Abstract
High-performance liquid chromatography is the prevalent method used to estimate Camptothecin quantitatively. In the present study thin layer chromatography and UV spectrophotometric method was established as a sensitive, economical and accurate method of detection and estimation of Camptothecin. The method was developed on TLC aluminum plates (60F254 from E-Merck Ltd.) precoated with silica gel using solvent system ethyl acetate: methanol (7.5:2.5, v/v) which gives a dark blue spot of Camptothecin at 254 nm (Rf value 0.46 cm). Spectrometric analysis of Camptothecin was carried out at the absorbance of 254 nm. Leaves and stems of Nothapodytes foetida and Ophiorrhiza mungos were extracted, subjected to TLC and UV spectrophotometric analysis and maximum absorption of CPT in ethyl acetate was observed at 254 nm. The quantification by this method was comparable to data obtained by HPLC. The optimized method was simple, repeatable, precise and cost effective, useful when there is large number of crude samples. In the present study this method was used to screen high yielding lines of CPT producing plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Camptothecin (CPT) is a cytotoxic quinoline alkaloid (Fig. 1), first isolated by Wall and Wani (1996) from Camptotheca acuminate belonging to family Nyssaceae. Camptothecin and its derivatives was found to inhibit DNA topoisomerase activity and at present the semi synthetic water soluble CPT analogs known as topotecan and irinotecan are used as anti-cancer agents (Aimi et al. 1989; Arisawa et al. 1981; Hsiang et al. 1985; Yasuyo Yamazaki et al. 2003). Among the various plant sources for anti-tumor compounds like Vinca alkaloid Vincristine (Noble 1990), Etoposide derived from Podophyllum peltatum (Thurston et al. 1989), Flavopiridol a synthetic flavone derived from the plant alkaloid rohitukine which was isolated from the leaves and stems of Amoora rohituka and later from Dysoxylum binectariferum (Kelland 2000). A number of additional plant derived agents are currently under investigation. Homoharringtonine is an alkaloid isolated from the Chinese tree, Cephalotaxus harringtonia has shown efficiency against various leukemia, 4-Ipomoeaonol is a pneumotoxic furan derivative isolated from sweet potato Ipomoea batatas (Rehm and Devor 1993). It is believed that CPT is the third most important alkaloid sought after by pharmaceutical companies around the world which was initially isolated from the bark and wood of Camptotheca acuminate (Nyssaceae) and act by inhibiting topoisomerase I. Subsequently, Camptothecin has been found in other plant species, including Nothapodytes foetida, Merrilliodendron megacarpum, Pyrenacantha klaineana, Ophiorrhiza mungos, Ophiorrhiza pumila, Ophiorrhiza filistipula, Ophiorrhiza trichocarpon, Ervatamia heyneana and Mostuea brunonis. Nothapodytes foetida could be one of the potent source of CPT which is extracted from the wood and bark of the tree (Govindachari and Viswanathan 1972). Camptothecin and its analogues are the only group of naturally occurring compounds that inhibit topoisomerase I, which is necessary for DNA and RNA synthesis. The chemistry and biology of both the natural and the synthetic analogues have been studied in much detail and as a result water soluble, highly potent and highly specific derivatives of Camptothecin have been produced. Because of rapidly growing global demand for these Camptothecin analogs, increased interest of research in various areas such as cancer has been evidenced.
Currently CPT and analogues are produced from natural sources, Camptotheca acuminate and Nothapodytes foetida (Govindachari and Viswanathan 1972; Wall and Wani 1996). Both these plants are trees with prolonged juvenile period and restricted cultivation areas. Even areas under cultivation take years to yield the commercially viable quantity of CPT. This has resulted in threat to the wild resources. Considering the importance of CPT as anti-cancerous compound there is difficulty in procurement of raw material. Hence, plant tissue culture is used as a major tool in propagating the elite species. Screening of high yielding lines in plant tissue culture studies result in large number of samples for quantification (Fulzele et al. 2001; Martin et al. 2008; Puri et al. 1999; Rai 2002). High performance liquid chromatography (Ahmed et al. 1998; Tsai et al. 1999; Zhao et al. 2006), High pressure thin layer chromatography methods (Vidya Dighe et al. 2012) have witnessed their use in quantification of CPT, however, published literature indicates the need for identification and optimization of simple and cost effective method for quantification of CPT when there are many samples. This compound is very commonly quantified by HPLC method (Sakato et al. 1994; Singh et al. 2009.) However use of HPLC method for quantification is very expensive and demands a simple and cost effective method. Hence in the present study, a simple and accurate method is been proposed by coupling TLC and UV spectrophotometer analysis. Also HPLC analysis was performed to compare and confirm the accuracy of the results.
