Abstract
The objective of this work was to determine if concentration of milk using ultrafiltration (in the absence of diafiltration) affects the physico-chemical properties of the casein micelles. The milk, once concentrated, was brought back to its original concentration and the physico-chemical properties as well as its susceptibility to rennet induced gelation were assessed. Although much is understood on the renneting behavior of concentrated milk, no information is available on how (or if) ultrafiltration per se modifies the properties of the micelles. Two times (2×) or five times (5×) concentrated milk (based on volume reduction) was prepared by ultrafiltration. Casein micelles were redispersed in their corresponding permeates and dialyzed against untreated skim milk. The stability of the casein micelles, their scattering properties, and the renneting properties were observed under the same volume fraction and ionic conditions of the skim milk control. There was a higher susceptibility to aggregation and a lower gel stiffness for the reconstituted micelles from the 5× milk compared to the 2× milk. 5× Ultrafiltration induced losses of calcium from the micelle, while 2× milk showed similar value as the control. The results suggest that the process of ultrafiltration affected the internal structure of the casein micelles, and this caused changes in the light-scattering properties and renneting functionality.
超滤是否对酪蛋白胶束的物理化学特性产生持续性的影响?
摘要 本文的目的是确定超滤 (未经过二次超滤) 是否对酪蛋白胶束的物理化学性质产生影响。将浓缩后的牛奶还原到原奶的浓度, 然后评价了乳对凝乳酶的敏感性等物理化学性质。尽管对浓缩乳的凝乳特性已经有了深入的了解, 但是对超滤是否对酪蛋白胶束性质产生影响, 有关这方面的研究信息非常有限。制备2倍或5倍的超滤浓缩乳, 然后将浓缩乳重新分散到相应的透过液中并在未处理的脱脂奶中透析。以脱脂乳作对照组, 测定了酪蛋白胶束稳定性, 酪蛋白胶束的光散射特性及其凝乳特性。与2倍超滤浓缩物的还原奶相比, 5倍超滤浓缩物的还原奶中酪蛋白胶束凝聚敏感性较高但形成凝胶的硬度较低。5倍超滤浓缩过程导致了胶束中钙的丢失, 而2倍的超滤浓缩乳与对照组相似的性质。研究结果表明超滤过程影响了酪蛋白胶束的内部结构, 进而影响了光散射和凝乳特性的改变。
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Caseins are the main proteins in milk, and they are arranged in self-assemblies called casein micelles. There are four main caseins: αs1-casein, αs2-casein, ß-casein, and κ-casein. These proteins contain several phosphoserine residues in their structure with the exception of κ-casein which only has one phosphoseryl residue and is glycosylated. This makes αs-caseins and ß-casein prone to precipitation with excess Ca2+ ions, and these fractions are therefore often referred to as calcium sensitive caseins (Horne 2006). Caseins contain a number of proline residues, are characterized by hydrophobic domains in their structure and they are charged. They have little secondary and tertiary structure, and they do not show thermal transitions during heating at temperatures <100°C. Caseins associate extensively via hydrophobic and electrostatic interactions, and the casein micelle structure can be modified by even small changes in environmental conditions (De Kruif and Holt 2003).
The physical properties of the casein micelles have been well described: they are spherical in shape with an average diameter around 200 nm, surrounded by a κ-casein “hairy” layer which provides steric and charge stabilization (Holt et al. 1986; Horne 1998). The κ-casein also provides a charge distribution around the casein particles, yielding a zeta potential close to −20 mV (De Kruif and Holt 2003). Casein micelles can be easily modified by changes such as pH (Dalgleish and Law 1988), temperature (O'Connell and Fox 2003) or processing treatments as high pressure (Huppertz et al. 2004; Sandra and Dalgleish 2005) or spray drying (Singh 2007). It is important to point out that a very small change in the ionic equilibrium of milk will induce changes in the casein micelles’ structure and functionality, although these changes may not be easily observed.
Ultrafiltration (UF) is a pressure-driven membrane process in which membranes having molecular weight cutoff between 10 and 75 kg·mol−1 are used to separate macromolecules such as proteins, while small molecules (e.g., lactose and minerals) diffuse through the membrane into the permeate. In recent years, membrane filtration of milk has gained significant momentum in dairy technology, as with this process it is possible to modify milk functionality and produce value-added ingredients. Ultrafiltration of skim milk results in a retentate-containing caseins, whey proteins, and colloidal minerals at higher concentrations than those found in the original milk. The proportion of casein to colloidal calcium and phosphate may change during UF, especially when the process is extended to the highest levels of concentration (McKenna et al. 2000; Renner et al. 1991). The extent of the changes on colloidal calcium phosphate during UF depends mainly on processing conditions (such as temperature and pH of filtration), and micellar dissociation has been reported to occur during UF (Singh 2007). Changes in the chemical and physical properties of skim milk during membrane filtration have been extensively investigated (Jimenez-Lopez et al. 2008; Karlsson et al. 2005); the casein micelles are recognized as key factors in the processing performance during filtration (David et al. 2008; Piry et al. 2008; Rabiller-Baudry et al. 2005). However, the effects of UF on the structure function of the casein micelles have received very little attention. Milk protein concentrates are often referred to as a product “in which the casein is in a similar, micellar form to that found in milk and the whey proteins are also in their native form” (Mulhivill and Ennis 2003). On the other hand, recent work (Ferrer et al. 2008) demonstrated that casein micelles in milk protein concentrates behave differently during rennet gelation, even once resuspended at the same volume fraction of the original skim milk and after the serum composition has been re-equilibrated. Casein micelles functionality is modified during processing of milk protein concentrates (Singh 2007); however, it is not clear at this point which processing steps or factors are the most critical in affecting functionality.
While it seems safe to assume that caseins are still present as micellar structures in milk protein concentrates (David et al. 2008), much less is understood about the impact of concentration by ultrafiltration on the integrity of the casein micelles.
The objective of this work was to understand to what extent ultrafiltration, in the absence of diafiltration, affects the physical–chemical and functionality of the casein micelles. The research hypothesis is that changes occur in the casein micelles’ assemblies during concentration by ultrafiltration and that these changes affect the processing functionality of ultrafiltered milk. However, such research requires a distinction between the effect of concentration, volume fraction differences, and the actual changes (if any) to the casein micelle. A novel approach was therefore necessary in this research. Firstly, the filtration process was carried out reducing as much as possible temperature effects and keeping low transmembrane pressures. In addition, the properties of the casein micelles were studied after re-dispersing the concentrated systems to their original concentration, and the serum composition was also re-equilibrated. This allowed comparing samples under similar environmental conditions.
