Abstract
Headspace solid-phase microextraction–gas chromatography-mass spectrometry (HS-SPME–GC-MS) and multivariate statistical analysis (MVA) are widely employed in the analyses/comparison of cheese volatiles. One shortcoming of this approach is that the SPME fiber-coat polarity has a significant effect on the adsorption of volatiles and thus, on the results of MVA. The aim of this study was to test the applicability of different sets of MVA variables, obtained from HS-SPME–GC-MS experiments using the non-polar polydimethylsiloxane (PDMS) and the polar polyacrylate (PA) fibers, in the profiling of cheese volatile compounds and tracking ripening-induced changes. The volatile profiles and ripening-induced changes of two different types of Pasta-Filata “Pirotski kačkavalj” cheese (prepared from raw ewe’s or cow’s milk) were assessed at four different stages of ripening (1, 5, 20, and 30 days). The HS-SPME–GC-MS–MVA results showed that the distribution of volatile compounds into classes gives valuable information concerning cheese type and ripening phase. The extraction with a PA fiber gives a more realistic cheese volatile profile when the relative ratio of the constituent classes is in question, whereas the PDMS fiber is better in recognizing the contribution/concentration of single dominant volatile compounds. For a better and more complete description/profiling of cheese volatiles, we propose the use of combined (transformed) variables that include the information obtained from both PA and PDMS extraction experiments.
摘要
顶空固相微萃取-气质联用(HS-SPME-GC-MS)和多元统计分析(MVA)广泛应用于分析和比较干酪的挥发性化合物。该方法的一个缺点是SPME 纤维外套的极性对挥发性化合物的吸附影响较大,因此也影响了MVA分析的结果。本论文目的是应用不同组合的MVA变量,这些变量是通过HS-SPME-GC-MS实验并采用非极性聚二甲硅氧烷(PDMS)和极性聚丙烯酸酯(PA)纤维萃取跟踪干酪成熟过程中引发的挥发性化合物变化。分析了两种不同类型的Pasta-Filata 和“Pirotski kačkavalj干酪(来自羊奶和牛奶)在四个不同成熟阶段(1,5,20和30 d)所产生挥发性化合物的变化。HS-SPME-GC-MS-MVA实验结果对干酪的类型和成熟阶段挥发性化合物的分布给出了非常重要的信息。利用PA纤维萃取对需要讨论干酪中各种挥发性化合物组分的相对比例给出了更理想的结果。然而PDMS纤维萃取对了解单个主要的挥发性化合物的分布和浓度方面更有优势。为了更好更全面的描述干酪的挥发性化合物,建议将PA和PDMS两种萃取实验的信息结合起来分析干酪成熟过程中挥发性化合物的变化。
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The unique flavor of different cheese varieties is due to the complex balance of the taste, aroma, and texture characteristics. Volatile compounds play a particularly important role in this respect (Arora et al. 1995; Januszkiewicz et al. 2008; Mondello et al. 2005; Peres et al. 2001; Pinho et al. 2002; Ziino et al. 2005). However, during cheese ripening, the cheese volatile/flavor profiles can change dramatically due the complex biochemical and chemical processes of lipolysis, proteolysis, and glycolysis (Fox and Wallace 1997). Due to the complexity of cheese volatile profiles (mixtures of a large number of compounds belonging to different chemical classes: alcohols, esters, acid, ketones, etc.), the monitoring of ripening-induced changes is not a straightforward task. In fact, for reasons of reliability and clarity, it is often necessary to perform multivariate statistical analyses (MVA, e.g., agglomerative hierarchical cluster analysis (AHC) and principal component analysis (PCA)) as a means of differentiating personal bias from statistical significance (Delgado et al. 2010). The variables used for MVA analyses (input data set) of cheese volatile profiles are usually obtained from gas chromatography (GC) and gas chromatography-mass spectrometry (GC-MS) experiments (Arora et al. 1995; Januszkiewicz et al. 2008; Mondello et al. 2005; Peres et al. 2001; Pinho et al. 2002, 2003; Ziino et al. 2005). Headspace solid-phase microextraction (HS-SPME) is a widely employed extraction method for GC and GC-MS analyses of cheese volatiles due to its relative simplicity and cost-effectiveness (Januszkiewicz et al. 2008; Mondello et al. 2005). Nevertheless, it has previously been demonstrated that the fiber-coat polarity has a significant effect on the adsorption of volatiles (Mondello et al. 2005; Pinho et al. 2003). Hence, one could justly formulate a question—Is it really reasonable to use data obtained from this type of sampling/analyses (HS-SPME–GC-MS) for the comparison (variables for MVA) of different types of cheese, or for monitoring ripening-induced changes in cheese volatiles? And, if it is so, does the extraction with one particular type of fiber give better, more reliable, and more reproducible results? Furthermore, is it possible to solve this problem (fiber-dependent differences in the volatile profile of a sample) by using some transformed/combined variables for MVA, which would include information obtained from experiments based on the usage of different SPME stationary phases?
