Ginger, due to its characteristic aromatic and pungent flavor is one of the most widely spices used as a condiment for a variety of foods and beverages. In folk medicine, ginger the branched rhizome of Zingiber officinale Roscoe (Zingiberaceae) has been traditionally used as a diaphoretic, a carminative, and an antispasmodic and as an anti-emetic agent against motion sickness and hyperemesis gravidarum (Langner et al. 1998). Studies conducted in recent era have suggested about diverse biological roles that ginger may play in antioxidative, anti-inflammatory, anti-carcinogenic, anti-nausea, antithrombotic, hypolipidemic, cardiovascular and antibacterial processes (Grzannar et al. 2005; Kadnur and Goyal 2005; Stoilova et al. 2007; Nicoll and Henein 2009; Kumar et al. 2014; Xie et al. 2014). Various components of ginger have been reported to have antioxidant and anti-inflammatory activity, besides exhibiting anti-tumor activity in rodent chemical carcinogenesis (Koo et al. 2011; Park et al. 1998; Ali et al. 2007).
The main constituents of ginger include zingerone, paradol, gingerols, and shogaols (Ali et al. 2007). Among other constituents isolated, zingerone one of the active constituents of ginger responsible for its pungent taste is a phenolic alkanone containing vanilloid (3-methoxy-4-hydroxy benzene) group in its structure. Earlier reports revealed that zingerone plays significant roles in numerous functional responses of mammals, such as enhancing anti-inflammatory and antioxidant effects (Rao et al. 2009; Chung et al. 2009; Kumar et al. 2014; Xie et al. 2014), reducing radiation-induced turn down in endogenous antioxidant levels, scavenging radiation-induced free radicals (Rao and Rao 2010). Besides, zingerone might also wield useful therapeutic effects on hypermotility-induced diarrhea by abrogating undue gastrointestinal motility in rats (Iwami et al. 2011).
In view of the above facts, we hypothesize that the prophylactic treatment of zingerone might have protective effects against CDDP-induced jejunum toxicity by intervening with inflammatory pathway and oxidative processes. In the present study, we investigated the protective role of zingerone against CDDP induced oxidative stress, inflammatory responses and jejunal injure in Wistar rats.
Methods
Chemicals
Zingerone was purchased from SRL Ranbaxy, India. CDDP was purchased from Dr Reddy’s India. H2O2, magnesium chloride, sulphosalicylic acid, perchloric acid, TCA, Tween-20, Folin–Ciocalteau reagent, sodium potassium tartarate, di-sodium hydrogen phosphate, sodium di-hydrogen phosphate and sodium hydroxide were purchased from E. Merck Limited. All other chemicals and reagents were of the highest-purity grade commercially available.
Animals
For the experimental study, 4- to 6-week-old male albino rats (120–150 g) of the Wistar strain were obtained from the Central Animal House of university, All procedures for using experimental animals were checked and permitted by the ‘Institutional Animal Ethical Committee’ that is fully accredited by the Committee for Purpose of Control and Supervision on Experiments on Animals (CPCSEA). The animals were housed in polypropylene cages in groups of four rats per cage and were kept in a room maintained at 25–28 °C with a 12 h light–12 h dark cycle. They were allowed to acclimatize for 1 week before the experiments and were given free access to standard laboratory animal diet and water ad libitum.
Treatment regimen
To study the effect of prophylactic treatment with Zingerone on CDDP-induced oxidative stress responses in the jejunum, 25 male Wistar rats were randomly allocated to four groups of six rats each. The rats of Group I (control group) received double distilled water at the dose of 5 ml/kg body weight (b.wt.) once daily for 14 days, which was used as a vehicle for zingerone. Group III received zingerone orally at the dose of 25 mg/kg b.wt. once daily for 14 consecutive days. Groups IV received zingerone at the dose of 50 mg/kg b.wt. once daily for 14 days. Groups II, III and IV were given a single injection of CDDP at the dose of 7.5 mg/kg b.wt., intraperitoneally on day 14 after 1 h of the last treatment with zingerone. All the rats were anaesthetised with mild anaesthesia and killed by cervical dislocation after 24 h of the CDDP injection.