Results from this research may increase our ability to optimize ultrafiltration processes and to tailor specific processing functionalities to the micelles. No less important is the potential contribution of this research to the understanding of casein micelle structure and their stability.
2 Materials and methods
2.1 Casein micelles preparation
Fresh raw skim milk was obtained from Gay Lea Foods (Guelph, ON, Canada). Sodium azide (0.02% w/v) was added to prevent microbial growth and milk was kept at 4°C until further use. To assure complete separation of milk fat, cold milk was filtered four times through Whatman Fiberglass filters (Fisher Scientific, Whitby, ON, Canada).
Casein micelles were concentrated using a tangential flow filtration system (PUROSEP LT-2, NCSRT, Apex, NC, USA) composed of a 4-L feed tank, a Quattroflow pump that directs the retentate to a membrane module composed of polyethersulphone (PES; CONSEP 3000, NCSRT) with nominal molecular weight cutoff 10 kg·mol−1, size of 0.18 m2, 0.75 mm channel height and dead volume of 40 mL. Two pressure transmitter are connected to the inlet and outlet of the membrane and a flowmeter is positioned at the retentate exit of the membrane. Ultrafiltration was carried out by re-circulating the skim milk to the feed tank and measuring the permeate collected in a graduated cylinder. The volume reduction was based on permeate volume. Transmembrane pressure and cross-flow velocity were set to 1.7 bar and 12 L·min−1, respectively. Temperature was kept at 40°C using a water bath attached to a double-jacketed feed tank. Before starting the ultrafiltration experiment, the filtration system was drained, the retentate tank was filled with skim milk, the pump was started and at least 1 L of milk was discarded immediately after it exited the membrane outlet to rinse the system from any water remaining. Then the milk was recirculated at low transmembrane pressure to fill the membrane and permeate compartment. After 200 mL of permeate were collected, the system (with the exception of the membrane module) was drained. The UF was started with a new batch of milk.
Milk was concentrated (based on a volume reduction ratio) to two times the original volume (two times, 2×), based on volume reduction (i.e., volume of permeate collected). After collecting the 2× retentate, UF was continued until five times the original volume (five times, 5×) was achieved. Permeate fractions were collected immediately after the beginning of the ultrafiltration, as well as before collecting 2× and 5× retentate samples. The pH was measured with a pH transmitter (2100e, Mettler-Toledo GmbH, Germany). The cleaning procedure employed to regenerate the membranes was according to manufacturer instructions, and consisted in (1) rinse with deionized water; (2) recirculation with alkaline solution (DIVOS 110, JohnsonDiversey, Sturtevant, WI, USA) at 50°C for 30 min; (3) rinse with deionized water; (4) recirculation of acid solution (DIVOS 2, JohnsonDiversey) at 20°C for 20 min; and (5) a repetition of the steps from 2 to 4.
Retentates (2× and 5×) were recombined with appropriate volumes of milk permeate to prepare casein micelles suspensions at 10% volume fraction (ϕ = 0.1), the initial volume fraction of skim milk. To restore the initial serum composition and being able to compare samples during renneting experiments, the recombined samples were dialyzed (nominal cutoff of 6–8 kg·mol−1, Millipore Corp., Bedford, MA, USA) against 33 volumes of raw skim milk for 24 h, at 4°C. Samples originated from 2× retentates were named LUF and those originated from 5× retentates were named HUF. Thus, the sample nomenclature is summarized as follows:
2× and 5×
Retentates concentrated two and five times the original volume, based on permeate collected, corresponding to casein micelles concentrated at ϕ = 0.2 and φ = 0.5, respectively.
LUF-R and HUF-R
Casein micelles suspensions redispersed in their own permeate (obtained as explained above) and at ϕ = 0.1 from the 2× (LUF) and 5× (HUF).
LUF-D and HUF-D
Casein micelles suspensions redispersed in their own permeate, and then dialyzed against the original skim milk. These samples were also at ϕ = 0.1.
Sample protein content was determined using the Bio-rad DC protein assay (Bio-Rad Laboratories, Mississauga, ON, Canada) to ensure protein concentrations were as expected.
2.2 Stability of casein micelles modified by UF
To test the stability of the casein micelles against ultrafiltration, the particle size, zeta potential and turbidity parameter of the casein micelles (using diffusing wave spectroscopy (DWS), see below) was measured immediately after ultrafiltration (only 2× and 5× samples) and 24 h after UF (2×, LUF-R, LUF-D and 5×, HUF-R and HUF-D). The apparent diameter and zeta potential were measured using a dynamic light scattering (DLS) instrument (Zetasizer, Nano-ZS, Malvern Instruments, Malvern, UK). Samples were diluted ≈1,000 times in fresh permeate. Permeates were obtained from ultrafiltration at the same time as each sample and filtered through a 0.22-μm Millex-GV filter unit (Millipore, Bedford, MA, USA) prior to use.
2.2.1 Diffusing wave spectroscopy experiments
In addition to DLS, DWS was employed to measure the light-scattering properties of the casein micelles without sample dilution. DWS is based on the measurement of temporal fluctuations of light that has been multiple scattered by colloids in a sample (Alexander et al. 2006; Weitz et al. 1993). DWS has been successfully employed to study changes occurring in food emulsions (Gaygadzhiev et al. 2009) and milk systems (Dalgleish et al. 2004; Donato et al. 2007). These studies have yielded information on the static properties (positional correlation) of the colloidal system via the value of photon transport mean free path, l*, as well as dynamic properties via the decay time, \( {\tau} \). The parameter τ is used to calculate the apparent diffusion coefficient and particle radius. A more detailed description of DWS theory is found elsewhere (Weitz et al. 1993). One of the best features of this technique is the possibility of observing the dynamic and static behavior of colloids in concentrated systems, without the deleterious effect of dilution and in a steady (undisturbed) state. This feature makes this technique ideal for the present study, where casein micelles suspensions prepared by ultrafiltration had volume fractions that ranged from 0.1 to 0.5.
