Abstract
Fat replacers can be used to improve the sensory and functional properties of reduced-fat cheeses. The effect of gum tragacanth (GT) on the rheological, functional and sensory properties of half-fat and full-fat Cheddar cheese during ripening was investigated. Four Cheddar-style cheeses were made in triplicate: full-fat control (FFC), half-fat control (HFC), full fat + GT (FFGum) and half fat + GT (HFGum). Cheesemilk for the latter two cheeses was supplemented with GT at a level of 0.05% (w/v); all cheeses were ripened at 8 °C for 10 months. Moisture and moisture-to-protein ratio were increased by GT addition. GT addition resulted in decreased pH in both FFGum and HFGum cheeses during ripening, especially the FFGum cheese. GT appeared to affect proteolysis only in the FF cheeses. GT was successful in decreasing hardness and springiness values during ripening. GT increased meltability in the FFGum cheese and to a lesser extent in the HFGum cheese in late ripening. An increase in opaqueness was also observed due to GT addition regardless of fat level. Dynamic small amplitude oscillatory rheology showed a depression in LTmax and an increase in G′ at 75 °C caused by GT at 7 months of ripening. GT did not appear to have any impact on starter bacteria and NSLAB counts. Results from a consumer ranking preference test showed that the GT was not successful in fully mimicking the sensory properties of the FFC cheese. These results suggest that GT appears more suited to enhancing the textural and functional properties of half-fat Cheddar cheese than its sensory properties.
摘要
脂肪替代物能够用来改善低脂切达干酪的感官和功能特性。本文研究了黄芪胶(GT)对全脂和半脂切达干酪在成熟过程中的流变学、功能和感官特性的影响。四种类型的切达干酪在制作过程中重复三次,他们分别是全脂切达干酪对照组(FFC);半脂切达干酪对照组(HFC);全脂切达干酪+黄芪胶(FFGum)和半脂切达干酪+黄芪胶(FFGum),后两种切达干酪在制作过程中补充了含量为0.05% (w/v)的黄芪胶,所有的干酪在8 °C成熟10个月。加入黄芪胶(GT)的干酪水分含量及水分与蛋白的比例都增加。黄芪胶(GT)的加入导致FFGum 和 HFGum干酪在成熟过程中pH值下降。黄芪胶(GT)只影响FF干酪的蛋白酶解。黄芪胶(GT)能够成功的降低干酪在成熟过程中的硬度和弹性。黄芪胶(GT)能够增加FFGum的溶解能力,在FFGum干酪成熟的晚期溶解能力也有较小程度的增加。由于不考虑脂肪的含量,黄芪胶(GT)的加入能够增加干酪的透光性。动态小振幅振荡流变性结果表明,加入黄芪胶(GT)的干酪经7个月的成熟期后在75 °C最大行程长度缩短,贮能模量(G′)增加。消费者喜好排名测试结果表明黄芪胶(GT)不能成功的完全模拟FFC干酪的感官特性。这些结果表明相对于感官特性而言,黄芪胶(GT)似乎更适合提高半脂切达干酪的质地和功能特性。
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Health-conscious consumers now demand foods with lower fat contents that have sensory properties similar to their full-fat counterparts. However, most consumers are not willing to sacrifice flavour or texture for fat reduction in cheese (Childs and Drake 2009). Fat has an important role in the development of flavour, texture and appearance of cheese. Removal of fat from cheese can cause textural, functional and sensory defects such as rubbery texture, lack of flavour, bitterness, off-flavour, poor meltability and undesirable colour (Banks 2004; Johnson et al. 2009; Mistry 2001). The different composition of reduced-fat cheeses compared to full-fat variants alters the biochemical and microbiological changes during ripening that influence flavour and texture development (Fox and Wallace 1997).
One of the most important strategies for improving the properties of reduced fat cheese is to increase its moisture content sufficiently to provide a moisture-to-protein ratio (M:P) that is equal to or greater than its full-fat counterpart (Broadbent et al. 2001). The addition of fat replacers to cheesemilk is one method used to achieve this objective. Fat replacers are ingredients used to take the place of milk fat and to mimic its characteristics in cheese. One of the most important functional properties of ingredient cheese is meltability. Along with improving the sensory and textural properties of unmelted cheese, an ideal fat replacer should increase meltability in a reduced-fat cheese. These ingredients can be composed of protein, carbohydrate (fat mimetics) or fat (fat substitutes). Carbohydrate-based fat mimetics are polar, water-soluble compounds that act mainly by immobilising water thereby increasing the moisture content of the cheese. Commercially available carbohydrate-based fat mimetics such as Novagel™ and Stellar™ (McMahon et al. 1996); Perfectamyl gel and Satiagel (Kavas et al. 2004); Raftiline® (Koca and Metin 2004) along with β-glucan hydrocolloid suspensions (Konuklar et al. 2004) have had mixed success in altering both rheological and sensory properties of reduced- and low-fat cheeses.
