Abstract
The photosynthetic rates of leaves depend on the vertical position and cultivation conditions. However, few models have been proposed to express photosynthesis according to leaf position, and there was a lack of quantitative analysis between physiological indicators and model parameters. The objectives of this study were to analyze the leaf photosynthetic characteristics of paprika plants according to leaf vertical position using photosynthesis models, and to analyze the relationship between the total nitrogen content and the photosynthetic model parameters. Leaf photosynthetic rates at different vertical positions were measured under varying light intensities and CO2 concentrations in triplicate. Rectangular hyperbola and FvCB (Farquhar, von Caemmerer, and Berry) models were selected, calibrated, and validated as multivariable photosynthesis models. Total nitrogen contents and SPAD values were measured at each leaf position and the coefficients of the photosynthetic rate models were compared. The R2 values for the rectangular hyperbola and FvCB models were 0.86 and 0.91, and the RMSE values were 4.651 and 2.104, respectively. Total nitrogen content linearly increased with increasing vertical leaf position and it was linearly related to the maximum carboxylation capacity and maximum electron transport rate, estimated in the FvCB model. In this study, the FvCB model was considered more suitable for expressing the relationship between total nitrogen contents and plant’s physiological responses according to the vertical position of leaves. The vertical leaf photosynthetic rate models established in this study will contribute to determining optimal environmental conditions for maximizing crop photosynthesis and establish the criteria for precise CO2 enrichment in greenhouses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The photosynthetic rates of leaves depend on the vertical position and cultivation conditions. In order to estimate the overall photosynthetic response of a crop, it is important to understand how different photosynthetic characteristics depend on the vertical position (Jung et al. 2018). The light intensities at the bottom of densely-planted crops are greatly reduced by shading effects among adjacent plants (Chen et al. 1999). A decrease in light intensity will impair crop growth and consequently reduce the photosynthetic rate and crop production (Aminifard et al. 2012). In addition, nutrient and pigment contents such as nitrogen and chlorophyll affects the photosynthesis of crops. In order to increase the production of fruit vegetable crops, quantitative methods for assessing crop photosynthesis under various environmental and physiological conditions are necessary. Recently, modeling technique has attracted attention as a method to quantify environmental factors affecting photosynthesis (Medina-Ruíz et al. 2011; Noe and Giersch 2004).
In the past, simple photosynthesis models using a single variable were widely used, but recently, the use of complex multivariate models are increasing. Typically, simple multiplication models were used to quantify the photosynthetic rate for a single environmental factor (Jones et al. 1991; Park et al. 2016), but these models do not reflect the physiological characteristics, such as electron transfer rates of crops. This has led to an increasing demand for improved models. Rectangular hyperbola or negative exponential model has been mainly used to express photosynthetic rates for light intensity and CO2 concentration (Baker and Allen 1993; Valladares et al. 1997; Thornley 1974) developed a simple model reflecting the chemical reactions that occur in light and dark reactions of photosynthesis. The Farquhar, von Caemmerer, and Berry model (FvCB model) is the most widely used in recent years (Farquhar et al. 1980; Yin et al. 2009). The FvCB model uses complex expressions corresponding to the physiological response of crops, but is considered to be the most suitable for determining changes in photosynthetic rate due to various environmental factors (Kim et al. 2016; Qian et al. 2012). Previous studies have identified photosynthesis changes with leaf age (Constable and Rawson 1980), but using leaf position is practically convenient because paprika leaves occur regularly along the phyllotaxis. Photosynthetic rate models have not been accurately validated and compared for each vertical leaf position in the crop.
According to Shin et al. (2011), the whole-plant photosynthetic rate was 30 µmol CO2 m− 2 s− 1 under 3000 µmol m− 2 s− 1 of light intensity in the case of ‘Fiesta’ paprika. To reflect the photosynthetic rate of the whole plant, affected by the vertical position of the leaves, an adequate model incorporating environmental factors with photosynthetic rate is required. Kim et al. (2016) measured the photosynthetic rate for each leaf position, but no comparison was performed between photosynthesis models. In addition, nitrogen affects the production of biochemical substances such as proteins, amino acids, nucleic acids, enzymes, and chlorophyll in plants (Suharja and Sutarno 2009), and the nitrogen content in the plants depends on the light distribution pattern of the plant canopy (Ellsworth and Reich 1993). With recent advances in three-dimensional plant modeling, the vertical light distribution and subsequent physiological changes of plants have been studied (Le Roux et al. 1999a; Sinoquet et al. 2001). In this case, an appropriate photosynthesis model for each position is required. In addition, indicators, such as nitrogen content, express the physiological response of plants. Thus, the relationship between physiological indicator and model parameter can be analyzed to determine the crop growth condition. However, few attempts have been made to interpret physiological response of paprika by comparing several photosynthesis models.
The objectives of this study were to analyze the leaf photosynthetic characteristics of paprika plants according to the vertical position of leaves using photosynthesis models, and to analyze the relationship between the total nitrogen content and the photosynthetic model parameters.
2 Materials and methods
2.1 Cultivation conditions
To investigate the leaf photosynthetic rate of paprika plants (Capsicum annuum L. ‘Scirocco’), experiments were conducted in Venlo-type greenhouses of the Protected Horticulture Research Institute, National Institute of Horticultural and Herbal Sciences (RDA), Haman, Korea (35.2°N, 128.4°E) for two cultivation periods. The setting temperatures for ventilation during the day and heating at night in the greenhouse were 30 °C and to 21 °C, respectively. The plants were sown on a tray on February 08, 2018 and May 06, 2019, and transferred to cubes on March 05, 2018 and June 07, 2019, respectively. During the seedling period, the electrical conductivity (EC) of PBG paprika nutrient solutions was initially set to 0.8 dS m− 1, gradually increased by 0.2 dS m− 1 per week, and maintained at 2.5 dS m− 1 at the end. After raising seedlings, the plants were transplanted on the slabs with a planting density of 2 plants/m on April 06, 2018 and July 10, 2019. Four cubes were planted on each slab. After transplanting, nutrient solutions with EC 2.5 dS m− 1 and pH 6.0 were supplied 14 times a day at 33 mL per plant by drip irrigation.
2.2 Measurements of leaf photosynthetic rate, SPAD value, and leaf total nitrogen content
Leaf photosynthetic rates were measured twice over two years for model establishment and verification. On July 04, 2018 and October 07, 2019, the first measurements were conducted from 10:00 to 15:00 to avoid photosynthesis afternoon depression (Kim et al. 2016; Qian et al. 2012). Leaf photosynthetic rates were measured using a portable photosynthesis measuring device (LI-6400, LI-Cor. Inc., Lincoln, NE, USA) with a 6400-02B LED light source chamber. Light intensities were set to 0, 50, 100, 200, 400, 900, 1500 and 2000 µmol m− 2 s− 1, and CO2 concentrations in the chamber were set to 50, 100, 400, 800, and 1200 µmol mol− 1, as previously employed by Schaffer et al. (1997). A light response curve at 50 µmol mol− 1 CO2 level was derived and the same curve derived sequentially at the next CO2 level. Block temperature, relative humidity and flow rate of an infrared CO2 gas analyzer (6400-02B, LI-Cor. Inc., Lincoln, NE, USA) were controlled at 25 °C, 65–85%, and 500 µmol s− 1, respectively. To determine specifically how the photosynthetic rate model depends on each vertical leaf position, measurements of photosynthetic rate were made for eight levels of light intensities and five levels of CO2 concentrations for each vertical leaf position. The leaves used for the measurements were fully-expanded and the plants were in the reproductive phase. Measurements were taken in triplicate on different leaves of paprika at heights of 0, 25, 50, 75, 100, 125, and 150 cm as shown in Fig. 1.
The second measurements were conducted from 10:00 to 15:00 on July 05, 2018 and October 11, 2019, and the leaf photosynthetic rate was obtained to verify the model established in the first measurement. Light intensities were set to 0, 100, 400, 800, and 1200 µmol m− 2 s− 1, and CO2 concentrations were set to 100, 400, 800, and 1200 µmol mol− 1. The measurement method was the same as the first, and measurements were made on five levels of light intensity and four levels of CO2 concentration for each position of the leaves. Each measurement was performed in triplicate in different leaves.
The SPAD values were measured with a chlorophyll meter (SPAD-502, Minolta, Osaka, Japan) and recorded as a mean of 10 measurements for each individual leaf. Measurements were taken along the edge of the leaf and were measured three times on different leaves at each position. Leaves were sampled at each position and finely ground through a mill after freeze-drying. Leaf total nitrogen content was analyzed using the Kjeldahl method (Bremner 1960).
2.3 Estimation of intercellular CO2 concentration
In order to express the \(A\)/\({C}_{i}\) curve (where \(A\) is the net CO2 assimilation rate and \({C}_{i}\) is intercellular CO2 concentration) and to calculate leaf photosynthetic rate with the FvCB model using the measured atmospheric CO2 concentration, the relationship between the atmospheric and intercellular CO2 concentrations was regressed. Eight models are available that express the CO2 exchange between the atmosphere and the leaves of plants. In this study, the widely used Ball-Berry model was selected because of its simple equation (Katul et al. 2000):
where \({C}_{i}\) and \({C}_{a}\) are the intercellular and atmospheric CO2 concentrations (µmol mol− 1), respectively, \(m\) is an empirical parameter, and \(RH\) is the relative humidity. In other plant species \(m\) ranges from 3 to 10, but it has not been reported for paprika (Leuning 1995). To estimate the \(m\) value for paprika, \({C}_{i}\), \({C}_{a}\), and \(RH\) values were measured using the portable photosynthesis measuring device, and regression analysis was conducted using Eq. (1). Measured \({C}_{a}\) and \(RH\) values were used for all \({C}_{i}\) calculations through this experiment.
2.4 Leaf photosynthetic rate models
The first model for expressing leaf photosynthetic rates with varying light intensity and CO2 concentration was a rectangular hyperbola model established by Kaitala et al. (1982). The rectangular hyperbola model used in previous studies was expressed in the following equation:
where \(P\) is the leaf photosynthetic rate (µmol CO2 m− 2 s− 1), \(\alpha\) is the photochemical efficiency (µmol mol− 1), \(PPFD\) is the photosynthetic photon flux density (µmol m− 2 s− 1), \(\beta\) is the carboxylation conductance (s− 1), \({C}_{i}\) is the intercellular CO2 concentration (µmol mol− 1), and \(R\) is the respiration (µmol CO2 m− 2 s− 1).
Since it is difficult to reflect the effect of temperature change on leaf photosynthetic rate in the rectangular hyperbola model, a modified rectangular hyperbola model with temperature variable was used, which is based on empirical equations to express the change in photochemical efficiency and carboxylation conductance (Jung et al. 2017). In this study, an exponential equation was selected rather than quadratic equation because the leaf temperature variation was small. The photochemical efficiency and carboxylation conductance used in the rectangular hyperbola model are expressed in the following equations:
where \({T}_{l}\) is the leaf temperature (°C), and \(a\) and \(b\) are regression coefficients. The rectangular hyperbola model used in the analysis is expressed in the following equation:
where \(a\) and \(b\) are regression coefficients, \(PPFD\) is the photosynthetic photon flux density (µmol m− 2 s− 1), \({C}_{i}\) is the intercellular CO2 concentration (µmol mol− 1), and \(R\) is the respiration (µmol CO2 m− 2 s− 1).
The FvCB model was expressed in the following equations:
where \(P\) is the leaf photosynthetic rate (µmol CO2 m− 2 s− 1), \({A}_{c}\) is the ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) carboxylation limited photosynthesis rate (µmol CO2 m− 2 s− 1), \({A}_{j}\) is the ribulose-1,5-bisphosphate (RuBP) regeneration limited photosynthesis rate (µmol CO2 m− 2 s− 1), \({V}_{c}\) is the carboxylation capacity at a certain light intensity (µmol CO2 m− 2 s− 1), \({C}_{i}\) is the intercellular CO2 concentration (µmol mol− 1), \({\varGamma }^{*}\) is the CO2 compensation point (µmol mol− 1), \({K}_{c}\) is the Michaelis–Menten constant of Rubisco for CO2 (µmol mol− 1), \(O\) is the O2 concentration (210 mmol mol− 1), \({K}_{o}\) is the Michaelis–Menten constant of Rubisco for O2 (µmol mol− 1), \({V}_{cmax}\) is the maximum carboxylation capacity (µmol CO2 m− 2 s− 1), \(PPFD\) is the photosynthetic photon flux density (µmol m− 2 s− 1), \(J\) is the electron transport rate at a certain light intensity (µmol m− 2 s− 1), \({J}_{max}\) is the maximum electron transport rate (µmol m− 2 s− 1), \(\alpha\) is the efficiency of light energy conversion on an incident light basis (0.42 mol e− mol− 1 photon), and \(\theta\) is the curvature of the light response of \(J\) (0.25 dimensionless) (Qian et al. 2012). The Michaelis–Menten constants of Rubisco for CO2 and O2 in the model were calculated based on Arrhenius function.
Regression analysis was performed on each model using the SPSS statistical package (IBM, New York, NY, USA). The light intensity, leaf temperature, relative humidity, and CO2 concentration were set as input data in the model expression, and the measured photosynthetic rates were set as dependent variables to conduct a non-linear regression. All analyses were performed for each of the measured results for each vertical leaf position of the paprika.
2.5 Validation of leaf photosynthetic rate models
In the leaf photosynthetic rate model determined by regression analysis, the calculated leaf photosynthetic rate was compared with the measured leaf photosynthetic rate in the secondary measurement, which was different from the data used to establish the models under the specific light intensity and CO2 concentration. A regression analysis was performed on a 1:1 line using the SPSS statistical package (IBM), and a graph of the model was drawn using Sigmaplot (Systat Software, San Jose, CA, USA).
2.6 Analyses of photosynthetic parameters
The relationship between the SPAD value and the total nitrogen content measured by leaf positions was analyzed through linear regression. A primary linear expression was used for regression analysis. The values \({P}_{max}\), \({V}_{cmax}\), and \({J}_{max}\), estimated in the rectangular hyperbola and FvCB models, were found to be related to SPAD values or total nitrogen content. As before, regression analysis was performed using the primary linear expression. Regression analysis was performed on each model using the SPSS statistical package.
3 Results
3.1 Intercellular CO2 concentration
The intercellular CO2 concentration increased linearly with increasing atmospheric CO2 concentration (Fig. 2). As the atmospheric CO2 concentration increased from 100 to 1200 µmol mol− 1, the intercellular CO2 concentration increased from 100 to 1100 µmol mol− 1. The \(m\) value in Eq. (1) was estimated to be 3.177 at a relative humidity of 74%. The R2 and root mean square error (RMSE) values in the Ball–Berry model were estimated to be 0.63 and 0.539, respectively.
3.2 Vertical SPAD values and leaf total nitrogen content
The leaf SPAD values over 75 cm in the vertical position decreased linearly, while those under 75 cm showed no tendency (Fig. 3a). The maximum and minimum SPAD values measured were 48.5 at 0 cm and 72.2 at 125 cm, respectively. Leaf total nitrogen content linearly increased with increasing vertical leaf position (Fig. 3b). The maximum and minimum total nitrogen contents measured were 5.46% at 0 cm and 2.43% at 150 cm, respectively.
3.3 Regression analyses of rectangular hyperbola and FvCB models
For the rectangular hyperbola model, the regression coefficients of \(a\), \(b\), and \(R\) included in Eq. (5) were analyzed (Table 1). \(a\) showed a low value at 150 cm, and a decreasing tendency at other heights. As the height increased, \(b\) values decreased, and the result obtained at 0 cm was about 75% of the result obtained at 150 cm. \(R\) showed a low value at 150 cm, and a decreasing tendency at other heights. The results were substituted for Eq. 5 showing the leaf photosynthetic rate in three-dimensional space with light intensity and CO2 concentration on the X and Y axes (Fig. 4). The rectangular hyperbola model showed overestimated values at 25 and 50 cm under light intensity and high CO2 concentration conditions. The photosynthetic rates over 50 cm increased in the form of saturation curves with increasing light intensity and CO2 concentrations. The photosynthetic rates under 100 cm also increased in the form of saturation curves with increasing light intensity. With increasing CO2 concentration, however, the leaf photosynthetic rates increased linearly, without showing the form of a saturation curve.
Leaf photosynthetic rates (\(P\)) of hydroponically-grown paprika plants estimated using the rectangular hyperbola model with light intensity (\(PPFD\)) and intercellular CO2 concentration (\({C}_{i}\)) at vertical leaf positions of 0 (a), 25 (b), 50 (c), 75 (d), 125 (e), and 150 cm (f). The leaf temperature was constant at 25 °C. White dots are measured values of leaf photosynthetic rates and curved meshes are the regressed estimates from the rectangular hyperbola model
For the FvCB model, \({V}_{cmax}\) and \({J}_{max}\) included in Eqs. 8 and 10 were analyzed (Table 2). From 0 to 150 cm in the vertical position of the leaf, those two values showed decreasing tendencies, with higher accuracy in regression analysis at 0 cm. The results were substituted for Eqs. 6–10, showing the leaf photosynthetic rate in three-dimensional space with light intensity and CO2 concentration on the X and Y axes (Fig. 5). Over 50 cm, all light intensity conditions were shown to be the RuBP regeneration-limited zone under low CO2 concentration conditions. In the high CO2 concentration conditions, the light intensity within 600–1000 µmol m− 2 s− 1 was found to be the RuBP regeneration-limited zone. Similar patterns were observed under 75 cm, but the Rubisco carboxylation limited zone was found to be wider than the RuBP regeneration-limited zone.
Leaf photosynthetic rates (\(P\)) of hydroponically-grown paprika plants estimated using the FvCB model with light intensity (\(PPFD\)) and intercellular CO2 concentration (\({C}_{i}\)) at vertical leaf positions of 0 (a), 25 (b), 50 (c), 75 (d), 125 (e), and 150 cm (f). The leaf temperature was constant at 25 °C. White dots are measured values of leaf photosynthetic rates and curved meshes are the regressed estimates from the FvCB model
3.4 Validation of leaf photosynthetic rate models
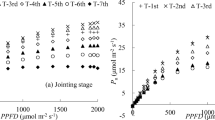
Rectangular hyperbola and FvCB model-estimated leaf photosynthetic rates for each height were compared to measured rates against a 1:1 regression line to evaluate model performance (Fig. 6). The R2 values and the RMSE values were 0.86 and 0.90, and 4.651 and 2.104 in the rectangular hyperbola and FvCB models, respectively.
3.5 Relationship among photosynthetic parameter, vertical SPAD value, and leaf total nitrogen content
There was a small negative relationship between the SPAD values and the total nitrogen contents, showing a negative slope as − 0.1010 (Fig. 7). The linear regression results for the parameters in the equation showed a low correlation with R2 = 0.33. The \(\alpha\) and \(\beta\) value estimated by the rectangular hyperbola model indicated a negative correlation with SPAD values, however, \(\beta\) value indicated a positive correlation with total nitrogen content. Similarly, the \({V}_{cmax}\) and \({J}_{max}\) values estimated by the FvCB model indicated a negative correlation with SPAD value and a positive correlation with total nitrogen content (Fig. 8). Estimating photosynthetic model parameters from the SPAD values resulted in low accuracy across all regressions. The regression results of the relationship between total nitrogen content and photosynthetic rate model parameters were generally more accurate than using SPAD values, but the maximum R2 value was as low as 0.61.
Relationships between the estimated values of \(\alpha\) and \(\beta\) (rectangular hyperbola model, a and b), \({V}_{cmax}\) (FvCB model, c and d), \({J}_{max}\) (FvCB model, e and f), and the measured SPAD values (a, c, and e) and total nitrogen content (b, d, and f), respectively, of hydroponically-grown paprika plants. The solid line indicates the regressed primary linear equation
4 Discussion
For the rectangular hyperbola model, the photochemical efficiency and carboxylation conductance were used to express plant physiological reactions as regressing parameters in the model. The photochemical efficiency increased toward the bottom leaves of the plants, but the bottom leaves showed low leaf photosynthetic rates. In the previous study, photochemical efficiency and carboxylation conductance of willow trees were 0.00028 µmol− 1 m2 and 0.001053 kg CO2 m− 3 s− 1, respectively (Kaitala et al. 1982). In addition, the photochemical efficiency measured in orache plants was known to be 0.177 µmol CO2 mol− 1 (Marshall and Biscoe 1980). This value was similar to the photochemical efficiency of leaf positions over 50 cm estimated in the rectangular hyperbola model in this study. Thus, the rectangular hyperbola model was suitably established through regression analysis. However, the rectangular hyperbola model showed overestimated values under high light intensity and CO2 concentration conditions especially at the positions at 25 and 50 cm (Fig. 4b, c). The lack of accuracy in the position revealed that the most active photosynthetic responses is a factor that reduces the reliability of the model. In addition, a modified rectangular hyperbola model with temperature variables was also developed, but empirical equations were used to express the change in photochemical efficiency and carboxylation conductance (Jung et al. 2017). However, the temperature changes occurred during photosynthesis measurements in this experiment were too small to use the quadratic empirical models as used in the previous study.
For the FvCB models, the Michaelis–Menten constants for CO2 and O2 concentrations were used to express photosynthetic reactions that vary with temperature (Qian et al. 2012). In terms of reflecting temperature, the FvCB model can more accurately represent the leaf photosynthetic rate compared to the rectangular hyperbola model. Our results showed the lowest R2 value with the FvCB model (Fig. 6), but the R2 value was more than 0.90, indicating that the model itself is reliable. For shaded leaves at the bottom of the canopy in various crops, decreases in Rubisco content and RuBP regeneration capacity were reported (Baker and McKiernan 1988; Evans 1993; Osborne et al. 1998). As previously reported, this study also showed a tendency for the maximum carboxylation capacity and the maximum electron transport rate to decrease from the upper leaf to the lower leaf (Table 2). At leaf positions of 0–50 cm, where photosynthesis actively occurs, the FvCB model was more accurate than the rectangular hyperbola model under high light intensity and CO2 concentration conditions.
Regressions using measured leaf photosynthetic rates were often inaccurate in the middle and bottom leaves of the plant canopy. By using a 3D plant model and simulations, Sinoquet et al. (1998) reported that the light intensity on the middle leaves of plants varied significantly. More adequate leaf photosynthetic rate models need to be applied to the top or outer leaves at high light intensity and the inner leaves at low light intensity. The bottom leaves showed smaller differences in light distribution compared to the middle and top leaves. Therefore, the leaf photosynthetic rate did not significantly change with changing environmental factors in the bottom leaves (Léchaudel et al. 2013). Photosynthesis varies depending on the leaf position due to changes in physiological and anatomical characteristics, such as leaf cell structure and chlorophyll content, according to environmental conditions (Larbi et al. 2015). In general, shaded leaves at the bottom of plants have low photosynthetic capacity and nitrogen content, resulting in insufficient photosynthesis even with increased CO2 concentrations (Del Pozo et al. 2007). In order to develop a more accurate model, it is necessary to identify the relationship between anatomical leaf structure and physiological indicators according to the vertical positions.
In this study, the nitrogen content at the bottom leaves was lower (Fig. 3b). According to Mavengahama et al. (2006), the optimum level of leaf nitrogen content for 6-weeks old paprika was approximately 3.7%. The total nitrogen content in this experiment was up to 5%, but there was no stress responses. The vertical distribution of total nitrogen content in paprika found herein was consistent with the measurements obtained in deciduous forests for species such as maple, oak, and walnut (Ellsworth and Reich 1993; Le Roux et al. 1999b). It is interpreted that the nitrogen is allocated to the top of the plant due to the need for photosynthesis-related enzymes. The distribution and allocation of nitrogen is a contentious topic, but it is generally known to be related with light intensity (Thornley 2004). Models having physiological characteristics with nitrogen distribution will be useful for farmers growing paprika at a high planting density.
According to Sun et al. (2019), SPAD values measured in tomatoes were 31.46–60.90, similar to those of paprika in this study. Pestana et al. (2001) observed that chlorophyll contents and SPAD values represent exponential relationship through orange trees. Díaz-Pérez (2013) also reported that chlorophyll contents in paprika were not correlated with leaf nitrogen content. The bottom leaves adapted to low light intensity had larger chloroplast size and chlorophyll contents than the top leaves adapted to high light intensity. In this study, the SPAD values according to leaf position was consistent with the previous studies (Figs. 3, 7). In addition, the SPAD values were less accurate in estimating parameters of photosynthetic rate models (Fig. 8). Considering photosynthetic mechanism, total nitrogen contents is more suitable for expressing the plant physiological responses than SPAD values. The SPAD values and nitrogen contents according to leaf position are proportional to accumulated light intensity (Thornley 2004; Yu et al. 2016). Because the light intensity exponentially decreases in crop canopy, it is likely that the SPAD values and nitrogen contents also tend to be the same. Thus, the relationship between the light intensity and SPAD value or nitrogen content of each leaf can be analyzed first with an exponential model, which can improve the accuracy rather than using only the parameters of the photosynthesis model.
5 Conclusions
Leaf photosynthetic rates of hydroponically-grown paprika plants were analyzed according to vertical position using two different multivariable photosynthetic rate models. The validation results showed that the R2 values of rectangular hyperbola and FvCB models were as high as 0.86 and 0.90 with RMSE values of 4.651 and 2.104, respectively. However, the R2 values of SPAD values and total nitrogen contents at the maximum electron transport rate in the FvCB model were as low as 0.45 and 0.61, respectively. Total nitrogen content linearly increased with increasing vertical leaf position and has a close relation with the maximum carboxylation capacity and maximum electron transport rate in the FvCB model. Compare to the rectangular hyperbola model, the FvCB model showed reliable values under high light intensity and CO2 concentration conditions at a position where photosynthesis was very active. It is desirable to use the FvCB model that expresses the relationship between total nitrogen contents and plant’s physiological responses according to the vertical position of leaves. The vertical leaf photosynthetic rate models established in this study will contribute to determine optimal environmental conditions for maximizing crop photosynthesis in greenhouses and to establish the criteria for precise CO2 enrichment.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Aminifard MH, Aroiee H, Ameri A, Fatemi H (2012) Effect of plant density and nitrogen fertilizer on growth, yield, and fruit quality of sweet pepper (Capsicum annum L.). Afr J Agric Res 7:859–866. https://doi.org/10.5897/ajar10.505
Baker JT, Allen LH Jr (1993) Contrasting crop species responses to CO2 and temperature: rice, soybean, and citrus. Vegetatio 104:239–260. https://doi.org/10.1007/978-94-011-1797-5_16
Baker NR, McKiernan M (1988) Modifications to the photosynthetic apparatus of higher plants in response to changes in the light environment. Biol J Linn Soc 34:193–203. https://doi.org/10.1111/j.1095-8312.1988.tb01958.x
Bremner JM (1960) Determination of nitrogen in soil by the Kjeldahl method. J Agric Sci 55:11–33. https://doi.org/10.1017/S0021859600021572
Chen JM, Liu J, Cihlar J, Goulden ML (1999) Daily canopy photosynthesis model through temporal and spatial scaling for remote sensing applications. Ecol Model 124:99–119. https://doi.org/10.1016/s0304-3800(99)00156-8
Constable GA, Rawson HM (1980) Effect of leaf position, expansion and age on photosynthesis, transpiration and water use efficiency of cotton. Funct Plant Biol 7:89–100. https://doi.org/10.1071/pp9800089
Del Pozo A, Perez P, Gutierrez D, Alonso A, Morcuende R, Martinez-Carrasco R (2007) Gas exchange acclimation to elevated CO2 in upper-sunlit and lower-shaded canopy leaves in relation to nitrogen acquisition and partitioning in wheat grown in field chambers. Environ Exp Bot 59:371–380. https://doi.org/10.1016/j.envexpbot.2006.04.009
Díaz-Pérez JC (2013) Bell pepper (Capsicum annum L.) crop as affected by shade level: microenvironment, plant growth, leaf gas exchange, and leaf mineral nutrient concentration. HortScience 48:175–182. https://doi.org/10.21273/hortsci.48.2.175
Ellsworth DS, Reich PB (1993) Canopy structure and vertical patterns of photosynthesis and related leaf traits in a deciduous forest. Oecologia 96:169–178. https://doi.org/10.1007/bf00317729
Evans JR (1993) Photosynthetic acclimation and nitrogen partitioning within a Lucerne canopy. I. canopy characteristics. Aust J Plant Physiol 20:55–67. https://doi.org/10.1071/pp9930055
Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149:78–90. https://doi.org/10.1007/bf00386231
Jones JW, Dayan E, Allen LH, van Keulen H, Challa H (1991) A dynamic tomato growth and yield model (TOMGRO). Trans Am Soc Agric Eng 34:663–672. https://doi.org/10.13031/2013.31715
Jung DH, Lee JW, Kang WH, Hwang IH, Son JE (2018) Estimation of whole plant photosynthetic rate of Irwin mango under artificial and natural lights using three-dimensional plant model and ray-tracing. Int J Mol Sci 19:152. https://doi.org/10.3390/ijms19010152
Jung DH, Yoon HI, Son JE (2017) Development of a three-variable canopy photosynthetic rate model of romaine lettuce (Lactuca sativa L.) grown in plant factory modules using light intensity, temperature, and growth stage. Protec Hortic Plant Fact 26:268–275. https://doi.org/10.12791/ksbec.2017.26.4.268
Kaitala V, Hari P, Vapaavuori E, Salminen R (1982) A dynamic model for photosynthesis. Ann Bot 50:385–396. https://doi.org/10.1093/oxfordjournals.aob.a086378
Katul GG, Ellsworth DS, