Abstract
The nutritional needs of bees are receiving renewed attention in the context of declining bee populations and changes in land use that threaten floral resources. We present a comprehensive analysis of the nutritional composition of sunflower (Helianthus annuus L.) pollen, comparing hand-collected, bee-collected and stored pollen. As found in previous studies, the protein content of sunflower pollen was relatively low compared to other important bee forage plants. In the cultivars tested, two essential amino acids, methionine and tryptophan, are likely to be below the minimum requirements for honeybees. Fatty acid composition showed lauric acid to be most abundant, followed by palmitic and α-linolenic acids. While sunflower offers abundant and accessible pollen, its quality may hinder bee development when it is an exclusive pollen source, and the cultivars of such mass-flowering crops may vary in value for pollinators.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Bees are essential pollinators, but their populations are declining at the same time as demand for pollination services increases. The nutritional needs of bees are currently receiving increased attention: for example, will land use changes permit bees to maintain nutritional balance on available floral resources, and what are the interactions between nutrition and the impact of diseases and pesticides? Pollen is more crucial than nectar because it provides the nutritional requirements of developing bees (larvae and young adults) in terms of protein, lipid, vitamins and minerals. Most analyses of the chemical composition of pollens have been broad surveys of a range of plant species (Todd and Bretherick, 1942; Somerville and Nicol, 2006; Weiner et al. 2010). Because it is often difficult to collect sufficient pollen for analyses, many researchers have used bee-collected pollens (but see Roulston et al. 2000; Weiner et al. 2010). In addition, the focus has been on the protein content of pollen, and more recently the amino acid profile, because of the importance for brood rearing.
We have previously provided a detailed analysis of the pollen chemistry of Aloe greatheadii var davyana (Asphodelaceae), an important indigenous bee plant in South Africa, comparing hand-collected, bee-collected and stored pollen (Human and Nicolson, 2006). Here we present a comparable analysis of the pollen of sunflower, Helianthus annuus L. (Asteraceae). This species, native to North America (Neff and Simpson, 1990), is a major annual oilseed crop worldwide, and pollination by honeybees improves seed production, seed mass and oil content (Krause and Wilson, 1981; Nderitu et al. 2008; Carvalheiro et al. 2011). In his study of honeybee behaviour on sunflowers, Free (1964) suggested that bees visit the florets mainly for nectar and discard excess pollen because it is less attractive than other pollens. The unattractiveness of sunflower pollen to honeybees has been commented on by other authors (Tepedino and Parker, 1982). Sunflower pollen is assumed to be of poor quality for bees because of its low protein content (Pernal and Currie, 2000; Somerville and Nicol, 2006; Tasei and Aupinel, 2008). Here we reassess the quality of sunflower pollen by analysing samples collected during a study investigating the benefits of patches of natural habitat and weeds for sunflower pollination in South Africa (Carvalheiro et al. 2011).
2 Methods
2.1 Study site and plant species
Sunflower pollen was obtained from commercial sunflower fields near Bela Bela in Limpopo Province, South Africa, during the 2009 flowering season. Beekeepers move their hives to these fields during flowering but are not paid for pollination services. Bee-collected and stored pollen from the Monsanto 6822 cultivar was collected from three adjacent hives: a bottom-fitting pollen trap was used to obtain bee-collected pollen directly from bees returning to the hives, while stored pollen was removed from 10–15 adjacent cells in frames of the three hives, giving two samples per hive. At the same time sunflowers were picked in the field and brought back to the laboratory so that fresh pollen could be collected by gently brushing the anthers with a paintbrush. Fresh pollen was also obtained from flowers of a second cultivar, Monsanto DK4040, grown on the experimental farm at the University of Pretoria. These two hybrids do not share a parent line (Wikus Boshoff, unpublished data). All pollen samples were stored frozen for 1 year before analysis.
2.2 Water content
Pollen samples (0.3 g) were dried to constant weight at 65 °C in order to obtain water content as a percentage of fresh weight (AOAC, 2000).