Post-mitochondrial supernatant preparation and estimation of different parameters
Jejunums were removed quickly, cleaned free of irrelevant material and immediately perfused with ice-cold saline (0.85 % NaCl). The jejunums (10 % w/v) were homogenized in chilled phosphate buffer (0.1 M, pH 7.4) using a Potter Elvehjen homogeniser. The homogenate was filtered through muslin cloth, and centrifuged at 3000 rpm for 10 min at 48C in a Remi Cooling Centrifuge (C-24 DL) to separate the nuclear debris. The aliquot so obtained was centrifuged at 12 000 rpm for 20 min at 48C to obtain post-mitochondrial supernatant (PMS), which was used as a source of various enzymes.
Measurement of lipid peroxidation
The assay for membrane lipid peroxidation (LPO) was done by the method of Wright et al. 1981, with some modifications. The reaction mixture in a total volume of 3.0 ml contained 1.0 ml tissue homogenate, 1.0 ml of TCA (10 %) and 1.0 ml thiobarbituric acid (0.67 %). All the test tubes were placed in a boiling-water bath for a period of 45 min. The tubes were then shifted to an ice-bath and centrifuged at 2500 g for 10 min. The amount of malondialdehyde (MDA) formed in each of the samples was assessed by measuring the optical density of the supernatant at 532 nm. The results were expressed as the nmol MDA formed/g tissue by using a molar extinction coefficient of 1.56 £ 105/M per cm.
Measurement of xanthine oxidase activity
The activity of xanthine oxidase (XO) was assayed by the method of Stripe and Della Corte 1969. The reaction mixture consisted of 0.2 ml PMS which was incubated for 5 min at 378C with 0.8 ml phosphate buffer (0.1 M, pH 7.4). The reaction was started by adding 0.1 ml xanthine (9 mM) and kept at 378C for 20 min. The reaction was terminated by the addition of 0.5 ml ice-cold perchloric acid (10 % (v/v)). After 10 min, 2.4 ml of distilled water were added and centrifuged at 4000 rpm for 10 min and mg uric acid formed/min per mg protein was recorded at 290 nm.
Measurement of reduced glutathione level
The GSH content in jejunum was determined by the method of Jollow et al. 1974 in which 1.0 ml of PMS fraction (10 %) was mixed with 1.0 ml of sulphosalicylic acid (4 %). The samples were incubated at 48C for at least 1 h and then subjected to centrifugationat 1200 g for 15 min at 48C. The assay mixture contained 0.4 ml filtered aliquot, 2.2 ml phosphate buffer (0.1 M, pH 7.4) and 0.4 ml 5, 50-dithio-bis-(2-nitrobenzoic acid; 10 mM) in a total volume of 3.0 ml. The yellow colour developed was read immediately at 412 nm on a spectrophotometer (Milton Roy Model-21 D). The GSH content was calculated as mmol 5,50-dithio-bis-(2-nitrobenzoic acid) conjugate formed/g tissue using a molar extinction coefficient of 13.6 £ 103/M per cm.
Measurement of glutathione peroxidase activity
The glutathione peroxidase (GPx) activity was calculated by the method of Mohandas et al. 1984. A total of 2 ml volume consisted of 0.1 ml EDTA (1 mM), 0.1 ml sodium azide (1 mM), 1.44 ml phosphate buffer (0.1 M, pH 7.4), 0.05 ml GR (1 IU/ml), 0.05 ml GSH (1 mM), 0.1 ml NADPH (0.2 mM) and 0.01 ml H2O2 (0.25 mM) and 0.1 ml 10 % PMS. The depletion of NADPH at 340 nm was recorded at 258C. The enzyme activity was calculated as mmol NADPH oxidised/min per mg protein with the molar extinction coefficient of 6.22 £ 103/M per cm.