The light source employed for DWS was a solid-state, continuous wave 532 nm laser with maximum power of 2 W (Verdi V2 from Coherent, Santa Clara, CA, USA). The scattered light was collected and fed via two matched photomultipliers (HC120-03, Hamamatsu, Loveland, OH, USA) to the correlator (FLEX2K-12 × 2, Bridgewater, NJ, USA). The scattered light intensity was collected in transmission mode. The sample (≈1.5 mL) was poured into an optical-flat-faced glass cuvette (Hellma Canada Ltd., Concord, ON, Canada) with a 5 mm path length. The cuvette was immersed in a small water bath at 30°C. Measurements were acquired during 3 min with intervals of 1 s (for stability measurements) and during 1 min with 1-s interval, for a total time of 3 h, for renneting experiments. To take in consideration possible intensity fluctuations of the laser, standard latex spheres of 260 nm diameter (Portland Duke Scientific, Palo Alto, CA, USA) were used to calibrate the laser intensity daily. Data was analyzed using DWS-Fit software developed specifically for this equipment by Mediavention Inc. (Guelph, ON, Canada).
2.3 Determination of calcium by ion chromatography
To determine changes in the equilibrium between micellar and soluble calcium and the presence of free caseins, the samples were centrifuged at 25,000×g using a Beckman Coulter OptimaTM LE-80 K Ultracentrifuge with rotor type 70.1 Ti (Beckman Coulter Canada Inc., Mississauga, Canada) for 30 min at 20°C. Samples and serum phases from the centrifugation were frozen at −18°C, until further analysis.
The amount of calcium in the samples was measured using non-suppressed ion chromatography (Rahimi-Yazdi et al. 2010). For the determination of soluble cations (defined as the total cations in serum after centrifugation), 1 mL of centrifugal supernatant and 200 μL of 1 mol·L−1 HCl were mixed in a volumetric flask, and volume was adjusted to 100 mL with high-performance liquid chromatography (HPLC) grade water. For the determination of total (soluble and insoluble) calcium 666 μL of sample, 200 μL of HCl (1 mol·L−1) and 466 μL of HPLC water were mixed in a 1.5-mL eppendorf microcentrifuge tube. The samples were centrifuged at room temperature for 15 min at 4,500×g (eppendorf centrifuge 5415D, Brinkmann Instruments, Ltd., Mississauga, Canada) to precipitate the proteins. The supernatant (1,333 μL) was then diluted to 100 mL with HPLC water. A preparative chromatography was carried out to decrease the interference of di- and tri-carboxylic acids available in milk, mainly citrate, as they interfere with the detection of cations, as described in detail elsewhere (Rahimi-Yazdi et al. 2010).
The elution was carried out using a 861 Advanced Compact Ion Chromatography (Ω Metrohm ion analysis, Metrohm Ltd., Herisau, Switzerland), composed of an injection valve, a high pressure pump, and a conductivity detector. The samples were loaded with an 838 sample processor into the 833 ion chromatography (IC) Liquid Handling Dialysis Unit (both Metrohm). The system was controlled by the IC Net 2.3 software (Metrohm).
The samples were eluted isocratically with a mobile phase consisting of 1.7 mmol·L−1 nitric acid and 1 mmol·L−1 pyridine-2, 6-dicarboxylic acid, 0.9 mL·min−1. An acceptor solution (2 mmol·L−1 nitric acid) was used, and 20 μL were injected in the column (Metrosep C2-150, Metrohm). Both column and detector temperatures were kept at 30°C. Calcium, magnesium and potassium standard solutions (1–10 ppm) were prepared from 1,000 ppm concentrated standards (TraceCERT®, Fluka, Sigma Steinheim, Germany).
The levels of insoluble minerals were calculated as follows:
The volume factor (VF) was calculated considering the effects of the dilution, the non solvent water and the volume occupied by the casein micelles according to Mermet (Gaucheron 2004) with slight modifications:
Where f is the fat content, cn is the casein content, h is the solvent water, p the protein content and δm and δsn are the densities of milk and supernatants, respectively. The value 1.36 was derived by assuming 3 g of H20/g of protein in the micelles, and a concentration of 22 mg·mL−1 of caseins in skim milk. Table 1 summarizes the values used in Eq. 2 for the skim milk control (SM) and the 2× and 5× milk.
The densities of the samples were calculated according to the composition of the samples using:
Where m x is the mass fraction of each component and ρ x is the density of the component. Table 1 shows the values used for these calculations. Total protein and caseins presented in Table 1 were obtained by measuring nitrogen (Dumas, Horiba Scientific, Mississagua, ON, Canada) in skim milk and its corresponding supernatants and multiplying by the factor 6.34 to obtain total protein and serum protein. When required, the calculated density was used to calculate protein amounts in kg·L−1 from kg·kg−1.
2.4 Determination of free caseins by ion exchange chromatography
The extent of dissociation of the caseins from the casein micelles was measured using ion exchange chromatography, as previously reported (Holland et al. 2010). The separation was carried out with a gradient of NaCl in urea/sodium acetate buffer, pH 3.5 (buffer A: 6 mol·L−1 urea, 0.2 mol·L−1 sodium acetate, and buffer B: 6 mol·L−1 urea, 0.2 mol·L−1 sodium acetate, 1 mol·L−1 NaCl). Supernatants were separated by centrifuging at 25,000×g for 30 min at 20°C (Beckman Coulter). Urea (3.6 g) and sodium acetate (0.013 g) were added to 5 mL of supernatant, and 5 mL of buffer A. The mix was adjusted to pH 7. After stirring for 30 min, β-mercaptoethanol (0.150 mL) was added and the mix was stirred for an additional 30 min. The pH was adjusted to 3.5 and the total volume was brought to 15 mL with Buffer A. Samples were then filtered through a 0.45-μm syringe filter and injected (500 μL) in a 1-mL cation exchange HP SP column (Amersham Biosciences, Piscataway, NJ, USA). Samples were eluted at 1 mL·min−1 using an ÄKTApurifier 900 series (GE Biosciences, Baie d'Urfé, Canada), equipped with a UV-900 detector (280 nm). The caseins were eluted on a gradient by increasing the volume of buffer B from 0% the first 2 min, to 16% the next 4 min, to 23% in 5 min, 31% in 2 min, 40% in 3 min, 50% in 2 min, and hold for 2 min more; with a final step to 100% B and hold for 3 min.
2.5 Renneting experiments
Only skim milk, LUF-D and HUF-D samples were used for renneting experiments, as the ionic composition of the serum phase needs to be comparable; casein micelles after ultrafiltration were redispersed to ϕ = 0.1 and dialysed against skim milk as explained above. Samples were equilibrated for 30 min at 30°C in a water bath before adding a 1 mol·L−1 CaCl2 (to a final concentration of 8.9 mmol·L−1). If needed, the pH was adjusted to 6.7 with NaOH 0.1 meq·L−1. A freshly diluted rennet solution (1%; Chymostar double strength rennet, 500 international milk clotting units mL−1; Danisco, Madison, WI) was added and mixed thoroughly for 15 s to a final concentration of 0.035 IMU mL−1. The samples were immediately transferred to test tubes for casein macropeptide release experiments, to the rheometer or to the optical glass cuvettes for the DWS experiment. All experiments were carried out in triplicate.