Bearing all this in mind, the main aim of this research was to test the applicability of different sets of MVA variables to profile cheese volatile compounds and monitor ripening-induced changes. The different sets of MVA variables include: the original data (relative amounts individual volatile compounds, obtained directly from HS-SPME–GC-MS experiments); transformed data (relative amounts of each class of compounds); and combined data (created by averaging the relative amounts of individual compounds obtained using both types of stationary phases).
HS-SPME–GC-MS analyses of Pasta Filata cheeses were performed using two SPME fibers of different polarity (polar: polyacrylate (PA) and non-polar: polydimethylsiloxane (PDMS)). “Pirotski kačkavalj” cheeses made from cow’s and ewe’s milk were analyzed at four stages of ripening. Results on the chemical composition of the volatile profiles of the two types of Pirotski kačkavalj cheese are reported here for the first time.
2 Materials and methods
2.1 Manufacture and sampling of cheese
Three batches of 12 0.5-kg cheeses (two different types of Pasta Filata “Pirotski kačkavalj” cheese) were manufactured in a small, certified dairy factory, Dairy School “Dr Obren Pejić”, in Pirot (Southeast Serbia) in three sequential periods within the cheese making season (i.e., November, February, and May) from raw ewe’s (EC) and cow’s milk (CC) according to traditional practices (Milosavljević et al. 2010). Three cheeses of each batch were randomly selected on the day of manufacture and after 5, 20, and 30 days of ripening. Samples of each type of cheese were taken from the inner part of the cheese and were stored at ∼−18 °C (during 1–5 weeks) prior to analysis.
2.2 Extraction of volatile compounds
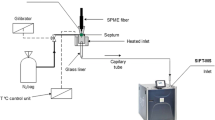
Cheese volatile compounds were extracted by SPME using two types of commercially available fibers (PDMS, 100 µm film thickness and PA, 85 µm film thickness). The SPME fibers were housed in their manual holder (Supelco, Bellefonte, PA, USA).
All extractions were performed as follows—2 g of each cheese sample (eight samples in total) was placed in a 50-mL headspace glass vial sealed tightly with a crimp cap and PTFE/silicone septum. The vial was immersed at 40 mm in a thermostated waterbath at 60 °C, and the volatile compounds were extracted over 20 min using the two SPME fiber coatings maintained 10 mm above the molten samples which were continuously stirred with a magnetic stirring bar. After extraction, the samples were directly desorbed (without delay) by manual injection into the GC injection port, held at 250 °C, using a specially designed splitless 0.75 mm ID inlet liner to suit these purposes (also purchased from Supelco).
2.3 Gas chromatography-mass spectrometry (GC-MS)
Separation and identification of the volatile compounds extracted as described above were performed by GC-MS. The instruments used and the experimental conditions are identical to those described in Radulović and Blagojević (2010), except that the samples were manually injected by inserting the saturated fiber into the inlet liner for 10 min. The GC oven temperature was held at 45 °C for 5 min, then raised to 250 °C at a heating rate of 10 °C.min−1, and then held isothermally for 10 min at 250 °C (carrier gas: helium at 1.0 mL.min−1). The relative abundance (percent peak area) of three independent replicate samples of each compound identified by GC-MS without the use of correction factors is given in Table S1. Qualitative analysis was performed in the same way as described in Radulović and Blagojević (2010) and was based on (a) comparison of the mass spectra, (b) order of elution, and (c) co-injection of authentic standards (the corresponding pure compounds were added/spiked to cheese samples prior to HS-SPME). Standard (pure) compounds were obtained from Merck, Germany or Sigma-Aldrich, USA. All analyses were performed in triplicate, and there were no statistically significant differences between the obtained results.