2.3 Protein
The Dumas method (AOAC, 2000) was used to determine crude protein content (in duplicate) through the determination of total nitrogen content using an elemental analyser (model FP-428; Leco instruments, Mississauga, Canada), calibrated against known standards. Pollen samples (0.2 g) weighed into a combustion boat were combusted at 950 °C. Nitrogen values obtained were multiplied by a conversion factor of 6.25 in order to determine total crude protein.
2.4 Amino acids
Pollen samples (0.02 g) were analysed in duplicate for protein-bound amino acids by the PicoTag® method (3.9 mm × 15 cm column) using a Waters HPLC amino acid analyser (Waters, Millipore Corp., Milford, MA). Samples were hydrolysed with 6 N HCl, derivatised with phenylisothiocyanate to produce phenylthiocarbamyl amino acids and then analysed by reverse-phase HPLC. Water/acetonitrile (60:40) and 0.14 M sodium acetate trihydrate were used as buffers. A UV spectrophotometer was used to detect absorbance at 254 nm, the column operating at 46 °C with a flow rate of 1 mL/min (Bidlingmeyer et al. 1984).
Methionine and tryptophan could not be measured by this method owing to their instability during acid hydrolysis, and were analysed separately. For the sulphur amino acids cystine and methionine, samples were pre-oxidised with performic acid at 0 °C overnight, the reaction stopped with hydrogen bromide, then treated as above. For tryptophan, samples were hydrolysed with saturated barium hydroxide at 110 °C for 16 h, then analysed by reverse-phase HPLC using a Symmetry column (4.6 × 150 mm) with detection at 285 nm.
2.5 Lipid content
The total lipid content of dried pollen was obtained, in duplicate, through the chloroform–methanol extraction method described by Folch et al. (1957) and the lipid fraction was estimated from the difference in mass.
2.6 Fatty acids
Standard procedures were used for methylation of lipids, using 0.7 g pollen per analysis, prior to determination of fatty acid composition (Genet et al. 2004). Fatty acids were identified using a Varian (Varian Ass Inc 1985, USA) 3300 FID chromatograph, with WCOT fused silica capillary columns (CPSIL 88; 100 m, 0.25 mm). Column temperature was 140–240 °C, while the injector port and FID were maintained at 250 °C. The carrier gas was helium at a flow rate of 50 mL/min. Fatty acids were identified by comparison with the relative retention times of fatty acid methyl ester peaks in standards obtained from Sigma (Taufkirchen, Germany).
2.7 Ash
Pollen samples (0.2 g) in porcelain crucibles were placed in a temperature-controlled furnace, preheated to 600 °C, for 2 h and afterwards transferred to a desiccator, cooled and weighed immediately (AOAC, 2000).
2.8 Statistical analysis
Nutritional data for hand-collected, bee-collected and stored pollens did not meet the assumptions for parametric statistics; variances were not homogeneous and data did not conform to a normal distribution. Statistical comparisons were made using Kruskal–Wallis ANOVA with multiple comparisons of mean ranks for all groups. Values are given throughout as means ± SD (n = 6), with the exception of methionine, cystine and tryptophan (n = 2), which were excluded from statistical analysis.
3 Results
3.1 Nutritional composition
The nutritional composition of the three categories of sunflower pollen (Monsanto 6822) is presented in Table I, together with that of hand-collected pollen for the second cultivar (Monsanto DK4040). For all samples the quantity of carbohydrate was obtained by difference.
Hand-collected pollens of the two cultivars did not differ in any of the parameters measured. However, there were significant differences between hand-collected, bee-collected and stored pollen of cultivar Monsanto 6822 in all components except ash. The water content of hand-collected pollen was significantly lower than that of bee-collected (P < 0.001) and stored pollen (P < 0.05), but the latter pollen types did not differ. Protein and lipid levels were lower in stored pollen than in hand-collected pollen, while carbohydrate levels were higher (P < 0.05); bee-collected pollen did not differ significantly from hand-collected pollen in these parameters, probably because of the low sample size.