2.5.1 Casein macropeptide release
The amount of casein macropeptide (CMP) released during renneting was monitored using reverse phase HPLC (ThermoFisher, Burlington, Canada) according to Lopez-Fandiño et al. (1993) with slight modifications. The hydrolysis was stopped at different time intervals by diluting the samples with 4% tricholoroacetic acid to a final concentration of 2%. Supernatants (1 mL) were centrifuged at 4,500×g for 15 min at 20°C in an Eppendorf microcentrifuge (Fisher Scientific, Whitby, Canada). The supernatant was filtered (0.45 μm) and injected (100 μL) on a reverse phase column (μRPC C2/C18 ST 4.6/100, Piscataway, NJ, USA) with a Vydac C-4 guard column (Mandel, Guelph, ON, Canada). A gradient of 0.1% trifluoracetic acid in water mixed with solvent B (0.1% trifluoracetic acid in 90% acetonitrile) was eluted at a flow rate of 1 mL·min−1, as follows: 82% solvent A for 5.4 min, solvent A reduced to 61% for 35 min, solvent A to 0% in another 6 min, solvent B at 100% for 5 min and back to 82% solvent A. The total area detected at 214 nm for the CMP peaks was compared with that derived from the total CMP area of a control skim milk after 3 h of renneting (assumed to be 100% of CMP released).
2.5.2 Rheological and light-scattering properties of rennet-induced gels
Immediately after rennet addition, 20 mL of the sample were placed in a concentric cylinder geometry (inner and outer cylinder diameters were 28 and 30 mm) of a controlled-stress rheometer AR1000 (TA Instruments, New Castle, DE, USA) at 30°C. Evaporation was avoided using a vapor trap. The elastic modulus (G′), the viscous modulus (G″) and phase angle (δ) were recorded continuously in dynamic low-amplitude oscillatory mode with 1 Hz frequency and 0.01 strain. The initial stress was 0.018 Pa and it was maintained until constant strain was reached. Data were collected starting 5 min after the addition of rennet and continuing for 80 min after the gelling point (defined as δ < 45°). After this point, a stress sweep was carried out by increasing the stress from 0.05 to 200 Pa, keeping the frequency constant at 1 Hz. The strain value at the end of the linear viscoelastic region (defined as the point when G′ decreased by 20%) was recorded and used for statistical analysis. Simultaneously the gelation was followed by DWS, by placing 2.5 mL of the renneted sample in an optical flat-faced glass cuvette and bringing it to the DWS setup as explained above.
2.6 Statistical analysis
PROC-MIX was used to calculate significant differences using SAS (SAS, version 8.2, Cary, NC, USA). Significant differences (P < 0.05) were calculated for the values of different variables measured in three independent filtration experiments. Standard deviations are also presented in graph and tables.
3 Results and discussion
3.1 Effect of concentration on the diffusivity of the casein micelles
To determine the stability of casein micelles that had been concentrated to 2× or 5× (that is, two or five times the casein micelles volume fraction), the average size of the casein micelles was continuously monitored with DLS and DWS for 3 h. No changes were noted over time. In addition, the sizes were not significantly different within 24 h of storage at 4°C. The values of the diffusion coefficient measured by DWS as a function of casein volume fraction were compared with the values derived from the theoretical hard-sphere self-diffusion behavior calculated by the Beenakker–Mazur formalism (Weitz et al. 1993). In fact, the concentration dependence of the normalized self-diffusion coefficient of a colloidal system containing hard spheres can be written, within approximation as:
where, D o is the diffusion coefficient of a particle at infinite dilution and ϕ is the volume fraction (Weitz et al. 1993). It is worthy to note that Eq. 4 was developed for a monodispersed colloidal suspension to account for hydrodynamic effects, assuming constant serum properties. In a polydisperse system such as milk, the restriction of the diffusivity as a result of the high volume fraction (e.g., 5× retentates) is lower, since the small particles are allowed to fit within the interparticle spaces. In a polydisperse system, therefore, the average diffusion coefficient at high volume fraction would have a higher value than that calculated for a monodisperse system with the same average particle size.
In accordance with the theory, the normalized diffusivity of casein micelles in the ultrafiltered retentates decreased gradually with an increase of the volume fraction, for a monodispersed system of hard spheres the diffusivity (D/D0) would decrease from 0.83 at ϕ = 0.1–0.67 and 0.31 at ϕ = 0.2 and 0.5, respectively. In the 2× retentate, at ϕ = 0.2, the experimental value of the diffusion coefficient (D/D 0) (0.61 ± 0.03) matched that calculated using Eq. 4 within a 10% error. However, when casein micelles were concentrated to 5×, experimental data was lower than the theoretical calculation by 33.6% (D/D 0 = 0.20 ± 0.05). In a suspension such as skim milk, the average separation between casein micelles can be easily calculated and yields around one particle diameter. But under concentrated conditions such as those in 5× retentate, casein micelles are as close as 1/3 of the particle diameter. Even at this close proximity, a polydisperse system of hard sphere would have a D/D 0 higher than 0.31 ± 0.03. The diffusivity values of casein micelles at 5× indicates that other interparticle forces (i.e., electrostatic, steric) and the interactions between particles, other than the hydrodynamic forces accounted for in the hard-sphere theory, become more significant.
3.2 Effect of concentration on soluble calcium and soluble caseins
During ultrafiltration there was a small decrease in the pH of the milk, from 6.7 to 6.5, at the end of the 5× concentration. This change in pH is in agreement with previous reports (Karlsson et al. 2005), and was accompanied by a change in the cation profile of the milk. Table 2 shows the average values of 3 cations (Ca2+, Mg2+, K+) in skim milk and the undiluted 2× and 5× retentates. The average concentrations of the cations in skim milk are in broad agreement with literature values (Holt 1997).