2.4 Multivariate statistical analysis (MVA)
PCA and AHC were performed using the Excel program plug-in XLSTAT version 2011.3.02 and according to Radulović and Blagojević (2010). AHC was determined using Pearson dissimilarity where the aggregation criterion were simple linkage, unweighted pair-group average and complete linkage, and Euclidean distance where the aggregation criterion were weighted pair-group average, unweighted pair-group average, and Ward’s method. Euclidian distance dissimilarities of the analyzed samples were within the interval [0, 2,000–12,000]. PCA of the Pearson (n) type was performed. Axes (F1 and F2 factors—the first and second principal components) of the resulting biplots refer to the ordination scores obtained for the samples. Both MVA methods were applied utilizing following types of variables: PA and PDMS original variables (relative amounts of individual compounds, as determined from HS-SP–GC-MS experiments with PA and PDMS fibers), PA and PDMS transformed variables (relative amounts of classes of compounds, obtained from the experiments with PA and PDMS fibers), and original-combined and transformed-combined variables (these were created by averaging (weight factor of 0.5) of the relative amounts of individual constituents/class of constituents obtained from the experiments with both types of stationary phases).
3 Results and discussion
3.1 Volatile compounds of the two types of Pirotski kačkavalj cheese
The main compounds identified in the cheeses are listed in Table 1 (all identified compounds, 73 in total, are given in the Electronic supplementary material). The major classes of volatile compounds identified in both cheese types were short- and medium-chain fatty acids, alcohols, esters, and ketones. Some of the identified compounds were unique to just one sample/ripening period or reported for the first time in cheese samples. For example, methyl 2-hydroxy-4-methylpentanoate has not been previously reported in any other type of cheese prepared from cow’s milk. However, this compound, (bio)synthetically related to leucine, was previously detected as a volatile metabolite in a number of different plant species (Knudsten et al. 1994). Perhaps, the origin of this ester in milk/cheese is directly connected to its presence in the fodder. The same may be true for several aliphatic and aromatic hydrocarbons detected (Viallon et al. 1999). Some of the compounds identified were characteristic for only one cheese type/ripening period and could also be products of lipid autooxidation or carotene degradation, formed during ripening (Barbieri et al. 1994; Johnson et al. 1969). Diethyl ether, dichloromethane, and chloroform were most probably contaminants introduced during the isolation procedure, during storage or from detergents used during cleaning of the plant and equipment.
3.2 Extraction of volatile compounds: influence of the stationary phase type on the resulting volatile profile
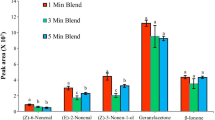
The two types of fiber stationary phases were selected due to their differentiating capacity for adsorption of volatiles (Mondello et al. 2005; Pinho et al. 2003). It has been shown that the non-polar PDMS fiber was satisfactory for compounds of low to medium polarity whereas the PA stationary phase is more suitable for the extraction of highly polar compounds of a broader volatility range (Mondello et al. 2005). The data presented in Table 1 could be used to justify and once again emphasize the necessity to use at least two fiber types, of different coating polarity, for HS-SPME extraction. The sensitivity of the PDMS phase for the extraction of octanoic acid (and other longer chain aliphatic compounds) was obviously higher than that of the PA fiber (Table 1). On the other hand, the PA fiber more effectively adsorbed ethanol and the rest of the detected acids. The results also show that both fibers had similar sensitivity for alcohols other than ethanol. When reaching conclusions on this “functional group affinity” of a particular stationary phase, one should bear in mind the changes (i.e., the decrease) of polarity of members of a (homologous) series of compounds with an increase in the number of carbon atoms in their alkyl chains. Once more, it is important to note that the use of just one type of stationary phase for HS-SPME of volatile compounds would have easily given false (over- or understating the relative contribution of certain compound(s)) results concerning the most dominant volatile compounds present in these cheese samples.
MVA analyses (see Electronic supplementary material Fig. S2) revealed that, in some cases, no significant differences were detected between the PDMS and PA cheese volatile profiles. Nevertheless, a substantial number of PA volatile profiles were recognized as statistically different from their corresponding non-polar fiber counterparts. The differences between the volatile profiles obtained through the use of different SPME phases (non-polar and polar) become even more pronounced if the sums of the volatile compound classes are used as variables. It is interesting to note that all four cheese samples made from cow’s milk and the 1-day-old cheese sample made from ewe’s milk had very similar volatile profiles (both fibers), whereas the volatile profiles of the three older ewe’s cheese samples were very different depending on the fiber used. It seems that some more pronounced chemical changes occur during the ripening of ewe’s milk cheese in comparison to those associated with cow’s cheese (this could be a consequence of the transformation of one class of compounds to another of different polarity).
