Abstract
Herbal remedies for human brain disorders are much preferred over synthetic drugs because of various side effects of synthetic drugs ranging from sleep disorders to withdrawal syndromes. Passiflora incarnata Linn (Passifloraceae) has been widely used in traditional medicine in West India, Mexico, the Netherlands, and South America. It has been used as an anxiolytic and sedative-hypnotic since ancient times. The main constituents of leaves of P. incarnata are flavonoid and indole alkaloids based on β -carboline ring system viz., harman, harmine, harmalol and harmaline. Harmine and harmaline alkaloids are reported to be effective antiparkinsonian compounds. The objective of present investigation was to evaluate antiparkinsonian activity and antioxidant activity of butanol extract of P. incarnata leaves (BEPI). Antiparkinsonian activity of BEPI was studied using haloperidol induced catalepsy and tacrine induced jaw movements. The antioxidant activity of BEPI was studied using DPPH scavenging assay and H2O2 scavenging assay. The results of present study has shown that butanol extract of P. incarnata leaves (BEPI) (150 mg kg−1 and 300 mg kg−1, i.p.) significantly (p ≤ 0.001) reduced the duration of haloperidol induced catalepsy as well as tacrine induced jaw movements. BEPI has also shown significant antioxidant effect. The result obtained in current study indicates that BEPI posses antiparkinsonian as well as antioxidant activity which may be useful in symptomatic relief of PD and to prevent progression of PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a slowly progressive neurodegenerative disease. In PD, there is a loss of dopaminergic neurons projecting from substania nigra pars compacta (SNPC) to neostratum leading to the imbalance between dopamine and acetylcholine. The mainstay of PD treatment has been the use of drugs which facilitate the presynaptic release of dopamine and anticholinergic drugs (Sharma and Sharma 2007). Neuroprotective therapies hold promise for slowing progression of the disease because they can protect the cells that produce dopamine. Oxidative stress plays an important role in the pathogenesis of PD (Sudha et al. 2003). Neurons of substantia nigra (SN) may be particularly vulnerable to oxidant stress, because the oxidative metabolism of dopamine has the potential to generate cytotoxic free radicals. Dopamine can be oxidized by either monoamine oxidase or undergo autooxidation to generate hydrogen peroxide (H2O2). H2O2 can damage the neuron directly or indirectly through the formation of hydroxyl radicals in presence of ferrous ions. Neuromelanin present within the SN neurons has the potential to promote site-specific accumulation and reduction of iron thereby potentiating iron-induced lipid peroxidation and consequent cell death. H2O2 is normally detoxified by reduced glutathione (GSH) in the reaction catalyzed by GP, thus an increased rate of dopamine turnover or a deficiency of GSH could lead to oxidative stress. Thus, it appears that free radicals may be one of the important agents responsible for destruction of SN neurons, thereby leading to PD (Youdim et al. 1993; Youdim and Riederer 1997). Antioxidants have been studied for their effectiveness in reducing these deleterious effects and neuronal death in many in vitro and in vivo studies. An increasing number of studies demonstrated the efficacy of polyphenolic antioxidants from fruits and vegetables to reduce or to block neuronal death occurring in the pathophysiology of these disorders (De Rijk et al. 1997; Charles 2006). Several agents already in therapeutic use might exert some of their effects by antioxidant action, including selegiline (deprenyl), apomorphine and nitecapone (Halliwell 2001).
Passiflora incarnata Linn (Passifloraceae) has been widely used in traditional medicine in West India, Mexico, the Netherlands and South America. It has been used as an anxiolytic and sedative-hypnotic since ancient time. Aerial parts of P. incarnata have been used as sedative, anxiolytic, antispasmodic, analgesic, anticonvulsant, and wormicidal and also in whooping cough, bronchitis, asthma, and other tough coughs. P. incarnata is also known to possess antitussive, analgesic, anticonvulsant, antiasthmatic, aphrodisiac and anti-inflammatory activity (Dhawan et al. 2004).
The main constituents of leaves of P. incarnata are flavonoid (0.25 %) such as vitexin, isovitexin, orientin, isoorientin, apigenin and kampferol. Besides flavonoid, various indole alkaloids based on ß -carboline ring system viz., harman, harmine, harmalol and harmaline are reported to be present in P. incarnata. In addition, various other phytoconstituents reported to be present in P. incarnata include: carbohydrates, essential oil, amino acids and a cyanogenic glycoside gyanocardin (Soulimani et al. 1997). Harmine and harmaline alkaloids are reported to be effective anti-Parkinson compounds (Jean and Richard 2004). Hence, the aim of present investigation was to study antiparkinsonian activity of P. incarnata leaves.