3.2 Amino acids
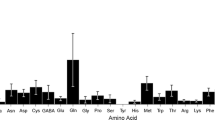
Table II presents data on the amino acid composition of sunflower pollen. Because these data are given as grams per 100 g protein, amino acid concentrations are not diluted by the addition of nectar to the pollen during collection by bees. Proline was the only amino acid that differed between hand-collected pollens of the two cultivars (P < 0.05). Another seven essential amino acids did not differ in concentration between hand-collected, bee-collected and stored pollen of Monsanto 6822, with the exception of arginine, where bee-collected and stored pollen differed (P < 0.05). The levels of these amino acids in stored pollen all met the requirements for honeybees as determined by de Groot (1953). The last two essential amino acids, methionine and tryptophan, were well below the minimum requirements for honeybees (but only two samples were available and additional analysis is needed).
Three non-essential amino acids (proline, serine and tyrosine) were higher in stored than in fresh pollen (P < 0.05), but the levels in bee-collected pollen did not differ significantly from the other types. The total concentrations of essential and non-essential amino acids did not differ across pollen types.
3.3 Fatty acids
Eight fatty acids were detected in sunflower pollen, and together they made up half the mass of pollen lipids. The most abundant fatty acids were lauric, palmitic and α-linolenic acids (Table III). Hand-collected pollens of the two cultivars did not differ in fatty acid composition. Very few differences in fatty acid composition were found across pollen types. Stored pollen was higher in oleic acid (P < 0.05) than hand-collected pollen, while bee-collected and hand-collected pollen differed in eicosenoic acid concentrations (P < 0.05). There were no differences in fatty acid composition between bee-collected and stored pollens. Saturated, unsaturated and total fatty acids did not differ across pollen types.
4 Discussion
Nectar and pollen are combined in the provisions of bees, but the nectar component is often ignored or underestimated (Roulston et al. 2000; Nicolson, 2011). As a result of added nectar sugars, water and carbohydrate increased in bee-collected and stored sunflower pollen, while the protein, lipid and ash contents decreased. Half the dry mass of bee-collected sunflower pollen consists of nectar sugars, even though the prominent pollenkitt helps to stick sunflower pollen grains together (Human and Nicolson, 2003). The bulk of the sugar is added at the collection stage, and we found little difference in nutritional composition between bee-collected and stored pollen (see also Herbert and Shimanuki, 1978). The function of stored pollen or bee bread in preserving and processing nutrients is still largely unknown (Anderson et al. 2011). Because the extent of dilution with nectar sugars at the collection stage is not predictable, it is not possible to apply a correction factor to chemical analyses of bee-collected pellets (Roulston and Cane, 2000; Nicolson, 2011; Leonhardt and Blűthgen, 2012).
The protein in sunflower pollen appears to be deficient in both quantity and quality. Where quantity is concerned, our data confirm the low protein content that has been consistently reported in analyses of bee-collected sunflower pollen (Kleinschmidt and Kondos, 1976; Rayner and Langridge, 1985; Schmidt et al. 1995; Pernal and Currie, 2000; Somerville and Nicol, 2006; Tasei and Aupinel, 2008). Notably, the protein content of bee-collected sunflower pollen was at the bottom of the range for 62 plant species used by beekeepers in south-east Australia (Somerville and Nicol, 2006).
The quality of pollen protein depends on the amounts of essential amino acids relative to bee requirements (de Groot, 1953). We found deficiencies in methionine and tryptophan in the two cultivars tested in this study. Three other reports on amino acids in bee-collected sunflower pollen did not indicate amino acid deficiencies (Wille et al. 1985; Rayner and Langridge, 1985; Somerville and Nicol, 2006). However, conversion of the data of Wille et al. (1985) to grams per 100 g protein (based on a protein concentration of 14 % in bee-collected pollen) gives rather high values for all amino acids, including methionine. In addition, only the study of Rayner and Langridge (1985) included tryptophan: this amino acid is not routinely determined because of the additional alkaline hydrolysis required (Gilliam et al. 1980). In a recent study sampling a broad range of pollens, tryptophan was low in many species (Weiner et al. 2010). Isoleucine is often deficient in pollen, especially eucalypt pollens (Somerville and Nicol, 2006). Most analyses have been of bound amino acids, and separate analyses of water-soluble and protein-bound amino acids show that the latter are dominant in terms of concentration (González Paramás et al. 2006; Weiner et al. 2010). Analysis of free amino acids in a single sample of hand-collected Monsanto DK4040 pollen (data not shown) gave a total amino acid concentration that was only 2 % of the concentration of protein-bound amino acids.