Measurement of superoxide dismutase activity
The superoxide dismutase (SOD) activity was measured by the method of Marklund and Marklund 1974. The reaction mixture consisted of 2.875 ml Tris–HCl buffer (50 mM, pH 8.5), pyrogallol (24 mM in 10 mM HCl) and 100 ml PMS in a total volume of 3 ml. The enzyme activity was measured at 420 nm and was expressed as units/mg protein. Here, one unit of enzyme is defined as the enzyme activity that inhibits the auto-oxidation of pyrogallol by 50 %.
Measurement of catalase activity
The catalase (CAT) activity was measured by the method of Claiborne 1985. In brief, the assay mixture consisted of 2.0 ml phosphate buffer (0.1 M, pH 7.4), 0.95 ml H2O2 (0.019 M) and 0.05 ml of PMS (10 %) in a final volume of 3.0 ml. Changes in absorbance were recorded at 240 nm. The CAT activity was calculated in terms of nmol H2O2 consumed/min per mg protein.
Measurement of glutathione reductase activity
The GR activity was measured by the method of Carlberg and Mannervik 1975. The assay system consisted of 1.65 ml phosphate buffer (0.1 M, pH 7.6), 0.1 ml EDTA (0.5 mM), 0.05 ml oxidizsed glutathione (1.0 mM), 0.1 ml NADPH (0.1 mM) and 0.1 ml of 10 % PMS in a total volume of 2.0 ml. The enzyme activity was assessed at 25 °C by measuring the disappearance of NADPH at 340 nm and was calculated as nmol NADPH oxidised/ min per mg protein using a molar extinction coefficient of 6.22 × 103/M per cm.
Measurement of glutathione-S-transferase activity
The glutathione-S-transferase (GST) activity was measured by the method of Habig et al. 1974. The reaction mixture consisted of 2.4 ml phosphate buffer (0.1 M, pH 6.5), 0.2 ml GSH (1.0 mM), 0.2 ml 1-chloro-2,4-dinitrobenzene (1.0 mM) and 0.2 ml of cytosolic fraction in a total volume of 3.0 ml. The changes in absorbance were recorded at 340 nm and the enzyme activity was calculated as mmol 1-chloro-2, 4-dinitrobenzene conjugate formed/min per mg protein using a molar extinction coefficient of 9.6 × 103/M per cm.
NF-κB estimation in nuclear fraction
NF-κB content translocated to nucleus was estimated by using an ELISA kit (NF- κB p65 ELISA) (Invitrogen Corporation, CA, USA) in nuclear fraction of jejunum tissue according to protocol provided by the manufacturer.
Assay for nitrite levels
Nitrite assay was done using Griess reagent by the method of Green et al. 1982 with some modifications. In brief, 100 μl of Griess reagent (1:1 solution of 1 % sulfanilamide in 5 % phosphoric acid and 0.1 % naphthylethylene diamine dihydrochloride in water) was added to 100 μl of PMS incubate for 5–10 min at room temperature protected from light. Purple/magenta color began to form immediately. Absorbance was measured at 546 nm, nitrite concentration was calculated using a standard curve for sodium nitrite, and nitrite levels were expressed as l mol/mg protein.
Measurement of protein
The protein concentration in all samples was determined by the method of Lowry et al. 1951 using bovine serum albumin as the standard.
Assay for TNF-α Level
TNF-α protein level was measured by enzyme-linked immunosorbent assay (ELISA) kit (eBioscience, Inc., San Diego, USA). Analysis was performed according to the manufacturer’s instruction.
Statistical analysis
The data from individual groups are presented as the mean ± standard error of the mean (SEM). Differences between groups were analysed by using one way analysis of variance (ANOVA) followed by Tukey–Kramer multiple comparisons test and minimum criterion for statistical significance was set at p < 0.05 for all comparisons.
Results
Effect of prophylactic treatment of zingerone against CDDP-induced reduced glutathione depletion in the jejunum
The level of GSH was depleted significantly (P < 0.01) in the CDDP-treated group (Group II) as compared to the control group (Group I). Zingerone pretreatment showed a significant increase in the level of GSH in Group III (P < 0.05) and Group IV (P < 0.001) when compared with Group II (Table 1).
Effect of zingerone supplementation and CDDP on the activities of glutathione-dependent enzymes in the jejunum
CDDP treatment caused a significant decrease in the activities of GPx (P < 0.001), GST (P < 0.001) and GR (P < 0.01) in Group II as compared to Group I. Zingerone supplementation at the dose of 25 mg/kg b.wt. significantly increased the activity of GPx (P < 0.01) and GST (P < 0.05), GR (P < 0.01) in Group III as compared to Group II. But the higher dose of zingerone (50 mg/kg b.wt.) significantly attenuated the activities of GPx (P < 0.01), GST (P < 0.001), GR (P < 0.01) in Group IV as compared to Group II (Table 1).
Effect of prophylactic treatment of zingerone against CDDP-induced lipid peroxidation
The level of MDA was significantly enhanced (P < 0.001) in Group II as compared to Group I. Zingerone pretreatment significantly decreased the level of MDA in Group III (P < 0.05) and Group IV (P < 0.001), respectively, as compared to Group II (Table 2).
Effect of zingerone pretreatment and CDDP on the xanthine oxidase activity in jejunum
The activity of XO was significantly increased (P < 0.001) in Group II as compared to Group I. Zingerone pretreatment significantly decreased the activity of XO in Group III (P < 0.01) and Group IV (P < 0.001) as compared to Group II (Table 2).
Effect of zingerone supplementation and CDDP on the activities of antioxidant enzymes in the jejunum
The activities of CAT and SOD were decreased significantly (P < 0.001 and P < 0.01 respectively), in Group II as compared to Group I. Zingerone pretreatment at the dose of 25 mg/kg b.wt. significantly augmented the activities of CAT (P < 0.01) and SOD (P < 0.05) in Group III as compared to Group II. The higher dose of zingerone (50 mg/kg b.wt.) also showed significant increase in the activities of CAT (P < 0.001) and SOD (P < 0.01) in Group IV as compared to Group II (Table 2).
Effect of zingerone and CDDP on NFκB
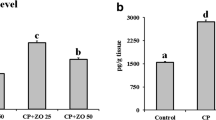
Level of NFκB was found elevated significantly in CDDP-treated group in comparison with Group I (P < 0.001). Pretreatment with zingerone in groups III and IV significantly (P < 0.05, P < 0.01) decreased the NFκB level (Fig. 1).
Effect of zingerone and CDDP treatment on TNF-α levels
We have assessed the effect zingerone on CDDP-induced jejunal TNF-α (Fig. 2). We found that there was a significant difference in the level of proinflammatory cytokines between Group I (control group) and CDPP-treated Group II (P < 0.001). Pre-treatment with zingerone significantly inhibit their production in the Group III (P < 0.05) & Group IV (P < 0.01) when compared with the only CDDP-treated Group II.
Effect of zingerone pre-treatment on CDDP induced increase in TNF-α. Values are expressed as mean ± SEM. (n = 6). *** p < 0.001 shows significant difference in Group II (CDDP 7.5 mg/kg b.wt) when compared with Group I. # p < 0.01 shows significant difference in the Group III (CDDP 7.5 mg/kg b.wt + zingerone 25 mg/kg b.wt) when compared with Group II and ## p < 0.001 also shows significant difference in Group IV (CDDP 7.5 mg/kg b.wt + zingerone 50 mg/kg b.wt) as compared to Group II
Effect of zingerone and CDDP on the NO production
Administration of CDDP resulted in the elevated jejunum NO production in the Group II as compared with the group I (p < 0.001). We observed that pre-treatment with zingerone was significantly effective in reducing NO production in Group III & IV when compared with the Group II (p < 0.01, p < 0.001) (Fig. 3).