Gum tragacanth (GT) is a dried gummy exudate which is obtained from the stems of Asiatic species of Astragalus (Leguminosae). It consists of water-soluble tragacanthin, water-swellable bassorin (60–70% of polymer) and up to 4% protein (Whistler 1993). GT is stable over a wide range of pH and has a molecular weight of 840 kg.mol−1. It is widely used as a stabiliser, emulsifier and thickener in the food, pharmaceutical and cosmetic industries. GT is an anionic polysaccharide containing carboxylic groups. It is believed that when GT is added to an aqueous system of casein micelles, the tragacanthin fraction adsorbs onto the micelle surface, modifying the electrostatic repulsion and enhancing steric repulsion between micelles, while the non-adsorbing bassorin fraction can increase the viscosity of the aqueous phase (Azarikia and Abbasi 2010).
Nasirian et al. (2010) used GT successfully to mimic the sensory properties of fat in reduced-fat dairy cream due to its favourable emulsification properties. Rahimi et al. (2007) found that GT can be used to improve the rheological and sensory properties of low-fat Iranian White Cheese when added at levels up to 0.75 g.kg−1 milk. However, a level of 1 g.kg−1 milk caused a negative effect on sensory texture scores. Aziznia et al. (2009) speculated that increasing GT level above 0.5 g.L−1 in non-fat yoghurt led to an increased distance between casein micelles due to increased steric stabilisation and therefore less contact and structure in the yoghurt gel. Evidently, the level of GT addition to dairy products is of critical importance.
The objective of this study was to evaluate the effects of supplementing cheesemilk with gum tragacanth on the rheological and sensory properties of half-fat and full-fat Cheddar cheeses during ripening.
2 Materials and methods
2.1 Cheese manufacture
Raw bovine milk was standardised with cream to a fat content of 3.5% for full-fat control (FFC) milk and 1.5% for half-fat control (HFC) milk. Milks were batch pasteurised at 63 °C for 30 min. A 2 kg quantity of each milk type was heated to 45 °C and supplemented with 25 g food grade gum tragacanth (Cake-stuff, Lesmahagow, Lanarkshire, Scotland) by mixing at 3,200 rpm using a Silverson L4RT blender (Silverson Machines Ltd, Chesham, Buckinghamshire, UK). This mixture was then flash pasteurised at 75 °C for 30 s. The supplemented milks were recombined with bulk milk in cheese vats containing the respective milk type. Four Cheddar-style cheeses were manufactured according to standard protocol on a 50-kg scale in the food processing facilities at University College, Cork. R-604Y (Chr. Hansen Ltd., Little Island, Co. Cork, Ireland) was used as the starter culture at a level of 0.02% (w/v). Chymosin (Maxiren 180; DSM Food Specialities, Delft, Netherlands), at a strength of 180 IMCU ml−1, was added to the cheesemilk at a level of 0.3 mL.L−1. Coagulum was cut at equal firmness (measured subjectively). Curd was cooked from 31–39 °C over 30 min and held at this temperature until whey was drained at pH 6.2. The curd was cheddared until pH 5.4 was reached and was then milled and dry salted at a level 2.5% w/w NaCl. The salted curd was transferred to rectangular moulds 25.4 × 20.3 cm and pressed overnight at 5 g.cm−2. The cheeses were vacuum packaged and ripened at 8 °C for a period of 10 months. Three independent cheese-making trials were performed.
2.2 Chemical analysis
Compositional analysis was performed on the cheeses at day 14 of ripening. The moisture contents of the cheeses were determined by an oven drying method (IDF 1982), protein by the macro-Kjeldahl procedure (IDF 1986), fat by the Gerber method (IIRS 1955), salt by a titrimetric method using potentiometric end-point determination (Fox 1963). Cheese pH was determined by measuring the pH of homogenised cheese slurry made from 10 g cheese and 10 g water at room temperature. Proteolysis was assessed by determining the levels of pH 4.6-soluble nitrogen as percent of total nitrogen (pH4.6SN%TN) at 1 and 6 months of ripening. Urea–polyacrylamide electrophoresis was carried out directly on the cheeses using the procedure described by O’Mahony et al. (2005). Urea–PAGE gels were loaded on an equal protein basis for all cheeses.