Genotypic differences have been recorded in nectar production of oilseed rape Brassica napus (Pierre et al. 1999) and in nectar sugar composition of sunflowers (Pham-Delègue et al. 1990), and we may also expect variability in pollen nutrients among cultivars. Pollen analysis is more complex than that of nectar, and data are scarce, although the percentage of individual amino acids was found to be similar in pollens of citrus cultivars (Gilliam et al. 1980). Cultivars are not bred for the characteristics of their pollen or nectar, and the type of cultivar used may constrain the usefulness of mass-flowering crops for pollinators. At present there is not enough information available to distinguish cultivar differences from discrepancies between samples analysed in the same or different laboratories (Somerville, 2001; Somerville and Nicol, 2006).
High lipid levels in sunflower pollen are considered attractive to bees, but some components may have inhibitory effects (Singh et al. 1999). Roulston and Cane (2000) reported a wide range (0.8 % dry mass in eucalypt pollen to 18.9 % dry mass in dandelion pollen) for ether-extractable materials in dry pollen. Half of the lipid content in our sunflower pollen samples consisted of long-chain fatty acids. Lauric acid was the most abundant (one third of the total), followed by palmitic and α-linolenic acids. The apparent increase in fatty acids in bee-collected and stored sunflower pollen, though not significant, could be due to microbial modifications or contact with beeswax during storage.
This fatty acid profile differs from previous analyses of fatty acids in sunflower pollen in its high lauric acid content. Farag et al. (1978) found that myristic acid made up almost half of the total in bee-collected pollen, while Schulz et al. (2000), in a comprehensive (but not quantitative) survey of lipids of fresh pollen, gave the major fatty acid as eicosenoic acid, with the pollenkitt lipids resembling the total extract. Loublier et al. (1991) also extracted the pollenkitt and recorded high levels of palmitic, α-linolenic and eicosenoic acids. As in the case of amino acids, there may be differences in fatty acid profile between cultivars, locations, years, methods of analysis and laboratories (Somerville, 2001). The high proportion of lauric acid in our samples is interesting because it was the most effective of the fatty acids found to show antimicrobial activity against Bacillus larvae, the causative agent of American foulbrood disease (Feldlaufer et al. 1993). While oleic and palmitic acids are important in bee nutrition, myristic, linoleic and linolenic acids have antimicrobial and antifungal activity (Manning, 2001). Among the plant species included in Manning’s review, lauric acid is uncommon, except in dandelion (Standifer, 1966) and two other species of Asteraceae, which in combination with our data suggests a possible taxonomic basis for fatty acid composition. In addition, eicosenoic acid (gadoleic acid, C20:1) is absent from the species listed by Manning (2001), although we found this to be a major fatty acid in pollen of both sunflower and A. greatheadii var davyana (Human and Nicolson, 2006). This aloe pollen has a similar lipid content to sunflower pollen, but a greater diversity and total quantity of fatty acids.
Detailed studies on wild sunflower in its native range in Texas and Kansas have shown that many bee species take advantage of the open and accessible flowers with abundant pollen and nectar (Neff and Simpson, 1990; Minckley et al. 1994). However, the pollen protein content of Asteraceae is at the low end of the spectrum for bee-pollinated plants (Roulston et al. 2000), and beekeepers consider these pollens to be a poor resource (Schmidt et al. 1987). Very poor development of two species of Osmia, generalist solitary bees, was recorded on the pollen of dandelion Tanacetum vulgare (Asteraceae) (Sedivy et al. 2011), and the inability of honeybees to rear brood on dandelion pollen has been attributed to deficiencies in several amino acids (Loper and Cohen, 1987). Bumblebees (Bombus terrestris) in micro-colonies performed poorly and reared very small larvae on sunflower pollen, compared to other pollen sources (Tasei and Aupinel, 2008). Of 60 species of bees of the genus Colletes examined by Müller and Kuhlmann (2008), the pollen specialists concentrated on species of Asteraceae whereas the pollen generalists avoided this family, suggesting that bees may need physiological adaptations, such as detoxification abilities, to successfully utilise the pollen of Asteraceae. In addition to its nutrient deficiencies, the pollen of this family may contain defensive compounds, such as pyrrolizidine alkaloids in Senecio (Reinhard et al. 2009). The extent of pollen digestion is also an important factor in comparing the nutritional value of different pollens to bees (Peng et al. 1985; Roulston and Cane, 2000). For honeybee workers collected from sunflower fields, we have previously recorded an extraction efficiency, adjusted for the empty grains in fresh pollen, of 69 % (Human et al. 2007).