Materials and methods
The plant materials Nothapodytes foetida, Ophiorhiza mungos were collected from different geographic locations (T1-Amboli, T2-Agumbe, T3-Belgaum, T4-Coorg, T5-Chikmanglore, T6-Karod, T7-Kemmangundi, T8-Mahabaleshwar, T9-Sagar and T10-Kodchadry) and authentic standard of CPT was procured from Rishi Herbal Technologies pvt. Ltd. Bangalore. Analytical grade solvents were obtained from (Finar chemicals Ltd., SDFC Ltd., NICE Chemicals Pvt. Ltd., India) and pre coated silica plates 60F254 from E MERCK Ltd., India.
Extraction
Five grams of dried plant material is powdered and extracted twice with petroleum ether and thrice with methanol in soxhlet extraction unit. Combined methanolic extract is evaporated to dryness using rotoflash evaporator. The concentrated methanolic extract is diluted with water to get aqueous methanolic extract. Aqueous methanolic extract is separated with chloroform and chloroform phase is evaporated to dryness.
Sample preparation
Pre weighed and dried chloroform extract is dissolved in 3 ml of ethyl acetate. Fifty microliter of this sample solution is used for spotting on a TLC plate.
Standard preparation
Known amount of standard is dissolved in ethyl acetate. Fifty microliter of this standard solution is spotted along with unknown samples.
Calibration curve for standard camptothecin
Different stock solutions with concentration, 0.2, 0.4, 0.6, 0.8, and 1 ng/ml of standard CPT were prepared in ethyl acetate. From each concentration, 50 μl was spotted on a TLC plate. The data of peak height/area versus CPT concentration were treated by linear least square regression.
TLC method
Known amounts of dried chloroform extract of plant samples were dissolved in 3 ml of ethyl acetate and 50 μl of this solution was spotted in the form of dots of diameter 1 cm each on TLC aluminum plates (60F254 from E MERCK Ltd.) precoated with silica gel, and space between two spots was 1 cm. The chromatogram was developed in a glass chamber saturated (30 min prior) with mobile phase (ethyl acetate: methanol 7.5:2.5) at room temperature. The developed chromatogram was observed under UV (254 nm). The spots corresponding to standard were scraped out and then dissolved in 1 ml of ethyl acetate and filtered with 0.22 μm filter. The filtrate was made up to 3 ml with ethyl acetate and absorbance was recorded using a spectrophotometer at 254 nm using a suitable blank. The mean absorbance value of the amounts studied in triplicates was plotted against concentration of CPT to get a calibration curve. Unknown sample absorbance was recorded and plotted to derive the concentration of CPT using standard curve.
HPLC method
Quantitative HPLC was carried out on Waters associate using Novapak C18 column (150 mm) with mobile phase acetonitrile: methanol (1:1 v/v) and preparation buffer (10 ml acetic acid and 600 ml distilled water). The flow rate was adjusted to 1 ml/min, sample run time was 30 min and the detector was set at 254 nm. The concentration of CPT in different samples was measured by standard calibration curves of authentic samples obtained from Rishi Herbal Technologies, Bangalore, India.
Results and discussion
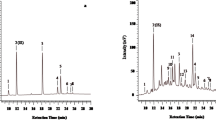
The extractions of CPT and TLC procedures (Table 1) are discussed earlier by many authors (Fulzele and Satdive 2005; Roja and Heble 1994). However to develop this cost effective method good extraction and separation of the spots on the TLC plate are required for the accuracy. Three different TLC methods were performed using solvent system acetonitrile: methanol (7:3 v/v), acetonitrile: methanol: acetic acid (7:2:0.1 v/v/v), ethyl acetate: methanol (7.5:2.5 v/v) were tried to get best resolution. The methods reported by (Table 1) and the methods standardized in the present study were compared and the resolution was best in the mobile phase ethyl acetate: methanol (7.5:2.5 v/v). The amounts of CPT standard and their corresponding absorbance values were plotted to device a calibration curve (Fig. 2) the graph show a collinear relationship. Figure 3 shows the TLC results as compared with standard CPT. The test samples T1-T10 follow the similar Rf as that of standard. The technique found to be sensitive, since the concentration of standard CPT taken was in ng and appreciable linear curve with the same was obtained, hence the present study is sensitive and reliable even at lower concentrations of CPT. The method was applied to plant parts of Nothapodytes foetida and Ophiorizza mungos collected from different geographical locations. Nothapodytes foetida plant parts collected from amboli region was found to contain 0.084 % w/w with that of sagar region containing 0.018 % w/w, Kodchadry being reported for the first time and contains 0.035 % w/w and Ophiorizza mungos plant parts collected from Kemmanagundi region contain 0.036 % w/w with that of karod region being 0.010 % w/w. The accuracy of the results obtained by TLC method was confirmed with HPLC (Figs. 4 and 5) and found to be comparable (Fig. 6). In our investigations dealing with selection of high yielding lines both in wild and in cell lines result in hundreds of samples. The present study clearly mentions about the need for identification of a method which is simple. Hence a protocol is required to facilitate easy detection of CPT. The developed method is simple, easy to use and cost effective. The technique is found suitable with an advantage of avoiding extra purification and mobile phase consumption per sample is extremely low thereby reducing the acquisition and disposal cost. The technique can have an upper hand over traditional techniques in processing the sample and standard simultaneously under similar conditions giving better analytical precision and accuracy. The novelty of the method lies in providing extreme flexibility for various steps i.e., selection of stationary phase, mobile phase, developing method, detection with or without pre or post column derivatization. This does not require any sophisticated procedures like HPLC-UV, HPLC-MS, HPLC-GC, etc. which are tedious with processing and operation procedures. Using the basis of the reported protocol a validated method can be developed for estimation of CPT content in biological samples.
Conclusion
The proposed technique is of great importance in using strong reagents for the purpose of molecule identification and CO TLC can be developed in practice were several analyst can simultaneously work on the same system. Technically it is very simple to learn and operate in which no skilled person are required. Whole spectrum can be seen at a glance with negligible wear and tear and hence low maintenance cost is required. The critical steps in HPLC like choice of solvent to dissolve a sample can be overwhelmed by completely removing the solvent from sample before developing a chromatogram. Having benefit of using turbid samples purification can be avoided. Also, solvents need no prior treatment like filtration and degassing, analytical grade are suitable for analysis.
Interference and contamination possibility can be minimized from previous analysis as fresh stationary phase and mobile phase are used for each analysis. Corrosive, UV absorbing, mobile phase having pH 8 can also be used which is not possible in HPLC. Hence, quantitative measurement by UV-Visible and fluorescence is possible.
Abbreviations
- CPT:
-
Camptothecin
- TLC:
-
Thin layer chromatography
- HPLC:
-
High performance liquid chromatography
References
Ahmed F, Vyas V, Saleem A, Li XG, Zamek R, Cornfield A, Haluska P, Ibrahim N, Rubin EH, Gupta E (1998) High-performance liquid chromatographic quantitation of total and lactone 20(S) camptothecin in patients receiving oral 20(S) camptothecin. J Chromatogram B: Biomed Sci Appl 707:227–233
Aimi N, Nishimura M, Miwa A, Hoshino H, Sakai S, Haginiwa J (1989) Pumiloside and deoxypumiloside, plausible intermediates of camoptothecin biosynthesis. Tetrahedron Lett 30:4991–4994
Arisawa M, Gunasekera SP, Cordell GA, Farnsworth NR (1981) Plant anticancer agents XXI. Constituents of Merrilliodendron megacarpum. Plant Med 43:404–407
Dighe V, Parekh G, Mestry D (2012) Quantitation of camptothecin from Ervatamia Heyneana (Wall.) T. cooke stem powder using high performance thin layer chromatography. Int J Pharm Bio Sci 3(3):230–237
Fulzele DP, Satdive RK (2005) Comparision of technique for the extraction of the anti-cancer drug camptothecin from Nothapodytes foetida. J Chromatogr A 1063(1–2):9–13
Fulzele DP, Satdive RK, Pol BB (2001) Growth and production of camptothecin by cell suspension culture of Nothapodytes foetida. Plant Med 67:150–152
Govindachari TR, Viswanathan N (1972) Alkaloids of Mappia foetida. Phytochemistry 11:3529–3531
Hsiang YH, Hertzberg R, Hecht, Liu LF (1985) Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem 260:14873–14878
Kelland LR (2000) Flavopiridol, the first cyclin-dependent kinase inhibitor to enter the clinic: current status. Expert Opin Invest Drugs 9:2903–2911
Martin KP, Zhang C-L, Hembrom ME, Slater A, Madassery J (2008) Adventitious root induction in Ophiorrhiza prostrata, a tool for the production of CPT (an anticancer drug) and rapid propagation. Plant Biotechnol Rep 2(2):163–169. doi:10.1007/s11816-008-0057-4
Noble RL (1990) The discovery of the vinca alkaloids–chemotherapeutic agents against cancer. Biochem Cell Biol 68(12):1344–1351
Puri SC, Handa G, Gupta RK, Srivastava TN, Somal P, Sharma SN (1999) Quantitation of camptothecin in Nothapodytes foetida. J Indian Chem Soc 76:370–371
Rai VR (2002) Rapid clonal propagation of Nothapodytes foetida (Wight)-Sleumer a threatened Medicinal tree. In Vitro Cell Dev Biol—Plant 38:347–351
Rehm S, Devor DE (1993) Acute effects of 4-ipomeanol on experimental lung tumors with bronchiolar or alveolar cell features in Syrian hamsters or C3H/HeNCr mice. J Cancer Res Clin Oncol 120:41–50
Roja G, Heble MR (1994) The Quinoline alkaloids camptothecin and 9-methoxy camptothecin from tissue cultures and mature trees of Nothapodytes foetida. Phytochemistry 36:65–66
Sakato K, Tanaka H, Mukai N, Misawa M (1994) Isolation and identification of camptothecin from Camptotheca acuminate suspension cultures and mature trees of Nothapodytes foetida. Phytochemistry 36:65–66
Singh I, Kumaravadivel N, Gnanam R, Vellaikumar S (2009) RP-HPLC analysis of CPT content in Nothapodytes Nimmoniana an endangered medicinal plant. J Med Plant Res 4(3):255–259
Tafur S, Nelson JD, Delong DC, Svoboda GH (1976) Antiviral components of Ophiorrhiza mungos isolation of camptothecin and 10-methoxycamptothecin. Lloydia 39:261–262
Thurston LS, Imakura Y, Haruna M, Li DH, Liu ZC, Liu SY, Cheng YC, Lee KH (1989) Antitumour agents. 100. Inhibition of human DNA topoisomerase II by cytotoxic ether and ester derivatives of podophyllotoxin and.alpha.-peltatin. J Med Chem 32:604–608
Tsai TH, Tsai TR, Chen YF, Chou CJ, Chen CF (1999) Determination of unbound 20(S)-camptothecin in rat bile by on-line microdialysis coupled to microbore liquid chromatography with fluorescence detection. J Chromatogr B: Biomed Sci Appl 732(1):221–225
Van Hengel AJ, Harkes MP, Wichers HJ, Hesselink PG, Buitelaar RM (1992) Characterization of callus formation and camptothecin production by cell lines of Camptotheca acuminata. Plant Cell Tissue Organ Cult 28:11–18
Wall ME, Wani MC (1996) Camptothecin and taxol: from discovery to clinic. J Etnopharmacol 51:239–253
Yamazaki Y, Sudo H, Yamazaki M, Aimi N, Saito K (2003) Camptothecin biosynthetic genes in hairy roots of Ophiorrhiza pumila: cloning, characterization and differential expression in tissues and by stress compounds. Plant Cell Physiol 44(4):395–403
Zhao YX, Gao JQ, Qiao HL, Chen HL, Liang WQ (2006) Development and validation of a sensitive reversed-phase HPLC method to determine intracellular accumulation of hydroxycamptothecin. J Pharm Biomed Anal 41:1007–1010
Acknowledgements
The authors are thankful for the financial assistance provided by the National Medicinal Plants Board, New Delhi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lokesh, P., Balasubramanya, S. & Anuradha, M. Cost effective quantification of camptothecin and a comparative study of its content in Nothapodytes foetida and Ophiorrhiza mungos sourced from selected geographical locations. Orient Pharm Exp Med 14, 87–92 (2014). https://doi.org/10.1007/s13596-013-0124-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13596-013-0124-4