3.3 Different types of variables for the comparison of volatile profiles of different cheese types and the tracking of ripening-induced changes
The results of MVA analyses (Figs. 1 and 2) clearly showed that, in general, there are significant differences in the aroma profiles of Pirotski kačkavalj made from ewe’s and cow’s milk. Generally speaking, all MVA analyses clearly distinguished (with few exceptions that will be discussed latter) the two types of cheese, and the grouping of CC and EC samples is easily discernible from all the PCA biplots and AHC dendrograms. If considered alone, due to the “functional group affinity” of each of the SPME fiber stationary phases, the volatile profiles obtained by using a PDMS or PA fiber provide data which either over- or underestimated the relative amount of an individual volatile compound. It was our intention to check if this could be overcome by using some transformed/combined variables for MVA, which would include data obtained from experiments with different fiber stationary phases. In order to do that, additional variables were devised by combining (summing with the weight value equal to 0.5) relative amounts of individual compound/classes of compounds found in a cheese sample by each of the different fibers (PA and PDMS). The MVA results performed with these new variables are given in Fig. 3. Significant differences in the flavor profiles of the cheese samples prepared from different types of milk (ewe’s and cow’s) were still clearly highlighted.
Dendrograms (AHC analysis) and biplots (PCA analysis) representing chemical composition dissimilarity relationships of eight cheese samples using the original-combined (weight factor of 0.5) variables: a PA and PDMS-AHC, b PA and PDMS-PCA; and the transformed-combined (weight factor of 0.5) variables: c PA and PDMS-AHC, d PA and PDMS-AHC
As can be seen from Table 1 and Figs. 1, 2, and 3, significant differences in the volatile profiles of the two cheese types occurred during the ripening period. The relative amounts of some of the compounds/classes of compounds tended to increase during the cheese ripening (e.g., majority of acids), as seen in Table 1, while others decreased (e.g., ketones). Generally speaking, with ripening, a larger number of volatile compounds were identified in cheeses made using EC than in CC. It should be noted that the ripening-induced changes in the cheese volatile profiles are, most probably, the result of at least two different processes: (a) chemical/microbiological transformation of native raw milk constituents and (b) evaporation loss of the most volatile compounds. Thus, the cheese volatile profile could be considered to be the result of a complex and dynamic equilibrium.
The changes in the volatile profiles of both types of cheese are clearly shown in Figs. 2 and 3. The extraction of flavor compounds using the different fibers resulted in very different volatile profiles (subsection “3.2”). For that reason, ripening-induced changes in the volatile profiles of CC and EC samples were statistically analyzed taking into account the results obtained by both types of HS-SPME stationary phases (PA and PDMS) and by using several different types of variables: original (relative amounts of individual compounds), transformed (relative amounts of selected classes of compounds), and combined variables (the sum of original/transformed variables corresponding to the same sample, but obtained using different fibers, with the weight factor equal to 0.5). In a number of cases, for most of the CC cheese samples, almost all variables used gave comparable results. It is interesting to note that the volatile profile of the CC samples which had longer ripening times were more (statistically) similar to EC samples (generally considered as richer in flavor); see Figs. 1, 2, and 3. For example, as can be seen from Fig. 3, MVA analyses performed with both types of combined variables (relative amounts of individual compounds and classes of compounds), CC3 and CC4, samples were either placed within the same cluster with EC samples (AHC) or have values of score factors similar to those of certain EC samples. This perhaps could be used as an additional confirmation of the fact that cheese ripening is of critical importance for the full development of its flavor and aroma.
The results of MVA analyses obtained for the EC samples, using different sets of variables, were much less coherent. It seems, according to all performed PCA analyses (differences in the corresponding score factors), that EC undergoes a more pronounced and, more or less, constant change during ripening. According to the AHC analyses, samples EC2 and EC3 were recognized as statistically mutually very similar and were always placed within the same cluster of the corresponding dendrograms. In contrast, the clustering of EC1 and EC4 was not the same if EC-PDMS instead of EC-PA profiles were used as the input datasets for AHC. This again pinpoints the issue relating to differences in the affinity of the HS-SPME stationary phases to compounds of differing polarity. Interesting results were obtained when combined variables were used (averaging of the cheese profiles was achieved by combining the profiles obtained from both of the used types of fibers, with the weight factor of 0.5). For example, AHC analysis (average of the relative amounts of individual constituents as variables) resulted in dendrograms that are essentially the same as those obtained by the use of original variables derived from PDMS profiling. However, the results of the AHC based on combined–transformed variables (averaging of the relative amounts of classes of constituents) were analogous to those of the AHC with original PA variables. Thus, it could be speculated that, perhaps, PA gives a more realistic cheese profile when classes of constituents are in question, while PDMS is better at recognizing the specific dominant volatile/flavor compounds. MVA experiments with combined variables obtained with different weighting factors (0.8/0.2, 0.6/0.4, 0.4/0.6, and 0.2/0.8) were also performed and gave expected results (in the sense of resemblance to the MVA results obtained by using the corresponding (PA or PDMS) variables).