Materials and methods
Plant material
The fresh leaves of P. incarnata were collected in the month of June, July and August from local nursery in Pune region India. The plant was identified and authenticated by Dr. Dinesh Shirodkar, Botanical Survey of India, Pune, India. (Voucher Specimen No: PASSIN 3).
Preparation of extracts
Shade dried leaves (1,000 g) were powdered and macerated with ethanol for 48 h. The extract was evaporated to dryness. The leaf extract was suspended in water and fractioned successively with hexane, chloroform, ethyl acetate and n- butanol (BEPI). The percentage yields of extracts were 11.7 % w/w, 1.1 % w/w, 0.9 % w/w and 22.5 % w/w, respectively.
Chemicals
Ethanol, hexane, chloroform, ethyl acetate and n- butanol (S.D. Fine Chem. Limited, Mumbai, Maharashtra, India) of highest quality were used.
Drugs
Haloperidol (RPG Life Sciences Ltd, Mumbai), L –DOPA (Alembic Limited, Vadodara), tacrine (Sigma-Aldrich, Mumbai)
Dose selection
An acute toxicity study by European Medicines Agency, 2008 (Anonymous 2008) reported LD 50 of P. incarnata as 3,140 mg kg−1, i.p. in mice and 3,510 mg kg−1, i.p. in rats. Considering these LD 50 values, 1/10th (300 mg kg−1, i.p.) and 1/20th (150 mg kg−1, i.p.) dose of LD 50 was used in the present study.
Standardization of extract for Chrysin by RP-HPLC-UV method
Acid hydrolysis of the BEPI was done according to the method reported in the literature (Ali et al. 2004). 30 mg of BEPI were hydrolyzed with 2N HCl in methanol (30 ml) at 80 °C for 2 h. The liberated aglycone was extracted several times with ethyl acetate and examined by HPLC. The acidic mother liquor was neutralized with Na2CO3, filtered, and evaporated to dryness for examination of the sugar moiety. The ethyl acetate fraction was dried and this fraction was used for the Chrysin estimation.
The HPLC analysis was carried out using Waters HPLC system (Cyber Lab HPLC, LC-100) consisting of 515 binary pumps, 2487 dual wavelength UV detector, and Rheodyne manual injector with 20 micro liter loop. The chromatographic separation was achieved on reversed-phase analytical column (Variance C18 250 × 4.6 mm, Microsoft MV 100–5). The mobile phase consisted of acetonitrile. Mobile phase was filtered through a 0.45-μm membrane. The flow rate was set to 0.5 ml/min and was detected at 269 nm for determination of chrysin. The injection volume was 20 μl, and the peaks were identified by comparison with the retention times of authentic chrysin.
Animals
Swiss albino mice (25–30 g) and Wistar rats (120–150 g) of either sex was used. Animals were housed under standard conditions of temperature (24 ± 2 °C) and relative humidity (30–70 %) with a 12:12 h light: dark cycle. The animals were fed with standard pellet diet and water ad libitum. The institutional animal ethical committee approved all the experimental protocols. Experiments were carried out as per CPCSEA guidelines.
Anti-Parkinsonian activity
-
A]
Haloperidol induced catalepsy:
Adult male Swiss albino mice (25–30 g) were divided into four groups (n = 6). Mice were pretreated with vehicle, BEPI (150 and 300 mg kg−1, i.p), and L-DOPA (30 mg kg−1, i.p.) 30 min before haloperidol (1 mg kg−1, i.p.). The duration of catalepsy was measured at 0, 30, 60, 90, 120, and 150 min after haloperidol administration using bar test. Both the forepaws of the animals were placed on a wooden bar elevated three cm above the ground and duration of catalepsy was measured. A cut-off time was 300 s (Nair and Arjuman 2007).
-
B]
Tacrine induced jaw movements:
Rats divided into four groups and treated with vehicle, BEPI (150 and 300 mg kg−1, i.p), and L-DOPA. After 20 min, tacrine (2.5 mg kg−1, i.p.) was administered and the number of tremulous jaw movements and orofacial bursts were measured for 60 min. Tremulous jaw movements were defined as vertical deflections of the lower jaw not directed at a particular stimulus (Kasture et al. 2009).
Antioxidant activity
-
1]
DPPH (1, 1-diphenyl-2-picrylhydrazyl) assay
The free radical scavenging activity of the extract was measured in terms of hydrogen donating or radical scavenging ability using the stable free radical DPPH. 0.1 mM solution of DPPH in methanol was prepared and 1.0 ml of this solution was added to 3.0 ml of extract solution in water at various concentrations (2–1,000 μg ml−1). The mixture was incubated for 45 min at room temperature and the absorbance was measured at 517 nm against the corresponding blank solution. Ascorbic acid was used as reference. Percentage inhibition of DPPH free radical was calculated based on the control reading, (which contained DPPH and distilled water without any extract) using the following equation:
$$ \mathrm{DPPH}\kern0.5em \mathrm{Scavenged}\kern0.5em \left(\%\right)=\left[\left({\mathrm{A}}_{\mathrm{c}}-{\mathrm{A}}_{\mathrm{t}}\right)/{\mathrm{A}}_{\mathrm{c}}\right]\times 100 $$Where Ac and At are the absorbance of the control and extract or standard respectively (Jiin-Tzong et al. 2001).
-
2]
H2O2 Scavenging Activity
The H2O2 scavenging activity of the extract was determined according to the method of Ruch et al. A solution of H2O2 (40 mM) was prepared in phosphate buffer (pH 7.4). 3.4 ml (16–1,000 μg ml−1) extract in phosphate buffer were added to solution H2O2 (0.6 ml, 40 Mm). Absorbance was determined at 230 nm after 10 min against a blank solution containing phosphate buffer without hydrogen peroxide. The percentage of H2O2 scavenging of extract and ascorbic acid (standard compound) was calculated as:
$$ \%\kern0.5em \mathrm{Scavenged}\kern0.5em {\mathrm{H}}_2{\mathrm{O}}_2=\left[\left({\mathrm{A}}_{\mathrm{c}}-{\mathrm{A}}_{\mathrm{t}}\right)/{\mathrm{A}}_{\mathrm{c}}\right]\times 100 $$Where Ac and At are the absorbance of the control and extract or standard respectively (Ranju et al. 2011).
Results
In RP-HPLC-UV method authentic sample of chrysin showed retention at 3.11 min. n- butanol extract of P. incarnata leaves (BEPI) showed similar retention time as that of authentic chrysin. The result of HPLC analysis of BEPI have shown varied amount of chrysin. The results of HPLC analysis are given in Table 1.
Antiparkinsonian activity was studied using haloperidol induced catalepsy and tacrine induced jaw movements. In the present study, haloperidol (1 mg kg−1 i.p.) produced significant catalepsy at 30 min after haloperidol administration. However, maximum catalepsy was produced after 120 min in vehicle treated animals. The pretreatment with BEPI (150 mg kg−1 and 300 mg kg−1, i.p.) showed a significant (P < 0.001) reduction in the haloperidol induced catalepsy after 60 min onwards. Table 2 represents effect of BEPI on haloperidol induced catalepsy.
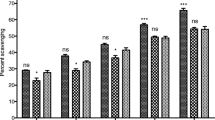
In the present study, tacrine (2.5 mg kg−1, i.p.) produced maximum numbers of tremulous jaw movements during 30–40 min interval. Pretreatment with BEPI (150 and 300 mg kg−1, i.p.) significantly reduced the tacrine induced jaw movements as well as number of bursts induced by tacrine. Figures 1, 2 and 3 represent effect of BEPI on tacrine induced tremulous jaw movements.
The antioxidant activity of BEPI was studied using DPPH (1, 1-diphenyl-2-picrylhydrazyl) scavenging assay and H2O2 scavenging assay and IC50 value of BEPI was found to be 37.59 and 31.92 μg/ml respectively (Table 3). Thus, BEPI was found to have significant antioxidant effect which is comparable to standard ascorbic acid. The results are demonstrated in Figs. 4 and 5.