Effect of zingerone pre-treatment on CDDP induced nitric oxide formation. Values are expressed as mean ± SEM. (n = 6). *** p < 0.001 shows significant difference in Group II (CDDP 7.5 mg/kg b.wt) when compared with Group I. ## p < 0.01 shows significant difference in the Group III (CDDP 7.5 mg/kg b.wt + zingerone 25 mg/kg b.wt) when compared with Group II and ### p < 0.001 also shows significant difference in Group IV (CDDP 7.5 mg/kg b.wt + zingerone 50 mg/kg b.wt) as compared to Group II
Discussion
In this study, we have observed the protective effects of zingerone against CDDP-induced jejunal toxicity in Wistar rats. The CDDP-induced renal toxicity is well documented (Miller et al. 2010; Eljack et al. 2014). CDDP-induced jejunal toxicity is still unclear but it may be through the formation of DNA adduct and the production of panoply of reactive oxygen species (ROS) by CDDP which leads to the condition of oxidative stress. Therefore, the natural compounds with antioxidant properties are gaining much attention. The present investigation was carried out to elucidate the effect of zingerone on CDDP-induced jejunal toxicity in rats and to assess its role in modulation of oxidative and inflammatory pathway.
Ginger, a pervasive herbal medicine having an antioxidant property has been given a scientific approval for use in problems related to oxidative process (Shukla and Singh 2007; Ali et al. 2007; Kim et al. 2007). Ginger owing to its practical potency, has been the focus of intensive scientific research over the past two decades (Langner et al. 1998; Shukla and Singh 2007). Among the key constituents of ginger, zingerone has been well recognized for its anti-mutagenic and anti-carcinogenic activities, and recently reported to have been associated with anti-inflammatory and anti-oxidative activities (Surh et al. 2001). In this study, we observed that pretreatment with zingerone demonstrates protection against CDDP-induced jejunal toxicity. The search for finding naturally occurring dietary antioxidants that can effectively protect against CDDP induced gastrointestinal toxicity is gaining much attention. In the present study, we have observed the protective effects of zingerone against CDDP-induced jejunal toxicity.
CDDP plays vital role in the induction of lipid peroxidation via production of free radicals like superoxide anion (O2•−), hydrogen peroxide (H2O2), hydroxyl radical (•OH) (Yao et al. 2007). Several studies have reported that oxidative stress observed as a result of CDDP treatment is marked by remarkable elevation in the level of malondialdehyde (MDA), a lipid peroxidation product (Khan, Khan et al. 2012; Rehman et al. 2014). Our results concur with the previous findings which revealed a significant increase in the level of MDA in rats treated with CDDP and pretreatment with zingerone considerably reduces the level of MDA. It was also observed that enhanced xanthine oxidase (XO) activity and CDDP-induced GSH depletion further substantiate the CDDP induced oxidative damage in the colon of Wistar rats. Zingerone supplementation therefore remarkably attenuates the GSH depletion and XO activity. XO is an enzyme that reduces O2 to superoxide anion radical (O2•−) and accordingly produce oxidative stress (Heunks and Dekhuijzen 2000) while GSH a tripeptide is a low molecular weight cellular antioxidant. GSH protects the peroxidation of lipid membrane by conjugating with the electrophile such as 4-Hydroxy-3-nonenal (HNE), and thus gets depleted in this conjugation reaction (Kawanishi and Yamamoto 1991). This conjugation reaction of GSH via sulphahydryl (SH) group to electrophile is catalyzed by glutathione-S-transferase (GST), an antioxidant enzyme which exhibits decreased activity during the process (Forman et al. 2009).
Moreover our study perceived that detoxifying enzymes like CAT, GPX, GR and Phase–II GST were decreased while as the SOD activity increased in CDDP treated rats. Zingerone pretreatment considerably attenuated the activities of these antioxidant and phase-II detoxifying enzymes. In our study, the increase in SOD activity in CDDP treated group is in agreement with the previous findings which demonstrate over-expression of SOD to lessen the CDDP toxicity during CDDP treatment (Vijayalakshmi et al. 2006). The GST enzyme detoxifies a number of ROS via catalyzing the conjugation with GSH (Manar et al. 2004). The diminished activities of antioxidant enzymes (viz., GPx, CAT and GR) and phase-II detoxifying enzymes (viz., GST) in CDDP-treated groups support the involvement of oxidative stress in the pathophysiology of CDDP-induced jejunum toxicity.
Inflammation induced as a result of oxidative stress also plays an important physiological role in CDDP induced hepatotoxicity via multiple intercalating pathways (Pala and Gurkan 2008). Most of the pathological conditions illustrated by oxidative stress consequently result in an increased level of nitrites (Federico et al. 2007). Increased levels of NO following CDDP treatment further react with the superoxide radical leading to the formation of the cytotoxic peroxynitrite which increases the chances of organ injury (Koppenol et al. 1992; Laskin 2009). Zingerone treatment attenuated this abnormal increase in the level of NO. NFκB activation is crucial in the expression of proinflammatory cytokines like TNF-α and other mediators involved in acute inflammatory responses and other conditions related to increased ROS generation. Inhibitors of NFκB have shown protection against CDDP induced toxicity. The proinflammatory cytokine TNF-α, also has been proven to play a vital role in the patho-mechanism of CDDP-induced injury. CDDP exposure elicits acute inflammatory responses prompted via induction of NFκB and TNF-α, which is in conformity with previous reports (Ramesh and Reeves 2002; Francescato et al. 2007). Our results showed decrease in the inflammatory responses involved in the jejunum toxicity caused by CDDP due to the decreased expression of both NFκB and TNF-α significantly by the prophylactic treatments of zingerone (Figs. 2 and 3). Further, stimulation of transcription factor NF-κB by TNF- α causes genes to generate potentially cell damaging g oxidative enzymes like NADP oxidase, cyclo-oxygenase (COX-2) and iNOS, besides release of TNF-α and other pro-inflammatory cytokines (Nanji et al. 2003).
Conclusion
The biochemical and molecular findings of the present study reveal the antioxidant and anti-inflammatory properties of zingerone against CDDP-induced Jejunal toxicity. The exact mechanism of zingerone’s protective action against CDDP although still unknown but plausible mechanism concluded from the findings of the present study suggests that zingerone’s protective effect against CDDP-induced jejunal toxicity probably might be through the attenuation of oxidative stress and inflammation. In addition, we have also demonstrated that zingerone aids in maintaining antioxidant armory and also suppresses activation of redox active transcription factor: NFκB. In view of the above findings, we hypothesize its use as a combinational therapy with CDDP but before that further studies are still needed to elucidate the exact protective mechanism of zingerone.
References
Adenis A, Carlier D et al (2005) Cytarabine and cisplatin as salvage therapy in patients with metastatic colorectal cancer who failed 5-fluorouracil + folinic acid regimen. French Northern Oncology Group. Am J Clin Oncol 18:158–160
Ali BH, Blunden G et al (2007) Some phytochemical, pharmacological and toxicological properties of ginger Zingiber officinalis Roscoe: a review of recent research. Food Chem Toxicol 46:409–20
Basu A, Krishnamurthy S (2010) Cellular responses to cisplatin-induced 501 DNA damage. J Nucleic Acids 1–16. http://dx.doi.org/10.4061/2010/201367
Carlberg I, Mannervik B (1975) Glutathione level in rat brain. J Biol Chem 250:4480–4575
Chung SW, Kim MK et al (2009) Peroxisome proliferator-activated receptor activation by a short term feeding of zingerone in aged rats. J Med Food 12:345–350
Claiborne A (1985) Catalase activity. In: Greenwald RA (ed) CRC handbook of methods in oxygen radical research. CRC Press, Boca Raton, pp 283–284
Eastman A (1985) Activation of programmed cell death by anticancer agents: cisplatin as a model system. Cancer Cells 2:275–280
Eljack ND, Ma HY, Drucker J, Shen C, Hambley TW, New EJ, Friedrich T, Clarke RJ (2014) Mechanisms of cell uptake and toxicity of the anticancer drug cisplatin. Metallomics 6(11):2126–2133
Federico A, Morgillo F et al (2007) Chronic inflammation and oxidative stress in human carcinogenesis. Int J Cancer 121:2381–2386
Forman HJ, Zhang H et al (2009) Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol Asp Med 30:1–12
Francescato HDC, Costa RS et al (2007) Parthenolide reduces cisplatin-induced renal damage. Toxicology 230:64–75
Green LC, Wagner DA et al (1982) Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem 126:131–138
Grzannar R, Lindmark L et al (2005) Gingere, an herbal medicinal product with broad anti-inflammatory actions. J Med Food 8:125–32
Guerrero-Beltrán CE, Calderón-Oliver M et al (2010) Sulforaphane protects against cisplatin-induced nephrotoxicity. Toxicol Lett 192:278–285
Habig WH, Pabst MJ et al (1974) Gluta thione-S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Heunks LM, Dekhuijzen PN (2000) Respiratory muscle function and free radicals: from cell to COPD. Thorax 55:704–716
Iwami M, Shiina T et al (2011) Inhibitory effects of zingerone, a pungent component of Zingiber officinale Roscoe, on colonic motility in rats. J Nat Med 65:89–94
Jollow DJ, Mitchell JR et al (1974) Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 11:151–169
Kadnur SV, Goyal RK (2005) Beneficial effects of Zingiber officinale Roscoe on fructose induced hyperlipidemia and hyperinsulinemia in rats. Indian J Exp Biol 43:1161–64
Kawanishi S, Yamamoto K (1991) Mechanism of site-specific DNA damage induced by methylhydrazines in the presence of copper (II) or manganese (III). Biochemistry 30:3069–3075
Khan R, Khan AQ et al (2012) Chrysin protects against cisplatin-induced colon toxicity via amelioration of oxidative stress and apoptosis: Probable role of p38MAPK and p53. Toxicol Appl Pharmacol 258(3):315–329
Kim JK, Kim Y et al (2007) Gingerol prevents UVB induced ROS production and COX-2 expression in vitro and in vivo. Free Radic Res 41:603–614
Koc A, Duru M et al (2005) Protective agent, erdosteine, against cisplatininduced hepatic oxidant injury in rats. Mol Cell Biochem 278:79–84
Koo KLK, Ammit AJ et al (2011) [6]-Gingerols and related analogues inhibit arachidonic acid-induced human platelet serotonin release and aggregation. Thromb Res 03:387–397
Koppenol WH, Moreno JJ et al (1992) Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chem Res Toxicol 5:834–842
Kumar L, Chhibber S, Harjai K (2014) Hepatoprotective effect of zingerone (4-(4-hydroxy-3-methoxyphenyl) butan-2-One) in lipopolysaccharide induced liver injury mouse model through down regulation of inflammatory mediators. Int J Pharmacogn Phytochem Res 6:308–314
Langner E, Greifenberg S et al (1998) Ginger: history and use. Adv Ther 15:25–44
Laskin DL (2009) Macrophages and inflammatory mediators in chemical toxicity: a battle of forces. Chem Res Toxicol 22:1376–1385
Longo V, Gervasi PG et al (2011) Cisplatin induced toxicity in rat tissues: the protective effect of Lisosan G. Food Chem Toxicol 49:233–237
Lowry OH, Rosebrough NJ et al (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Manar MH, Brown MR et al (2004) Association of glutathione- S-transferase-P1 (GST-P1) polymorphisms with bronchopulmonary dysplasia. J Perinatol 24:30–35
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
Miller RP, Tadagavadi RK, Ramesh G, Reeves WB (2010) Mechanisms of cisplatin nephrotoxicity.Toxins (Basel) 2:2490–2518
Mohandas M, Marshall JJ et al (1984) Differential distribution of glutathione and glutathione-related enzymes in rabbit kidney: possible implications in analgesic nephropathy. Biochem Pharmacol 33:1801–1807
Nanji AA, Jokelainen K et al (2003) Curcumin prevents alcohol-induced liver disease in rats by inhibiting the expression of NF-kappa B-dependent genes. Am J Physiol Gastrointest Liver Physiol 284:321–327
Nicoll R, Henein MY (2009) Ginger (Zingiber officinale Roscoe): a hot remedy for cardiovascular disease? Int J Cardiol 131:408–9