Diversity in bee diets is necessary to avoid nutritional deficiencies, such as in essential amino acids, and to dilute toxins, and helps to maintain honeybee immune systems (Alaux et al. 2010). This will be especially important when bees are foraging on mass flowering crops such as sunflower, and is a compelling reason for allowing weeds and natural vegetation to persist in large-scale agricultural systems (Schmidt et al. 1995; Carvalheiro et al. 2011).
References
Alaux, C., Ducloz, F., Crauser, D., Le Conte, Y. (2010) Diet effects on honeybee immunocompetence. Biol. Lett. 6, 562–565
Anderson, K.E., Sheehan, T.H., Eckholm, B.J., Mott, B.M., DeGrandi-Hoffman, G. (2011) An emerging paradigm of colony health: microbial balance of the honey bee and hive (Apis mellifera). Insectes Soc. 58, 431–444
AOAC (2000) Official methods of Analysis. Arlington, VA, USA
Bidlingmeyer, B.A., Cohen, S.A., Tarvin, T.L. (1984) Rapid analysis of amino acids using pre-column derivatization. J. Chromatogr. 336, 93–104
Carvalheiro, L.G., Veldtman, R., Shenkute, A.G., Tesfay, G.B., Pirk, C.W.W., Donaldson, J.S., Nicolson, S.W. (2011) Natural and within-farmland biodiversity enhances crop productivity. Ecol. Lett. 14, 251–259
de Groot, A.P. (1953) Protein and amino acid requirements of the honey bee (Apis mellifica L.). Physiol. Comp. Oecol. 3, 1–83
Farag, R.S., Youssef, A.M., Ewies, M.A., Hallabo, S.A.S. (1978) Long-chain fatty acids of six pollens collected by honeybees in Egypt. J. Apic. Res. 17, 100–104
Feldlaufer, M.F., Knox, D.A., Lusby, W.R., Shimanuki, H. (1993) Antimicrobial activity of fatty acids against Bacillus larvae, the causative agent of American foulbrood disease. Apidologie 24, 95–99
Folch, J., Lees, M., Sloane Stanley, G.H. (1957) A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226, 497–509
Free, J.B. (1964) The behaviour of honeybees on sunflowers (Helianthus annuus L.). J. Appl. Ecol. 1, 19–27
Genet, T., Labuschagne, M.T., Hugo, A. (2004) Capillary gas chromatography analysis of Ethiopian mustard to determine variability of fatty acid composition. J. Sci. Food Agric. 84, 1663–1670
Gilliam, M., McCaughey, W.F., Wintermute, B. (1980) Amino acids in pollens and nectars of citrus cultivars and in stored pollen and honey from honeybee colonies in citrus groves. J. Apic. Res. 19, 64–72
González Paramás, A.M., Gómez Bárez, J.A., Cordón Marcos, C., García-Villanova, R.J., Sánchez-Sánchez, J. (2006) HPLC-fluorimetric method for analysis of amino acids in products of the hive (honey and bee-pollen). Food Chem. 95, 148–156
Herbert, E.W., Shimanuki, H. (1978) Chemical composition and nutritive value of bee-collected and bee-stored pollen. Apidologie 9, 33–40
Human, H., Nicolson, S.W. (2003) Digestion of maize and sunflower pollen by the spotted maize beetle Astylus atromaculatus (Melyridae): is there a role for osmotic shock? J. Insect Physiol. 49, 633–643
Human, H., Nicolson, S.W. (2006) Nutritional content of fresh, bee-collected and stored pollen of Aloe greatheadii var. davyana (Asphodelaceae). Phytochemistry 67, 1486–1492
Human, H., Nicolson, S.W., Strauss, K., Pirk, C.W.W., Dietemann, V. (2007) Influence of pollen quality on ovarian development in honeybees (Apis mellifera scutellata). J. Insect Physiol. 53, 649–655
Kleinschmidt, G.J., Kondos, A.C. (1976) The influence of crude protein levels on colony production. Australian Beekeep. 80, 251–257
Krause, G.L., Wilson, W.T. (1981) Honey bee pollination and visitation patterns on hybrid oilseed sunflowers in central Wyoming (Hymenoptera: Apidae). J. Kansas Entomol. Soc. 54, 75–82
Leonhardt, S.D., Blűthgen, N. (2012) The same, but different: pollen foraging in honeybee and bumblebee colonies. Apidologie 43(4), 449–464
Loper, G.M., Cohen, A.C. (1987) Amino acid content of dandelion pollen, a honey bee (Hymenoptera: Apidae) nutritional evaluation. J. Econ. Entomol. 80, 14–17
Loublier, Y., Ducruet, V., Pham-Delegue, M.-H., Douault, P., Vear, F., Degas, C., Masson, C. (1991) The pollen as an attraction parameter for pollinators to sunflower (Helianthus annuus L.). Acta Hort 288, 394–398
Manning, R. (2001) Fatty acids in pollen: a review of their importance for honey bees. Bee World 82, 60–75
Minckley, R.L., Wcislo, W.T., Yanega, D., Buchmann, S.L. (1994) Behavior and phenology of a specialist bee (Dieunomia) and sunflower (Helianthus) pollen availability. Ecology 75, 1406–1419
Müller, A., Kuhlmann, M. (2008) Pollen hosts of western palaearctic bees of the genus Colletes (Hymenoptera: Colletidae): the Asteraceae paradox. Biol. J. Linn. Soc. 95, 719–733
Nderitu, J., Nyamasyo, G., Kasina, M., Oronje, M.L. (2008) Diversity of sunflower pollinators and their effect on seed yield in Makueni District, Eastern Kenya. Span. J. Agric. Res. 6, 271–278
Neff, J.L., Simpson, B.B. (1990) The roles of phenology and reward structure in the pollination biology of wild sunflower (Helianthus annuus L., Asteraceae). Isr. J. Bot. 39, 197–216
Nicolson, S.W. (2011) Bee food: the chemistry and nutritional value of nectar, pollen and mixtures of the two. Afr. Zool. 46, 197–204
Peng, Y.-S., Nasr, M.E., Marston, J.M., Fang, Y. (1985) The digestion of dandelion pollen by adult worker honeybees. Physiol. Entomol. 10, 75–82
Pernal, S.F., Currie, R.W. (2000) Pollen quality of fresh and 1-year-old single pollen diets for worker honey bees (Apis mellifera L.). Apidologie 31, 387–409
Pham-Delègue, M.H., Etievant, P., Guichard, E., Marilleau, R., Douault, P.H., Chauffaille, J., Masson, C. (1990) Chemicals involved in honeybee–sunflower relationship. J. Chem. Ecol. 16, 3053–3065
Pierre, J., Mesquida, J., Marilleau, R., Pham-Delègue, M.H., Renard, M. (1999) Nectar secretion in winter oilseed rape, Brassica napus—quantitative and qualitative variability among 71 genotypes. Plant Breeding 118, 471–476
Rayner, C.J., Langridge, D.F. (1985) Amino acids in bee-collected pollens from Australian indigenous and exotic plants. Aust. J. Exp. Agric. 25, 722–726
Reinhard, A., Janke, M., von der Ohe, W., Kempf, M., Theuring, C., Hartmann, T., Schreier, P., Beuerle, T. (2009) Feeding deterrence and detrimental effects of pyrrolizidine alkaloids fed to honey bees (Apis mellifera). J. Chem. Ecol. 35, 1086–1095
Roulston, T.H., Cane, J.H. (2000) Pollen nutritional content and digestibility for animals. Plant Syst. Evol. 222, 187–209
Roulston, T.H., Cane, J.H., Buchmann, S.L. (2000) What governs protein content of pollen: pollinator preferences, pollen–pistil interactions, or phylogeny? Ecol. Monogr. 70, 617–643
Schmidt, J.O., Thoenes, S.C., Levin, M.D. (1987) Survival of honey bees, Apis mellifera (Hymenoptera: Apidae), fed various pollen sources. Ann. Entomol. Soc. Am. 80, 176–183
Schmidt, L.S., Schmidt, J.O., Rao, H., Wang, W., Xu, L. (1995) Feeding preference and survival of young worker honey bees (Hymenoptera: Apidae) fed rape, sesame, and sunflower pollen. J. Econ. Entomol. 88, 1591–1595
Schulz, S., Arsene, C., Tauber, M., McNeil, J.N. (2000) Composition of lipids from sunflower pollen (Helianthus annuus). Phytochemistry 54, 325–336
Sedivy, C., Müller, A., Dorn, S. (2011) Closely related pollen generalist bees differ in their ability to develop on the same pollen diet: evidence for physiological adaptations to digest pollen. Funct. Ecol. 25, 718–725
Singh, S., Saini, K., Jain, K.L. (1999) Quantitative comparison of lipids in some pollens and their phagostimulatory effects in honey bees. J. Apic. Res. 38, 87–92
Somerville, D.C. (2001) Nutritional value of bee collected pollens. Report for the Rural Industries Research and Development Corporation, Australia
Somerville, D.C., Nicol, H.I. (2006) Crude protein and amino acid composition of honey bee-collected pollen pellets from south-east Australia and a note on laboratory disparity. Aust. J. Exp. Agric. 46, 141–149
Standifer, L.N. (1966) Fatty acids in dandelion pollen gathered by honey bees, Apis mellifera (Hymenoptera: Apidae). Ann. Entomol. Soc. Am. 59, 1005–1008
Tasei, J.-N., Aupinel, P. (2008) Nutritive value of 15 single pollens and pollen mixes tested on larvae produced by bumblebee workers (Bombus terrestris, Hymenoptera: Apidae). Apidologie 39, 397–409
Tepedino, V.J., Parker, F.D. (1982) Interspecific differences in the relative importance of pollen and nectar to bee species foraging on sunflower. Env. Entomol. 11, 246–250
Todd, F.E., Bretherick, O. (1942) The composition of pollens. J. Econ. Entomol. 35, 312–317
Weiner, C.N., Hilpert, A., Werner, M., Linsenmair, K.E., Blüthgen, N. (2010) Pollen amino acids and flower specialisation in solitary bees. Apidologie 41, 476–487
Wille, H., Wille, M., Kilchenmann, V., Imdorf, A., Bűhlmann, G. (1985) Pollenerte und Massenwechsel von drei Apis mellifera-Vőlkern auf demselben Bienenstand in zwei aufeinanderfolgenden Jahren. Rev. Suisse Zool. 92, 897–914
Acknowledgements
Amino acid analyses were carried out at the Southern African Grain Laboratory in Pretoria and all other analyses at Nutrilab, University of Pretoria. We thank A.G. Shenkute and G.B. Tesfay for helping with pollen collection, the National Research Foundation and the University of Pretoria for financial support, and the anonymous referees for useful comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Composition chimique du pollen “de faible qualité” du tournesol ( Helianthus annuus Asteraceae)
Nutriments / protéine / lipides / acides aminés / acides gras
Chemische Zusammensetzung des „minderwertigen“Pollens der Sonnenblume ( Helianthus annuus , Asteraceae)
Nährstoffe / Proteine / Lipide / Aminosäuren / Fettsäuren
Manuscript editor: Yves Le Conte
Rights and permissions
About this article
Cite this article
Nicolson, S.W., Human, H. Chemical composition of the ‘low quality’ pollen of sunflower (Helianthus annuus, Asteraceae). Apidologie 44, 144–152 (2013). https://doi.org/10.1007/s13592-012-0166-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13592-012-0166-5