2.3 Texture profile analysis
Texture profile analysis (TPA) was performed using a Texture Analyser TA-XT2i (Stable Micro Systems, Godalming, Surrey, UK) according to the method of O’Mahony et al. (2005), except cylindrical samples of dimensions: height 20 mm, diameter 20 mm were used. Hardness, cohesiveness and springiness were defined according to Bourne (1978). Five replicate samples from each cheese were compressed at each ripening time point.
2.4 Dynamic small amplitude oscillatory rheology
Rheological properties of the cheese samples were measured using a Carri-Med CSL2/100 Controlled Stress Rheometer (TA Instruments, Leatherhead, UK). Measuring geometry consisted of a 40-mm serrated stainless steel parallel plate above, a flat base plate below and a gap size of 1.8 mm. Cheese discs (40 mm diameter, 2 mm height) were glued to the base plate of the rheometer using cyanoacrylate glue in order to prevent slippage during measurement. The sample was compressed to the gap size and allowed rest for 10 min at 20 °C in order to allow stress relaxation prior to oscillation. Storage modulus (G′), loss modulus (G″) and loss tangent (LT) were recorded continuously at a low amplitude shear strain (0.05) at a frequency of 6.283 rad.s−1 over 20 min during which the temperature was increased from 20 to 82 °C. Each sample was analysed in triplicate.
2.5 Melt analysis
Melt analysis was carried out using a covered Schreiber test (Altan et al. 2005). Each cheese cylinder (5 mm height, 35 mm diameter) was placed in a covered glass Petri dish and then placed in an oven at 232 °C for 5 min. These were then removed and cooled for 30 min at room temperature. Measurements of the melt distance were made using electronic callipers. The diameter of the melted sample was measured at five different points and an average diameter was determined. Results were expressed as percentage increase in cheese diameter. Analysis on each cheese sample was performed in triplicate.
2.6 Colour analysis
Hunter L* values of cheese samples were measured at room temperature using a Minolta Colorimeter CR-300 (Minolta Camera Co., Osaka, Japan). Five different locations on the freshly cut surface of a cheese slice were measured for each cheese.
2.7 Consumer preference ranking test
A consumer acceptance sensory panel evaluated randomly coded samples of the full-fat control (FFC), half-fat control (HFC) and half-fat + GT (HFGum) cheeses. The acceptance panel consisted of 70 members (34% males and 66% females) ranging from 18 to 59 years old. Consumer panellists were students and staff members of University College Cork. Cheese blocks were cut into pieces (2.5 × 1 × 1 cm) and evaluated at room temperature. Evaluations were carried out in a panel room with individual booths. Normal lighting conditions were used, allowing the panellists also to discriminate on the basis of colour. Panellists completed a questionnaire asking their gender, age and frequency of Cheddar cheese consumption (never, 1 time/month, 1 time/week and >3 times/week). Panellists ranked the cheeses according to preference. Panellists (n = 5) who selected ‘never’ as their frequency of consumption were eliminated from data analysis. Sensory evaluation was carried out at 10 months of ripening.
2.8 Microbiological analysis
Samples (∼10 g) were taken aseptically from cheeses using a sterile cheese trier and placed into a stomacher bag. These samples were diluted 1:10 with sterile trisodium citrate (2% w/v) followed by homogenisation in a stomacher (Seward Stomacher 400; Seward Ltd., London, UK) for 4 min. Further dilutions were prepared depending on the stage of ripening. Starter bacteria were enumerated on LM17 agar (Terzaghi and Sandine 1975) incubated aerobically for 3 days at 30 °C. Non-starter lactic acid bacteria (NSLAB) were enumerated on Rogosa agar (Rogosa et al. 1951) incubated anaerobically for 5 days at 30 °C. Enumeration of starter bacteria and NSLAB were carried out in duplicate on all cheeses at months 3 and 6 of ripening.
2.9 Statistical analysis
ANOVA was carried out using the PASW Statistics Version 18 program (IBM, Armonk, NY, USA). The level of significance was determined at P < 0.05. For the consumer preference ranking test, a critical difference value was obtained from Basker tables (Resurreccion 1998).
3 Results and discussion
3.1 Chemical composition of cheeses
The composition of the cheeses at day 14 of ripening is shown in Table 1. The half-fat (HF) cheeses had a significantly higher (P < 0.05) moisture content compared to their full-fat (FF) counterparts. Addition of GT caused a significant (P < 0.05) increase in moisture content in both the FF and HF cheeses, which can be attributed to its water-binding properties. The negatively charged GT may interact with positively charged groups on caseins, resulting in bridging flocculation and/or steric stabilisation due to the low level of the polysaccharide present (Dickinson 1998). This situation may partially reduce coagulation during the renneting stage due to the increased distance between casein micelles and therefore reduction in hydrophobic association between para-casein micelles. An increased viscosity in the aqueous phase and immobilisation of water by GT may induce greater retention of whey in the initial para-casein gel matrix and reduce syneresis, conferring a more open protein matrix in the final cheese structure. Supplementing cheesemilk with 0.1% GT, Khosrowshahi (unpublished) observed an unsightly level of syneresis throughout ripening in both half-fat and full-fat Cheddar cheeses. Based on these findings, a GT supplementation level of 0.05% was chosen for the cheesemilk in the current study.
In agreement with Guinee et al. (2000a), the reduction in fat content of the HF cheeses led to increases in protein and moisture contents, and a decrease % MNFS compared to their FF counterparts. Moisture replaces the fat in the protein matrix but not on an equal basis. The increase in moisture caused by GT coincided with a significant (P < 0.05) decrease in fat content in both the FF and HF cheeses. The GT did not cause a significant change in protein content in either the FF or HF cheeses. As % MNFS did not change significantly due to addition of GT, it appears that the fat is replaced by water on a seemingly equal basis. However, GT addition increased the moisture-to-protein ratio (M:P) in both HF and FF cheeses.
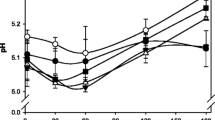
The pH of the HFC cheese was significantly higher than the FFC cheese during ripening, in agreement with Fenelon and Guinee (2000). These authors speculated that an increase in cheese pH with decreasing fat content may be attributed to the concomitant decrease in % MNFS, which has been found to lower the lactate-to-protein ratio. The higher protein content and lower M:P in the HF cheeses appear to give them a greater buffering capacity. The addition of GT generally caused a slight decrease in pH during ripening compared to the FF and HF control cheeses. This may be attributed to the increase in M:P caused by GT addition, which may reduce the buffering capacity. The FFGum cheese exhibited the largest decrease in pH. The lower pH in the FFGum cheese may be due in part to a higher retention of lactose post manufacture due to increased moisture retention and therefore, a higher level of lactate production in the cheese early during ripening.
3.2 Microbiological analysis
Starter cell counts (LM17 agar) decreased in all cheeses from 3 to 6 months (Table 2). Starter counts were generally higher in the HF cheeses possibly due to their increased moisture content. This is contrary to results reported by Laloy et al. (1996) who demonstrated that 50% reduced fat Cheddar cheeses had fewer starter cells than full fat cheeses. The presence of GT appears to have no effect on the number of starter bacteria. NSLAB counts increased from 3 to 6 months. Fat level and GT appeared to have no effect on NSLAB.
3.3 pH 4.6-soluble nitrogen as a percentage of total nitrogen
The level of pH4.6SN%TN significantly increased (P < 0.05) in all cheeses from 1 to 6 months during ripening (Table 1). In trials 2 and 3, there was a significant difference (P < 0.05) in pH4.6SN%TN between the FFC and HFC cheeses at 6 months of ripening. A number of studies have observed a decrease in pH4.6SN%TN with decreasing fat content throughout ripening (Fenelon et al. 2000; Guinee et al. 2000a, b; Rudan et al. 1999) and authors have speculated that the concomitant increases in % MNFS with fat level may be due to differences in chymosin retention. The same relationship of fat level with % MNFS occurred in the present study. pH4.6SN%TN values of the FFGum cheese were generally higher than the values of the HFC cheese at 1 and 6 months of ripening. As the pH of the FFGum cheese was much lower than that of the HFC cheese, increased residual chymosin activity at the lower pH cheese may be responsible.
3.4 Urea–PAGE
From the urea–PAGE electrophoretograms (Fig. 1), it appeared that fat reduction had an effect on the breakdown of αs1- and β-casein. The rate of degradation of αs1-casein was higher in the FF cheeses, whereas the rate of β-casein degradation appeared higher in the HF cheeses. Fenelon and Guinee (2000) reported a similar effect when fat level was reduced in Cheddar cheese. The FFGum cheese appeared to have increased hydrolysis of αs1-casein compared to the FFC cheese. The level of αs1-CN(f24-199) in the FFGum cheese was lower than that of the FFC cheese at 24 weeks of ripening. This may be attributed to increased cleavage of αs1-CN(f24-199) to (f102-199) due to increased residual chymosin activity at the lower pH of the FFGum cheese. There was a higher concentration of γ-caseins (β-CN f29-209, f106-209) and f108-209) in the FFC cheese at 16 weeks in comparison to the FFGum cheese. In cheese, β-casein is hydrolysed principally by plasmin (Sousa et al. 2001); therefore, the higher pH in the FFC cheese caused increased plasmin activity compared to the FFGum cheese. It appeared that the decrease in pH caused by addition of GT caused an increase in αs1-casein breakdown via enhanced chymosin activity and a reduction in β-casein breakdown via reduced plasmin activity. This effect of pH on proteolysis is in agreement with Watkinson et al. (2001). The slightly higher S/M in the FFGum cheese compared to the FFC cheese may have also caused a reduction in chymosin hydrolysis of β-casein (Kelly et al. 1996). GT did not appear to cause a difference in the breakdown of αs1- or β-casein in the HF cheeses.
3.5 Texture profile analysis
Hardness, cohesiveness and springiness values decreased during ripening for all cheeses. The decrease in hardness during ripening is widely attributed to a significant level of proteolytic breakdown of αs1-casein in the protein matrix (Creamer and Olson 1982). The decrease in hardness in early ripening may be largely due to solubilisation of colloidal calcium phosphate (CCP) leading to a decrease in the number of CCP crosslinks between caseins and a decrease in structural integrity of the para-casein matrix (Lucey et al. 2003). The HFC cheese was generally the hardest cheese throughout ripening and was significantly harder (P < 0.05) than the FFC cheese at most time points (Fig. 2). These results are in agreement with many studies which have shown that decreasing fat content in cheese increases hardness/firmness (e.g. Fenelon and Guinee 2000; Guinee et al. 2000a; Lteif et al. 2009). This is due to the reduction in filler volume and hence, a denser protein structure in this cheese. At most time points during ripening, there was no significant difference (P > 0.05) between the FFC and the HFGum cheeses. The increase in moisture content, M:P and physical disruption of the protein network caused by GT, create more inhomogeneities in the overall structure of the cheese, leading to a weaker protein structure. GT appeared to reduce hardness values in the HFGum cheese to a greater extent than in the FFGum cheese. Konuklar et al. (2004) also reported that carbohydrate-based fat mimetics can successfully decrease the hardness of reduced-fat Cheddar cheese. pH may have also had a notable contribution to the hardness values as HFC cheese has the highest hardness and pH values, while the opposite was true for the FFGum cheese. Decreasing the pH of cheese causes solubilisation of CCP cross-links, which may have contributed to the difference in hardness values observed between the cheeses during ripening.
The HFC cheese had significantly higher cohesiveness values compared to the FFC cheese. A decrease in cohesiveness with fat level has also been reported by Awad et al. (2005). At 1 week of ripening there was no significant difference between the FFC, HFC and HFGum cheeses, but a divergence was seen throughout ripening (Fig. 3) resulting in the HFC and HFGum cheeses having significantly higher (P < 0.05) cohesiveness than the FFC cheese in all trials at 6 months. In contrast to the effect of GT on cohesiveness during ripening, Koca and Metin (2004) observed an increase in cohesiveness in low-fat Kashar cheese supplemented with the carbohydrate-based fat mimetic Raftiline® after 60 days of ripening.
The HF cheeses had higher springiness than the FF cheeses at 1 week of ripening, but as the cheeses ripened, a convergence was seen (Fig. 4), which ultimately led to no significant difference (P < 0.05) at 6 months between the FFC and HFGum cheeses. The decrease in cohesiveness and springiness as in the case of hardness, is likely attributable to the proteolytic breakdown of the para-casein matrix during ripening.
3.6 Melt analysis
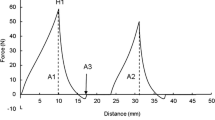
Results from the Schreiber melting test indicated that the HF cheeses melted much less than the FF cheeses at most ripening times (Fig. 5). These results are consistent with numerous studies that reported reducing the fat content of Cheddar cheese leads to a decrease in meltability (e.g. Guinee et al. 2000a; Konuklar et al. 2004; Rudan et al. 1999), with the main factors involved thought to be the higher protein content and lower M:P in the HF cheeses leading to an increased density of the protein matrix. The increase in meltability in all cheeses throughout ripening is likely to be due to degradation of the para-casein matrix and/or changes to its calcium equilibrium. GT addition caused a large increase the meltability in the FFGum cheese after 16 weeks of ripening. There appeared to be no difference in meltability between the HFC and HFGum cheeses at most time points during ripening. However, at 6 months in trial 2, there was no significant difference (P < 0.05) between the FFC and HFGum cheeses. In trial 3, there was a significant difference (P < 0.05) between the HFC and HFGum cheeses, with the HFGum cheese having a higher meltability. Koca and Metin (2004) observed an increase in meltability of low-fat Kashar cheese with Raftiline compared to a low-fat control at 90 days of ripening. McMahon et al. (1996) also reported an increase in meltability of reduced-fat cheeses during ripening with carbohydrate-based fat mimetics. Increases in meltability due to GT appear to be attributed mainly to the increase in moisture content, M:P and physical disruption of the protein network in the cheese by GT and decrease in pH. The low pH of the FFGum cheese led to increased proteolysis, and more extensive degradation of the para-casein matrix and consequently higher meltability. Increased solubilisation of CCP cross-links in the para-casein matrix leading to decreased structural integrity in the cheese may have also contributed to increased meltability. In reduced-fat cheeses, the increased evaporation at the surface of melting cheese due to lack of free oil causes more extensive protein aggregation, forming a thicker ‘skin’ on the cheese surface (Kim et al. 2011), leading to a reduction in melt area.
3.7 Dynamic small amplitude oscillatory rheology
Results from dynamic small amplitude oscillatory rheometry (DSAOR) at 7 months of ripening are shown in Table 3. The HF cheeses had a higher G′ at 40 °C, possibly reflecting the presence of a stronger protein matrix due to the increased protein content in these cheeses. The HFC cheese had lower LT values than the FFC cheese at 7 months. As a higher LT indicates a higher meltability (Lucey et al. 2003), the results for the FFC and HFC cheeses correlate well with the Schreiber meltability values. The temperatures at LTmax of the HF cheeses were ∼10 °C higher than those of the FF cheeses. GT caused a significant decrease (P < 0.05) in the LTmax and an increase in G′ at 75 °C in both the FF and HF cheeses. If intact GT still remains at 7 months, it may retard the phase separation of moisture from protein at high temperature. The protein component of GT may also have a role in depressing the LT. As GT can contain up to 4% protein, there may be interactions between the caseins and this protein fraction. The decrease in pH caused by GT may have also contributed to these results. Lee et al. (2010) also reported that cheese with low pH (<5.0), had a higher G′ at 40 °C and lower LTmax than cheeses with higher pH.
When comparing this decrease in ‘meltability’ caused by GT to the Schreiber meltability (Fig. 5), it should be noted that the gradual partitioning of the fat, moisture and protein occurring at the temperatures of the DSAOR test occur much faster during the Schreiber test due to rapid liquefaction of fat due to the instant exposure to 232 °C. Therefore, it is probable that interactions between GT and caseins at the temperature of the DSAOR may partially inhibit melting.
3.8 Colour analysis
L* values of cheeses during ripening are shown in Fig. 6. The FF cheeses had significantly higher (P < 0.05) L* values than the HF cheeses throughout ripening, which is in agreement with Rudan et al. (1999). The HF cheeses had much higher protein and lower M:P, MNFS and fat than the FF cheeses, which gives them a much denser protein matrix and less light-scattering centres (i.e. fat droplets and serum pockets). After 16 weeks of ripening, addition of GT appeared to increase significantly the L* values in both the FF and HF cheeses. Fat replacers, because of their particulate nature can act as light-scattering centres and increase the opaqueness of reduced fat cheese (McMahon et al. 1996). The increased moisture content and interruption of the para-casein matrix in cheeses containing GT gave the cheese a less dense protein network and an increased surface area occupied by scattering centres. This effect of GT is in agreement with Rahimi et al. (2007), who observed an increase in the L* values of low-fat Iranian White Cheese supplemented with GT.
3.9 Consumer preference ranking test
Table 4 shows the consumer preference ranking test scores for three of the experimental cheeses: FFC, HFC and HFGum at month 7 of ripening. The FFC cheese received the highest rank of the three cheeses. Results show that the HFGum cheese was overall the lowest ranked cheese when compared to the FFC and HFC cheeses. Rheological parameters obtained from TPA would suggest that the texture of the HFGum cheese should be more favourable than the HFC cheese, indicating flavour may have played a greater role than textural attributes. Rahimi et al. (2007) observed that the ability of GT to improve sensory properties of low-fat Iranian White cheese is highly dependent on the level of addition. The flavour of full-fat cheese varieties is one of the hardest attributes of cheese to mimic when producing reduced-fat cheeses. Flavour differences between full-fat and reduced-fat cheese is thought to be due to differences in composition, flavour release, structure and ripening biochemistry (Drake et al. 2010). A lower concentration of fat-derived volatile flavour compounds typical of full-fat Cheddar along with bitterness have been identified as reasons for atypical flavour in reduced-fat cheeses (Banks 2004; Drake et al. 2010; Mistry 2001). In addition, many panellists noticed an off-flavour in the HFGum cheese (not shown). Previous studies have observed unfavourable flavours in reduced-fat cheeses during ripening due to addition of carbohydrate-based fat mimetics (Koca and Metin 2004; Konuklar et al. 2004). Some fat replacers have been found to form volatile compounds on decomposition that give off-flavours in cheese during ripening (Suriyaphan et al. 1999).
4 Conclusions
This study indicates that GT can have a major effect on many of the rheological, sensory and functional properties of Cheddar cheese. The major effect of GT appears to be its ability to alter the composition and indirectly lower the pH of cheeses. The significant increases in moisture (P < 0.05) and M:P caused a decrease in TPA hardness and springiness in the HFGum cheese to values similar to the FFC. The increase in proteolysis in the FFGum cheese due to pH reduction may also be related to the increased meltability and lower values for TPA parameters in this cheese. Overall, the TPA parameters indicated that addition of GT to HF cheese at this level is successful in mimicking the hardness and springiness of the FFC cheese, which is a desired textural property. GT appeared to increase the meltability of the HFGum cheese at 6 months of ripening, which is highly desirable in the context of functionality. The effect of GT on the DSAOR parameters G′ and LT may show evidence of modification of casein interactions via the polysaccharide or protein component of GT at increased temperatures. From the consumer preference ranking test, it was evident that supplementing half-fat Cheddar cheese with this level of GT may not be suitable for use as a table cheese, but may be suitable as an ingredient cheese due to its favourable functional properties. A possible avenue for further investigation could involve the use of GT in low-fat Cheddar cheese.
References
Altan A, Turhan M, Gunasekaran S (2005) Comparison of covered and uncovered Schreiber test for cheese meltability evaluation. J Dairy Sci 88:857–861
Awad S, Hassan A, Muthukumarappan K (2005) Application of exopolysaccharide-producing cultures in making reduced fat Cheddar cheese. Textural and melting properties. J Dairy Sci 88:17–18
Azarikia F, Abbasi S (2010) On the stabilization mechanism of Doogh (Iranian yoghurt drink) by gum tragacanth. Food Hydrocoll 24:358–363
Aziznia S, Khosrowshahi A, Madadlou A, Rahimi J, Abbasi H (2009) Texture of nonfat yoghurt as influenced by whey protein concentrate and gum tragacanth as fat replacers. Int J Dairy Technol 62:405–410
Banks JM (2004) The technology of low-fat cheese manufacture. Int J Dairy Technol 57:199–207
Bourne MC (1978) Texture profile analysis. Food Technol 32(62–66):72
Broadbent JR, McMahon DJ, Oberg CJ, Welker DL (2001) Use of exopolysaccharide-producing cultures to improve the functionality of low fat cheese. Int Dairy J 11:433–439
Childs JL, Drake M (2009) Consumer perception of fat reduction in cheese. J Sens Stud 24:902–921
Creamer LK, Olson NF (1982) Rheological evaluation of maturing Cheddar cheese. J Food Sci 47(631–636):646
Dickinson E (1998) Stability and rheological implications of electrostatic milk protein–polysaccharide interactions. Trends Food Sci Technol 9:347–354
Drake MA, Miracle RE, McMahon DJ (2010) Impact of fat reduction on flavor and flavor chemistry of Cheddar cheeses. J Dairy Sci 93:5069–5081
Fenelon MA, Guinee TP (2000) Primary proteolysis and textural changes during ripening in Cheddar cheeses manufactured to different fat contents. Int Dairy J 10:151–158
Fenelon MA, O’Connor P, Guinee TP (2000) The effect of fat content on the microbiology and proteolysis in Cheddar cheese during ripening. J Dairy Sci 83:2173–2183
Fox PF (1963) Potentiometric determination of salt in cheese. J Dairy Sci 46:744–745
Fox PF, Wallace JM (1997) Formation of flavor compounds in cheese. Adv Appl Microbiol 45:17–85
Guinee TP, Auty MAE, Fenelon MA (2000a) The effect of fat content on the rheology, microstructure and heat-induced functional characteristics of Cheddar cheese. Int Dairy J 10:277–288
Guinee TP, Auty MAE, Mullins C, Corcoran MO, Mulholland EO (2000b) Preliminary observations on effects of fat content and degree of fat emulsification on the structure–functional relationship of Cheddar-type cheese. J Texture Stud 31:645–663
IDF (1982) Standard 4A, Cheese and processed cheese. Determination of the total solids content. Int Dairy Fed, Brussels
IDF (1986) Standard 20A, Determination of the nitrogen content (Kjeldahl method) and calculation of crude protein content. Int Dairy Fed, Brussels
IIRS (1955) Irish Standard 69, Determination of the percentage of fat in cheese. Inst Ind Res Stand, Dublin
Johnson ME, Kapoor R, McMahon DJ, McCoy DR, Narasimmon RG (2009) Reduction of sodium and fat levels in natural and processed cheeses: scientific and technological aspects. Compr Rev Food Sci Food Saf 8:252–268
Kavas G, Oysun G, Kinik O, Uysal H (2004) Effect of some fat replacers on chemical, physical and sensory attributes of low-fat white pickled cheese. Food Chem 88:381–388
Kelly M, Fox PF, McSweeney PLH (1996) Effect of salt-in-moisture on proteolysis in Cheddar-type cheese. Milchwissenschaft 51:498–501
Kim SY, Lim S, Gunasekaran S (2011) Protein interactions in reduced-fat and full-fat Cheddar cheeses during melting. Food Sci Technol 44:582–587
Koca N, Metin M (2004) Textural, melting and sensory properties of low-fat fresh Kashar cheeses produced by using fat replacers. Int Dairy J 14:365–373
Konuklar G, Inglett GE, Warner K, Carriere CJ (2004) Use of a beta-glucan hydrocolloidal suspension in the manufacture of low-fat Cheddar cheeses: textural properties by instrumental methods and sensory panels. Food Hydrocoll 18:535–545
Laloy E, Vuillemard JC, El Soda M, Simard RE (1996) Influence of the fat content of Cheddar cheese on retention and localization of starters. Int Dairy J 6:729–740
Lee MR, Johnson ME, Govindasamy-Lucey S, Jaeggi JJ, Lucey JA (2010) Insoluble calcium content and rheological properties of Colby cheese during ripening. J Dairy Sci 93:1844–1853
Lteif L, Olabi A, Baghdadi OK, Toufeili I (2009) The characterization of the physicochemical and sensory properties of full-fat, reduced-fat, and low-fat ovine and bovine Halloumi. J Dairy Sci 92:4135–4145
Lucey JA, Johnson ME, Horne DS (2003) Invited review: perspectives on the basis of the rheology and texture properties of cheese. J Dairy Sci 86:2725–2743
McMahon DJ, Alleyne MC, Fife RL, Oberg CJ (1996) Use of fat replacers in low fat Mozzarella cheese. J Dairy Sci 79:1911–1921
Mistry VV (2001) Low fat cheese technology. Int Dairy J 11:413–422
Nasirian R, Vaziri A, Safekordi AA, Ardjmand M (2010) Use of gum tragacanth in reduction of dairy cream fat. Milchwissenschaft 65:49–52
O’Mahony JA, Lucey JA, McSweeney PLH (2005) Chymosin-mediated proteolysis, calcium solubilization, and texture development during the ripening of Cheddar cheese. J Dairy Sci 88:3101–3114
Rahimi J, Khosrowshahi A, Madadlou A, Aziznia S (2007) Texture of low-fat Iranian White cheese as influenced by gum tragacanth as a fat replacer. J Dairy Sci 90:4058–4070
Resurreccion AVA (1998) Consumer sensory testing for product development. Aspen Publishers Inc., Gaithersburg
Rogosa M, Mitchell JA, Wiseman RF (1951) A selective medium for the isolation and enumeration of oral and faecal lactobacilli. J Bacteriol 62:132–133
Rudan MA, Barbano DM, Yun JJ, Kindstedt PS (1999) Effect of fat reduction on chemical composition, proteolysis, functionality, and yield of Mozzarella cheese. J Dairy Sci 82:661–672
Sousa MJ, Ardo Y, McSweeney PLH (2001) Advances in the study of proteolysis during cheese ripening. Int Dairy J 11:327–345
Suriyaphan O, Drake MA, Cadwallader KR (1999) Identification of volatile off-flavors in reduced-fat Cheddar cheeses containing lecithin. Food Sci Technol 32:250–254
Terzaghi BE, Sandine WE (1975) Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol 29:807–813
Watkinson P, Coker C, Crawford R, Dodds C, Johnston K, McKenna A, White N (2001) Effect of cheese pH and ripening time on model cheese textural properties and proteolysis. Int Dairy J 11:455–464
Whistler RL (1993) Exudate gums. In: Whistler RL, BeMiller JN (eds) Industrial gums: polysaccharides and their derivatives. Academic, San Diego, pp 309–339
Acknowledgments
This research was supported by a grant to D.R. Cooke by the Irish Research Council for Science Engineering and Technology.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Cooke, D.R., Khosrowshahi, A. & McSweeney, P.L.H. Effect of gum tragacanth on the rheological and functional properties of full-fat and half-fat Cheddar cheese. Dairy Sci. & Technol. 93, 45–62 (2013). https://doi.org/10.1007/s13594-012-0088-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13594-012-0088-z