Pala FS, Gurkan H (2008) The role of free radicals in ethiopathogenesis of diseases. Adv Mol Biol 1:1–9
Park KK, Chun KS et al (1998) Inhibitory eVects of [6]-gingerol, a major pungent principle of ginger, on phorbol ester-induced inXammation, epidermal ornithine decarboxylase activity and skin tumor promotion in ICR mice. Cancer Lett 129:139–144
Ramesh G, Reeves WB (2002) TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest 110:835–842
Rao BN, Rao BS (2010) Antagonistic effects of Zingerone, a phenolic alkanone against radiation-induced cytotoxicity, genotoxicity, apoptosis and oxidative stress in Chinese hamster lung fibroblast cells growing in vitro. Mutagenesis 25:577–87
Rao BN, Rao BS et al (2009) Radiomodifying and anticlastogenic effect of zingerone on Swiss albino mice exposed to whole body gamma radiation. Mutat Res 677:33–41
Rehman MU, Tahir M et al (2013) Chrysin suppresses renal carcinogenesis via amelioration of hyperproliferation, oxidative stress and inflammation: plausible role of NF-κB. Toxicol Lett 216(2–3):146–58
Rehman MU, Tahir M et al (2014) D-limonene suppresses doxorubicin-induced oxidative stress and inflammation via repression of COX-2, iNOS, and NFκB in kidneys of Wistar rats. Exp Biol Med 239(4):465–76
Saad SY, Najjar TA et al (2004) Role of nonselective adenosine receptor blockade and phosphodiesterase inhibition in cisplatin-induced nephrogonadal toxicity in rats. Clin Exp Pharmacol Physiol 31:862–867
Sherman SE, Gibson D et al (1985) X-ray structure of the major adduct of the anticancer drug cisplatin with DNA: cis-[Pt (NH3)2 {d (pGpG)}]. Science 230:412–417
Shukla Y, Singh M (2007) Cancer preventive properties of ginger: a brief review. Food Chem Toxicol 45:683–690
Stoilova I, Krastanov A et al (2007) Antioxidant activity of a ginger extract. Food Chem 102:764e70
Stripe F, Della Corte E (1969) The regulation of rat liver xanthine oxidase. J Biol Chem 244:3855–3863
Sun Y (1990) Free radicals, antioxidant enzymes and carcinogenesis. Free Radic Biol Med 8:583–599
Surh YJ, Chun K et al (2001) Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat Res 1:480–481
Vijayalakshmi B, Sesikeran B et al (2006) Chronic low vitamin intake potentiates cisplatin-induced intestinal epithelial cell apoptosis in WNIN rats. World J Gastroenterol 12:1078–1085
Wang G, Reed E et al (2004) Molecular basis of cellular response to cisplatin chemotherapy in non-small cell lung cancer. Oncol Rep 2:955–965
Wright JR, Colby HD et al (1981) Cytosolic factors which affect microsomal lipid peroxidation in lung and liver. Arch Biochem Biophys 206:296–304
Xie X, Sun S et al (2014) Zingerone attenuates lipopolysaccharide-induced acute lung injury in mice. Int Immunopharmacol 19:103–109
Yao X, Panichpisal K et al (2007) Cisplatin nephrotoxicity: a review. Am J Med Sci 334:115
Zhang X, Yeung ED et al (2010) Isoliquiritigenin, a natural anti-oxidant, selectively inhibits the proliferation of prostate cancer cells. Clin Exp Pharmacol Physiol 37:841–847
Zicca A, Cafaggi S et al (2004) Reduction of cisplatin hepatotoxicity by procainamide hydrochloride in rats. Eur J Pharmacol 442:265–272
Acknowledgments
The Authors are thankful to Vice Chancellor (Dr Tej Partab) for providing facilities to complete this research work.
Ethical statement
All procedures for using experimental animals were checked and permitted by the ‘Institutional Animal Ethical Committee’ that is fully accredited by the Committee for Purpose of Control and Supervision of Experiments on Animals (CPCSEA).
Conflict of Interest
The authors declare that they have no conflict interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rehman, M.U., Ahmad, B., Arif, A. et al. Zingerone protects against cisplatin-induced oxidative damage in the jejunum of Wistar rats. Orient Pharm Exp Med 15, 199–206 (2015). https://doi.org/10.1007/s13596-015-0187-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13596-015-0187-5