4 Conclusion
In this paper, the applicability of different types of variables in MVA profiling of cheese volatile compounds was explored. Our results have shown that, in addition to original variables (relative amounts of detected cheese volatile compounds), the distribution of volatile compounds into classes also gives valuable information concerning cheese type and ripening stage. Additionally, the extraction with a PA fiber gives a more realistic cheese profile when the relative ratio of the classes of constituents is in question, while PDMS is better in recognizing the contribution/concentration of the dominant volatile/flavor compounds. Also, combined variables that include information obtained from the extraction experiments using different types of stationary phases (PA and PDMS fibers) could be used for a better and more complete description/profiling of the aroma of cheese.
References
Arora G, Cormier F, Lee B (1995) Analysis of odor-active volatiles in cheddar cheese headspace by multidimensional GC/MS/sniffing. J Agric Food Chem 43:748–752
Barbieri G, Bolzoni L, Careri M, Mangia A, Parolari G, Spagnoli S, Virgili R (1994) Study of the volatile fraction of parmesan cheese. J Agric Food Chem 42:1170–1176
Delgado FJ, González-Crespo J, Cava R, García-Parra J, Ramírez R (2010) Characterisation by SPME–GC–MS of the volatile profile of a Spanish soft cheese P.D.O. Torta del Casar during ripening. Food Chem 118:182–189
Fox PF, Wallace JM (1997) Formation of flavour compounds in cheese. Adv Appl Microbiol 45:17–85
Januszkiewicz J, Sabik H, Azarnia S, Lee B (2008) Optimization of headspace solid-phase microextraction for the analysis of specific flavors in enzyme modified and natural cheddar cheese using factorial design and response surface methodology. J Chromatogr A 1195:16–24
Johnson AE, Nursten HE, Self R (1969) Aromatic hydrocarbons in foodstuffs and related materials. Chem Ind-London 1:10–12
Knudsten JT, Tollsten L, Bergstrom LG (1994) Floral scents—a checklist of volatile compounds isolated by head-space techniques. Phytochemistry 33:253–280
Milosavljević N, Joković N, Radulović N, Blagojević P, Savić D (2010) Major volatiles in “Pirotski kačkavalj” cheese made from sheep milk. Preh. ind.– Mleko i ml. proiz. 1–2:122–126
Mondello L, Costa R, Tranchid PQ, Chiofalo B, Zumbo A, Dugo P, Dugo G (2005) Determination of flavor components in Sicilian goat cheese by automated HS-SPME-GC. Flavour Frag J 20:659–665
Peres C, Viallon C, Berdague J-L (2001) Solid-phase microextraction-mass spectrometry: a new approach to the rapid characterization of cheeses. Anal Chem 73:1030–1036
Pinho O, Ferreira IM, Ferreira MA (2002) Solid-phase microextraction in combination with GC/MS for quantification of the major volatile free fatty acids in ewe cheese. Anal Chem 74:5199–5204
Pinho O, Pérès C, Ferreira IM (2003) Solid-phase microextraction of volatile compounds in “Terrincho” ewe cheese. Comparison of different fibers. J Chromatogr A 1011:1–9
Radulović NS, Blagojević PD (2010) Plant volatiles providing additional evidences to the occurrence of a wild-growing population of Calamintha vardarensis (Greuter et Burdet) Silic outside of its natural habitat. Chem Biodivers 7:2856–2868
Viallon C, Verdier-Metz I, Denoyer C, Pradel P, Coulon JB, Berdague JL (1999) Desorbed terpenes and sesquiterpenes from forages and cheeses. J Dairy Res 66:319–326
Ziino M, Condurso C, Romeo V, Giuffrida D, Verzera A (2005) Characterization of “Provola dei Nebrodi”, a typical Sicilian cheese, by volatiles analysis using SPME-GC/MS. Int Dairy J 15:585–593
Acknowledgments
The research has been supported by the Ministry of Education and Science of Serbia (Project 172061).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 466 kb)
About this article
Cite this article
Milosavljević, N.P., Blagojević, P.D., Savić, D.S. et al. Application of HS-SPME–GC-MS-derived variables for monitoring ripening-induced changes in cheese volatile compounds. Dairy Sci. & Technol. 92, 321–333 (2012). https://doi.org/10.1007/s13594-012-0070-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13594-012-0070-9
Keywords
- Volatile profiles
- Principal component analysis
- Agglomerative hierarchical cluster analysis
- Cheese ripening
- Pasta-Filata “Pirotski kačkavalj” cheese