Discussion
The present study evaluated the effects of n-butanol extract of P. incarnata leaves (BEPI) in experimentally induced animal model of PD. Neuroleptic-induced catalepsy in rodents was used as an animal model for screening drugs for Parkinsonism. It is a robust behavioural method for studying nigrostriatal function and its modulation by various neurotransmitters. Evidence indicates that drugs which potentiate or inhibit neuroleptic induced catalepsy in rodents might aggravate or reduce the extra pyramidal side effects, respectively. Drugs useful in the treatment of Parkinsonism inhibit haloperidol induced catalepsy (Nair and Arjuman 2007). Haloperidol blocks dopamine D2 receptors and produces a state of catalepsy in animals by reducing dopaminergic transmission in basal ganglion. Anticholinergic drugs are most effective in counteracting the catalepsy induced by haloperidol in experimental animals (Klemm 1985). In current study, pre-treatment with BEPI (150 mg kg−1 and 300 mg kg−1, i. p.) significantly reduced the haloperidol induced catalepsy which is indicative of its antiparkinsonian potential.
The acetylcholinesterase inhibitor, tacrine, induces a characteristic motor effect in rats known as tremulous jaw movements or vacuous jaw movements which shares some characteristics with human Parkinsonian tremors (Michael et al. 1997). Tacrine induced tremulous jaw movements in rat have been used as a model for screening antiparkinsonian activity (Salamone et al. 1998). DeBoer et al. reported that cholinergic stimulation induces Parkinsonian symptoms, and evidence indicates that dopamine antagonism or depletions can enhance the striatal acetylcholine release (DeBoer et al. 1993). It has been shown that antiparkinsonian agents like L-DOPA, apomorphine, amantadine, bromocriptine, and benzotropine effectively suppress tacrine induced tremulous jaw movements (Michael et al. 1997). In this investigation, pre-treatment with BEPI (150 and 300 mg kg−1, i.p.) significantly reduced the tacrine induced jaw movements as well as number of bursts induced by tacrine which signifies that BEPI possess potential antiparkinsonian activity.
According to the oxidative stress theory, patients with PD cannot detoxify potentially lethal endogenous or environmental oxidants such as hydrogen peroxide (H2O2) and free radicals (superoxide and nitric oxide) due to defective mitochondrial function. Thus, these potentially lethal oxidants oxidise cells and thereby cause cell injury (Kaufman and Milstein 2007). The use of haloperidol has been associated with an increased level of oxidative stress in the brain. This evidence suggests a possible role for antioxidants in the treatment of haloperidol-induced catalepsy (Rasheed et al. 2010).
Treatment of PD with the drug of choice, L-dopa, is limited only to the relief of symptoms, and long-term use may further add to the oxidative load by producing free radicals during normal metabolism and play a role in disease progression. Existing classes of drugs such as DA agonists, monoamine oxidase (MAO) inhibitors, catechol-O-methyltransferase inhibitors, and anticholinergic agents may be used in the early stages of the disease to relieve PD symptoms; none prevent the disease from progressing, and show debilitating side-effects with prolonged use. Therefore, it is of utmost importance to develop new agents that show or halt the rate of PD progression. The key therapy to ameliorate oxidative stress seen in PD is to repair the damage caused by free radicals before it is too late and to protect DAergic cells. Therefore, antioxidants might be one of the ideal agents to prevent free radical-mediated tissue destruction and inhibit some of the early degenerative events trafficking in the central nervous system that lead to neurodegeneration in PD and its experimental models. The protective effects of various antioxidants to modulate oxidative stress in experimental animal models of PD have been clearly shown, indicating that antioxidant therapy may be an attractive therapeutic approach to PD. To overcome free radical-mediated consequences of disease processes and drug therapies, antioxidants are now being looked upon as persuasive therapeutics against neuronal loss, as they have the capability to neutralize free radicals (Koppula et al. 2012).
Thus, to study the probable mechanism of action, the antioxidant activity of BEPI was studied using DPPH (1, 1-diphenyl-2-picrylhydrazyl) scavenging assay and H2O2 scavenging assay. It was found to have significant antioxidant effect which is comparable to standard ascorbic acid. Thus, it could be understood that BEPI posses antiparkinsonian as well as antioxidant activity which may be useful in symptomatic relief of PD and to prevent progression of PD.
Conclusion
The present investigation demonstrates that BEPI possesses significant antiparkinsonian activity as indicated by inhibition of haloperidol-induced catalepsy and reduction of the tacrine induced jaw movements. Further, it has been observed that BEPI possesses significant antioxidant activity. Thus, it may be concluded that bifunctional nature (antiparkinsonian as well as antioxidant activity) of BEPI may prove boon for treatment of Parkinson’s disease as well for preventing progressive neurodegeneration in Parkinson’s disease.
References
Ali EA, Ibtissam M, Rachida CT, Mohamed B, Rachid, Brahim EB, Mohammed L (2004) Flavone glycosides from Calycotome Villosa Subsp. Intermedia Molecules 9:568–573
Anonymous. assessment report on Passiflora incarnata L., herba (EMEA/HMPC/230961/2006) [monograph on internet]. London: European Medicines Agency; 2008 [cited 2008 Nov 15]. Available from http://www.emea.europa.eu
Charles R (2006) Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: a review of their intracellular targets. Eur J Pharmacol 545:51–64
De Rijk MC, Breteler MM, Den Breeijen JH, Launer LJ, Grobbee DE, Hofman A (1997) Dietary antioxidants and Parkinson disease: the Rotterdam study. Arch Neurol 54:762–765
DeBoer P, Abercrombie ED, Heeringa M, Westerink BHC (1993) Differential effect of systemic administration of bromocriptine and L-DOPA on the release of acetylcholine from striatum of intact and 6-OHDA-treated rats. Brain Res 608:198
Dhawan K, Kumar S, Sharma A (2004) Passiflora: a review update. J Ethnopharmacol 94:1–23
Halliwell B (2001) Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs & Aging 18:685–716
Jean PH, Richard CB (2004) Parkinson’s disease: medications. The National Parkinson Foundation, Inc., Florida
Jiin-Tzong G, Hui-Lien L, Shu-Hsiu C, Fang-I L, Chi-Yue C (2001) Antioxidant properties of the extracts from different parts of broccoli in Taiwan. Journal of Food and Drug Analysis 9:96–101
Kasture S, Pontis S, Annalisa P (2009) Assessment of symptomatic and neuroprotective efficacy of Mucuna pruriens seed extract in rodent model of parkinson’s disease. Neurotox Res 15:111–122
Kaufman DM, Milstein MJ (2007) Clinical neurology for psychiatrists: involuntary movement disorders. Saunders Elsevier, New York
Klemm WR (1985) Evidence for a cholinergic role in haloperidol induced catalepsy. Psychopharmacology (Berl) 85:139–142
Koppula S, Kumar H, More SV, Kim BW, Kim IS, Choi DK (2012) Recent advances on the neuroprotective potential of antioxidants in experimental models of Parkinson’s disease. Int J Mol Sci 13:10608–10629
Michael SC, Debbie LC, John DS (1997) Tremulous jaw movements induced by the acetylcholinesterase inhibitor tacrine: effects of antiparkinsonian drugs. European Journal of Pharmacology 322:137–145
Nair V, Arjuman A (2007) Effect of NR-ANX-C (a polyherbal formulation) on haloperidol induced catalepsy in albino mice. Indian J Med Res 126:480–484
Ranju P, Kundlik G, Nidhi S, Mohammed MH, Thirumoorthy N (2011) Antioxidant and free radical scavenging activity of ethanolic extract of Morinda citrifolia. Annals of Biological Research 2:127–131
Rasheed AS, Venkataraman S, Jayaveera KN, Fazil AM, Yasodha KJ, Aleem MA et al (2010) Evaluation of toxicological and antioxidant potential of Nardostachys jatamansi in reversing haloperidol-induced catalepsy in rats. International journal of general medicine 3:127–136
Salamone JD, Mayorga AJ, Trevitt JT, Cousins MS, Conlan A, Nawab A (1998) Tremulous jaw movements in rats: a model of parkinsonian tremor. Progress in Neurobiology 56:591–611
Sharma HL, Sharma KK (2007) Principles of pharmacology: drug therapy for neurodegenerative disorders, 1st edn. Paras Medical Publisher, Hyderadad
Soulimani R, Younos C, Jarmouni S, Bousta D, Misslin R, Mortier F (1997) Behavioural effects of P. incarnata L. and its indole alkaloid and flavonoid derivatives and maltol in the mouse. J Ethnopharmacol 57:11–20
Sudha K, Rao A, Rao S, Rao A (2003) Free radical toxicity and antioxidants in Parkinson’s disease. Neurol India 51:60–62
Youdim MB, Riederer P (1997) Understanding Parkinson’s disease. Sci Am 1:38–45
Youdim MB, Ben-Shachar D, Riederer P (1993) The possible role of iron in the etiopathology of Parkinson’s disease. Mov Disord 8:1–12
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ingale, S.P., Kasture, S.B. Antioxidant and antiparkinsonian activity of Passiflora incarnata leaves. Orient Pharm Exp Med 14, 231–236 (2014). https://doi.org/10.1007/s13596-014-0149-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13596-014-0149-3