It is well known that as the concentration takes place during ultrafiltration, the minerals associated with the proteins (such as calcium and magnesium) will increase in concentration (Green et al. 1984; Premaratne and Cousin 1991). However, the extent of concentration of the cations depends on the level of association of the minerals with the protein. As the level of concentration increases, the total calcium to protein ratios (moles Ca2+ per gram of protein, see Table 1 for protein concentrations) decreases from 0.94 ± 0.05 in SM to 0.77 ± 0.02 and 0.59 ± 0.02 for the 2× and 5× retentates, respectively. This is an obvious result of the soluble calcium permeating through the membrane.
Figure 1 shows the levels of total and soluble calcium of all the samples (concentrated, reconstituted before and after dialysis, as well as skim milk control) used in this research. The amount of soluble calcium of the UF samples was not significantly different from the skim milk control, with the exception of the HUF-R (reconstituted in permeate), where it was higher than the control. The amount of total calcium was significantly lower than skim milk for the 5× retentate, not only after reconstitution (HUF-R) but also after extensive dialysis against fresh raw milk (HUF-D; Fig.1). Table 3 shows the amount of insoluble calcium calculated using Eq. 1, with the parameters summarized in Table 1. The insoluble calcium concentration in skim milk was 23 ± 2 mmol·L−1, well in agreement with previously reported data on the concentration of colloidal calcium associated with the casein micelles (Holt 1997). While 2× retentates reached a similar value after reconstitution to the same volume fraction (LUF-R), the 5× retentates showed a low concentration of insoluble calcium (HUF-R). This statistically significant decrease in the insoluble calcium cannot be fully recovered after dialysis of the HUF milk, as the value for HUF-D is 20 ± 1 mmol·L−1. It has been previously reported that during UF some calcium precipitates on the membrane. This has been recognized as a major cause of reversible membrane fouling on PES membranes (Rabiller-Baudry et al. 2002). Working on a ZrO2-TiO2 microfiltration membrane, it was also shown that electrostatic interactions play an important role on fouling of the positively charged calcium and the negatively charged caseins on the negatively charged membrane (Jimenez-Lopez et al. 2008).
Amount of total (black bars) and soluble (grey bars) calcium measured by ion chromatography in skim milk (SM), concentrated 2× (LUF) and concentrated 5× (HUF) after redispersion in serum (R) and after (D) dialysis. The average and standard deviation of three independent measurements are presented. Letters indicate statistical differences (P < 0.05) in between the same fraction (total or soluble)
It is important to note the changes in the insoluble calcium to casein ratios (moles of insoluble Ca2+ per g of caseins): the original levels in SM (1 ± 0.09) decreased to 0.93 ± 0.04 in the 2× retentate and 0.76 ± 0.04 in the 5× retentate. These values correspond to a release of up to 5 mmol·L−1 of calcium from the insoluble fraction of skim during ultrafiltration. These values are similar to those reported in the literature (Le Graet and Gaucheron 1999), for casein micelles suspensions with similar protein levels as in the present research.
The solubilization of the colloidal calcium phosphate (CCP), may affect the internal organization of the micelles (Dalgleish and Law 1989; Holt and Horne 1996; Van Hooydonk et al. 1986). It is generally accepted that the structure of the casein micelles in milk is maintained by a combination of hydrophobic interactions and electrostatic associations between serine phosphates of the caseins and the CCP (Horne 2003). Therefore, it can be expected that even small changes in the calcium phosphate nanoclusters (which include CCP and calcium associated with phosphoserine residues) will induce internal rearrangements, one of these possible rearrangements being the solubilization of individual caseins from within the micelles (Dalgleish and Law 1988; Dalgleish and Law 1989).
To determine if concentration by ultrafiltration affected micellar composition, the presence of soluble caseins were tested by centrifuging the milk samples and analyzing the supernatants by ion exchange chromatography. There was no statistically significant change in the composition of the soluble proteins, as all peaks did not show significant differences in the elution or in the peak areas (data not shown). It is well known that pH-induced CCP solubilization results in the dissociation of individual caseins from the micelles (Van Hooydonk et al. 1986). However, it is also known that the dissociation is temperature dependent (Rose 1968) with almost no dissociation around 30°C (Dalgleish 1989) and in the pH range 6.7–6. The present experiments were carried out at 40°C.
3.3 Effect of concentration by UF on some physical properties of the casein micelles
The ζ-potential of the casein micelles was not statistically different for all the samples studied, SM (−15 ± 1 mV), LUF-R (−16 ± 1 mV), LUF-D (16 ± 1 mV), HUF-R (−12 ± 4 mV), and HUF-D (−14 ± 1 mV). It was therefore concluded that despite changes in the ionic composition of the serum occurring during ultrafiltration, these changes were not sufficient to modify the overall charge of the casein micelle. These results are in full agreement with the literature, as the ζ-potential of the casein micelles has been shown to be fairly stable in the range of pH between 6.7 and 6.5.
There seems to be disagreement on the effect of concentration by UF on the size of casein micelles, and this disagreement may be based on the use of different techniques (and the artifacts imposed by them) as well as differences in the actual UF process (especially if additional water is added for diafiltration). Electron microscopy observations on concentrated retentates reported no changes in the size of the casein micelles (McKenna et al. 2000), while others (Srilaorkul et al. 1991) showed a change in casein micelle size distribution when milk was concentrated by UF to 5×. Immediately after filtration, the apparent diameter of the casein micelles was measured using DLS, by diluting in permeate. The diameter of the micelles was comparable between SM (140 ± 8 nm) and 2× UF (134 ± 2 nm) but statistically significant in 5× UF (177 ± 6 nm). The larger size measured in these samples corresponded to the differences in the diffusion coefficient measured undiluted using DWS (see above). When the diameter was measured in the LUF-R and HUF-R (reconstituted in permeate) or after dialysis against milk, the apparent diameter measured by DLS was not different between the treatments.
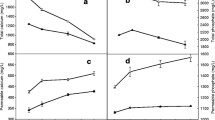
Figure 2 summarizes the light-scattering parameters measured using DWS, namely, radius and 1/l*. Fig. 2a shows the apparent radius for SM, and LUF and HUF reconstituted (LUF-R, HUF-R) to the SM volume fraction and the samples after dialysis against fresh skim milk (LUF-D and HUF-D). Fig. 2a clearly indicates that ultrafiltration did not induce changes in the apparent radius of the casein micelles for 2× retentate micelles. Statistical analysis between the treatments and within treatment (Proc-mix) did show differences between samples after reconstitution and dialysis (at P < 0.05). The results of size measurements by DLS and DWS led to the conclusion that at high concentration (5×), the casein micelle size may be affected, but the change was reversible.
Apparent radius (nm) (a) and 1/l* (b) in skim milk (SM) before (filled circle) and after 2× (LUF) and 5× (HUF) concentration by UF, samples were redispersed to 0.1 volume fraction (filled triangle) and dialyzed against SM (empty square). The average and standard deviation of three ultrafiltration experiments are presented
To gain more insight into the changes occurring during ultrafiltration, the static light-scattering properties of the various milk samples were also probed by DWS measuring the turbidity parameter, 1/l* (Fig. 2b). This parameter, which indicates the distance the photon travels before it becomes fully decorrelated, depends on size, shape, volume fraction, and refractive index contrast between the colloidal particles (micelles) and the serum and, under particular conditions, can give an indication of interparticle interactions in a scattering volume (Weitz et al. 1993). A 2× concentration and reconstitution did not induce a significant change in the 1/l* for LUF compared to the values of the original skim milk. No differences were noted for either LUF-R or LUF-D. In contrast, after concentrating the milk 5× by UF and re-dispersion (HUF-R), the casein micelles showed a significant decrease in the value of 1/l* compared to the control milk. In addition, the value of 1/l* continued to be low even after dialysis against milk (HUF-D).
Considering that all samples (SM, LUF and HUF, R or D) have the same volume fraction, apparent size (as shown in Fig. 2a), and surface properties (no differences in their ζ-potential), it can be safely assumed that the interparticle properties of the system were also constant. Therefore, changes in the refractive index contrast seemed to be the only possible source for these differences. In the case of the HUF-D and LUF-D, the reconstituted retentates were dialyzed against the original skim milk and therefore it is also possible to assume that these samples will have the same index of refraction in the serum phase background. The lower value of 1/l* of HUF, therefore, indicated a decrease in the refractive index of the casein micelles after the 5× concentration by UF and reconstitution. Since the refractive index of a casein micelle is a weighted contribution of that of the solvent and the protein, a decrease of contrast would indicate either an increase in water content or a decrease of protein content per unit area. As the amount of soluble caseins did not increase with ultrafiltration (see above), it was possible to conclude that this change in the refractive index of the casein micelle was caused by a localized spatial redistribution of mass within the micelles themselves (without effective changes in size) in response to a decreased level of CCP.
3.4 Renneting properties
The concentrated milk samples were reconstituted to the original volume fraction of milk and then dialyzed to ensure a comparable serum environment. This is of particular importance as the renneting properties are strongly affected by the ionic composition of the serum phase. These samples were subjected to renneting, to determine if any differences could be noticed in the casein micelles subjected to concentration by UF. The development of the elastic modulus (G′), and the phase angle (δ) for milk samples during renneting are summarized in Fig. 3. Statistical analysis showed that the ultrafiltration process did not affect the gelation point (Table 4). However, the rate of formation of the gel structure was significantly affected in the HUF-D milk, as indicated by the lower value of G′ compared to that of SM or LUF-D (see Table 4 for statistical analysis). It is important to note that all the samples had a similar concentration of soluble calcium. The difference may be explained by the lower level of insoluble calcium in the HUF-D samples compared to the LUF-D and SM (Table 3). The insoluble calcium was indeed not recovered after dialysis against the original milk. It has been previously shown that the removal of colloidal calcium phosphate at a fixed Ca2+ activity and pH reduces the renneting properties of milk (Lucey et al. 1996). The important role played by CCP in maintaining micellar integrity and functionality is well documented (Holt and Horne 1996; Van Hooydonk et al. 1986): the insoluble calcium phosphate is key in bridging the block copolymers (casein molecules) by keeping the balance between attractive and repulsive forces responsible for micelles formation and integrity, and for the formation of bonds during gelation. The development of the phase angle (δ) as a function of time after rennet addition is also shown in Fig. 3b. These values decreased at the beginning of gelation and reached a constant value around 19. There were no statistical differences between the plateau values amongst the different treatments (Table 4). It has been previously reported that decreased levels of insoluble calcium in the micelles cause an increase in the value of tan δ (Choi et al. 2007), indication of an increased susceptibility of the network bonds to rearrangements. However, although in the present work, the differences in the elastic modulus were significantly different between treatments, the values of δ were not different, most likely because the extent of the reduction of insoluble calcium was lower than in the previous studies.
Storage modulus (a) and phase angle (δ) (b) as a function of time after rennet addition to SM (empty circle), LUF-D (filled triangle), and HUF-D (filled circle) samples. The points shown are from one representative experiment, out of three separate ultrafiltration experiments. Note that all samples have same casein volume fraction and serum composition
The linear viscoelastic range (indicated as the percentage of strain needed to show a change in the elastic modulus) significantly decreased from 47 ± 2 and 48 ± 1% in SM and LUF-D respectively, to 39 ± 2% in HUF-D systems. This suggested that more brittle casein networks were formed in renneted gels made from HUF-D milk. Although the factors that determine the fracture properties of gels are not completely understood, they include the number of bonds per cross-section of the strand, the strength of each bond, and the tortuosity of the gel network (Lucey et al. 1996, 1997, 2003). In previous studies, the reduction of CCP has been accompanied by the release of caseins from the micelles (Dalgleish and Law 1988; Udabage et al. 2000), while others have linked CCP losses to the exposure of charged phosphoserine groups which could increase the local electrostatic repulsion between CN and eventually affect the gelling properties of the casein micelles (Lucey et al. 2003). Thus, gels made of casein micelles after HUF-D may have weaker bonds due to the loss of attractive insoluble calcium bridges and increased electrostatic repulsion between phosphoserine residues. It is important to note that in the present work, the soluble calcium around the casein micelles was comparable between the samples, while, in reality during cheesemaking, this may not be necessarily the case. It could be concluded that the overall viscoelastic properties were similar between the different retentate samples, with similar rearrangements, however the samples prepared with HUF-D milk showed a lower stiffness modulus, a shorter viscoelastic range and less or weaker linkages.
The initial stages of gelation during renneting of SM, LUF-D, and HUF-D was also observed in situ using DWS. Figure 4 shows the particle size and the turbidity parameter, 1/l*, as a function of time after rennet addition. The apparent radius was calculated from the values of diffusion coefficient; however, as the particles start aggregating and particle diffusion becomes restricted, the physical concept of apparent radius (as measured by DWS) becomes meaningless. The inset in Fig. 4a shows the apparent radius at the initial stages of the reaction (see scale). The slight decrease in the initial particle size was due to the cleaving of κ-casein by the action of rennet. In the cases of SM and LUF-D, there was a sudden and pronounced change in the apparent radius around 40 min, followed by a continuous and monotonic increase. This corresponded to the aggregation point of the casein micelles, and it occurred just before the increase in G′ (Fig. 4), both for SM and LUF-D. The observation that the onset of aggregation is noted first by DWS than by dynamic oscillatory rheology has been reported before (Sandra and Dalgleish 2007), and can be explained as a shift in the equilibrium of the colloidal system toward aggregation, not necessarily meaning that a gel structure is being formed yet.
Apparent radius (a) and 1/l ∗ (b) as a function of time during the first 60 min after rennet addition to SM (empty circle), LUF-D (filled triangle), and HUF-D (filled circle) samples. The results shown are from representative individual experiments, out of three independent experiments. Note that all samples have same casein volume fraction and serum composition
The case for HUF-D was significantly different than for SM and LUF-D. As expected, the initial particle size was similar to SM control (inset Fig. 4); however, the aggregation point (increase in radius) of the micelles appeared about 10 min earlier. The rate of increase was then similar to that of SM and LUF-D. It should be pointed out, once again, that the HUF-D had comparable colloidal and environmental characteristics as the other two milk systems.
The onset of aggregation for HUF-D occurred when around 75% of the CMP was released, while SM and LUF-D samples started aggregating when 90% of the CMP was released (data not shown). This important observation was evidence that the concentration by ultrafiltration to 5× somewhat destabilized the surface of the casein micelles, making the particles more prone to aggregation at lower levels of κ-casein cleavage. However, it is important to remember that LUF-D and HUF-D samples had comparable sizes as well as surface charge to those of skim milk control.
Figure 4b shows the development of the turbidity parameter 1/l* for SM, LUF-D, and HUF-D. The value of 1/l* was lower in the HUF-D than for LUF-D and SM, as previously discussed. This is a measure of interparticle interactions, as the structure factor of the micelles (physical characteristics as index contrast and concentration) remained constant. There was a continuous increase in the inter-micelle interaction as the rennet action progressed. There was no significant difference in the time-progression of the 1/l* for the three treatments, apart from the indication of differences in refractive index contrast which could possibly be related to interior protein rearrangements (see above).
4 Conclusions
In conclusion, the effects of concentration by ultrafiltration on the functional properties of milk products should not be interpreted as a mere effect of changes in the composition (e.g., higher protein concentration or ions) or processing such as heating, drying, or storage. Rather, for the first time, this research has shown that concentration by ultrafiltration per se induced irreversible changes that affect the functionality (renneting) of the casein micelles, especially after high levels of concentration. Although when making milk protein concentrates, little can be said about the effects of each processing parameter on the casein micelles, this work has shown that high levels of concentration by ultrafiltration changed the casein micelle, even in the absence of low or high temperatures, and at low (and controlled) levels of transmembrane pressure and fouling. Another important observation emerging from the current study is that the effects of concentration by ultrafiltration on casein micelles are not easily measurable with widespread techniques such as particle sizing and zeta potential measurements.
The unique supramolecular arrangement in the casein micelle is clearly a function of its environmental conditions. Although casein micelles have been shown to be fairly stable colloidal particles, this study demonstrated that casein micelles are irreversibly modified when concentrating with ultrafiltration, especially when reaching high concentration levels where interactions are increasing between the protein particles. These changes were reflected on the values of the turbidity parameter and the renneting functionality, when the retentates are reconstituted back to the original milk conditions. The loss of insoluble calcium phosphate and protein re-arrangements within the micelles are proposed as the main changes caused by high levels of concentration by UF. The differences in the renneting properties of casein micelles between low and high concentration levels in UF are explained by the loss of insoluble (colloidal) calcium phosphate and protein re-arrangements, as well as a change in the surface of the casein micelle, shown by a different susceptibility of caseins to rennet.
References
Alexander M, Corredig M, Dalgleish DG (2006) Diffusing wave spectroscopy of gelling food systems: the importance of the photon transport mean free path (l*) parameter. Food Hydrocoll 20:325–331
Choi J, Horne DS, Lucey JA (2007) Effect of insoluble calcium concentration on rennet coagulation properties of milk. J Dairy Sci 90:2612–2623
Dalgleish DG (1989) Caseins, casein micelles and caseinates. J Soc Dairy Technol 42:91–92
Dalgleish DG, Law AJR (1988) pH-Induced dissociation of bovine casein micelles.1. Analysis of liberated caseins. J Dairy Res 55:529–538
Dalgleish DG, Law AJR (1989) pH-Induced dissociation of bovine casein micelles.2. Mineral solubilization and its relation to casein release. J Dairy Res 56:727–735
Dalgleish DG, Alexander M, Corredig M (2004) Studies of the acid gelation of milk using ultrasonic spectroscopy and diffusing wave spectroscopy. Food Hydrocoll 18:747–755
David C, Pignon F, Narayanan T, Sztucki M, Gesan-Guiziou G, Magnin A (2008) Spatial and temporal in situ evolution of the concentration profile during casein micelle ultrafiltration probed by small-angle X-ray scattering. Langmuir 24:4523–4529
De Kruif CG, Holt C (2003) Casein micelle structure, functions and interactions. In: Fox PF, McSweeney PLH (eds) Advanced dairy chemistry, Vol. 1: proteins, part A, 3rd edn. Kluwer Academic/Plenum Publishers, New York, USA, pp 233–276
Donato L, Alexander M, Dalgleish DG (2007) Acid gelation in heated and unheated milks: interactions between serum protein complexes and the surfaces of casein micelles. J Agric Food Chem 55:4160–4168
Ferrer MA, Hill AR, Corredig M (2008) Rheological properties of rennet gels containing milk protein concentrates. J Dairy Sci 91:959–969
Gaucheron F (2004) Dosage des principaux minéraux du lait et des produits laitiers [Determination of minerals in milk and dairy products]. In: Gaucheron F (ed) Minéraux et produits laitiers [Minerals in dairy products]. Tec & Doc Lavoisier, Paris, France, pp 794–795
Gaygadzhiev Z, Alexander M, Corredig M (2009) Sodium caseinate-stabilized fat globules inhibition of the rennet-induced gelation of casein micelles studied by diffusing wave spectroscopy. Food Hydrocoll 23:1134–1138
Green ML, Scott KJ, Anderson M, Griffin MCA, Glover FA (1984) Chemical characterization of milk concentrated by ultrafiltration. J Dairy Res 51:267–278
Holland B, Rahimi-Yazdi S, Ion-Titapiccolo G, Corredig M (2010) Analysis of caseins by ion exchange chromatography. J Dairy Sci 93:893–900
Holt C (1997) The milk salts and their interaction with casein. In: Fox PF (ed) Advanced dairy chemistry, Vol. 3: lactose, water, salts and vitamins. Chapman and Hall, London, UK., pp 233–244
Holt C, Horne DS (1996) The hairy casein micelle: evolution of the concept and its implications for dairy technology. Neth Milk Dairy J 50:85–111
Holt C, Davies DT, Law AJR (1986) Effects of colloidal calcium-phosphate content and free calcium-ion concentration in the milk serum on the dissociation of bovine casein micelles. J Dairy Res 53:557–572
Horne DS (1998) Casein interactions: casting light on the black boxes, the structure in dairy products. Int Dairy J 8:171–177
Horne DS (2003) Casein micelles as hard spheres: limitations of the model in acidified gel formation. Coll Surf A–Physicochem Eng Aspects 213:255–263
Horne DS (2006) Casein micelle structure: models and muddles. Curr Opin Coll Interface Sci 11:148–153
Huppertz T, Fox PF, Kelly AL (2004) High pressure treatment of bovine milk: effects on casein micelles and whey proteins. J Dairy Res 71(1):97–106
Jimenez-Lopez AJE, Leconte N, Dehainault O, Geneste C, Fromont L, Gésan-Guiziou G (2008) Role of milk constituents on critical conditions and deposit structure in skimmilk microfiltration (0.1 μm). Sep Purif Technol 61:33–43
Karlsson AO, Ipsen R, Schrader K, Ardo Y (2005) Relationship between physical properties of casein micelles and rheology of skim milk concentrate. J Dairy Sci 88:3784–3797
Le Graet Y, Gaucheron F (1999) pH-Induced solubilization of minerals from casein micelles: influence of casein concentration and ionic strength. J Dairy Res 66:215–224
López-Fandiño R, Olano A, San José C, Ramos M (1993) Application of reversed-phase HPLC to the study of proteolysis in UHT milk. J Dairy Res 60(1):111–116
Lucey JA, Gorry C, O’Kennedy B, Kalab M, Tankinita R, Fox PF (1996) Effect of acidification and neutralization of milk on some physico-chemical properties of casein micelles. Int Dairy J 6:257–272
Lucey JA, Teo CT, Munro PA, Singh H (1997) Rheological properties at small (dynamic) and large (yield) deformations of acid gels made from heated milk. J Dairy Res 64:591–600
Lucey JA, Johnson ME, Horne DS (2003) Invited review: perspectives on the basis of the rheology and texture properties of cheese. J Dairy Sci 86:2725–2743
McKenna B.A. (2000) Effect of processing andstorage on reconstitution properties of whole milk and ultrafiltered milk powders, Ph.D Thesis, Massey University, Palmerston North, New Zealand.
Mulhivill DM, Ennis MP (2003) Functional milk proteins: production and utilisation. In: Fox PF, McSweeney PLH (eds) Advanced dairy chemistry, Vol. 1: proteins, part B, 3rd edn. Kluwer Academic/Plenum Publishers, New York, USA, pp 1175–1228
O'Connell JE, Fox PF (2003) Heat induced coagulation of milk. In: Fox PF, McSweeney PLH (eds) Advanced dairy chemistry, Vol. 1: proteins, part B, 3rd edn. Kluwer Academic/Plenum Publishers, New York, USA, pp 879–945
Piry A, Kuhnl W, Grein T, Tolkach A, Ripperger S, Kulozik U (2008) Length dependency of flux and protein permeation in crossflow microfiltration of skimmed milk. J Membr Sci 325:887–894
Premaratne RJ, Cousin MA (1991) Changes in the chemical composition during ultrafiltration of skim milk. J Dairy Sci 74:788–795
Rabiller-Baudry M, Le Maux M, Chaufer B, Begoin L (2002) Characterisation of cleaned and fouled membrane by ATR-FTIR and EDX analysis coupled with SEM: application to UF of skimmed milk with a PES membrane. Desalination 146:123–128
Rabiller-Baudry M, Gesan-Guiziou G, Roldan-Calbo D, Beaulieu S, Michel F (2005) Limiting flux in skimmed milk ultrafiltration: impact of electrostatic repulsion due to casein micelles. Desalination 175:49–59
Rahimi-Yazdi S, Ferrer MA, Corredig M (2010) Non-suppressed ion chromatographic determination of total calcium in milk. J Dairy Sci 93:1788–1793
Renner E., Abd El-Salam M.H. (1991) Application of ultrafiltration in the dairy industry, Elsevier LTD, London, UK.
Rose D (1968) Relation between micellar and serum casein in bovine milk. J Dairy Sci 51:1897–1902
Sandra S, Dalgleish DG (2005) Effects of ultra-high-pressure homogenization and heating on structural properties of casein micelles in reconstituted skim milk powder. Int Dairy J 15:1095–1104
Sandra S, Dalgleish DG (2007) The effect of ultra high-pressure homogenization (UHPH) on rennet coagulation properties of unheated and heated fresh skimmed milk. Int Dairy J 17:1043–1052
Singh H (2007) Interactions of milk proteins during the manufacture of milk powders. Lait 87:413–423
Srilaorkul S, Ozimek L, Ooraikul B, Hadziyev D, Wolfe F (1991) Effect of ultrafiltration of skim milk on casein micelle size distribution in retentate. J Dairy Sci 74:50–57
Udabage P, Mckinnon IR, Augustin MA (2000) Mineral and casein equilibria in milk: effects of added salts and calcium chelating agents. J Dairy Res 67:361–370
Van Hooydonk ACM, Hagedoorn HG, Boerrigter IJ (1986) pH-induced physico-chemical changes of casein micelles in milk and their effect on renneting. I. Effects of acidification on physico-chemical properties. Neth Milk Dairy J 40:281–296
Weitz DA, Zhu JX, Durian DJ, Gang H, Pine DJ (1993) Diffusing-wave spectroscopy—the technique and some applications. Phys Scr T49B:610–621
Acknowledgments
This work was funded by the Ontario Dairy Council—Natural Sciences and Engineering Council of Canada Industrial Research Chair program.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Ferrer, M.A., Alexander, M. & Corredig, M. Does ultrafiltration have a lasting effect on the physico-chemical properties of the casein micelles?. Dairy Science & Technol. 91, 151–170 (2011). https://doi.org/10.1007/s13594-011-0002-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13594-011-0002-0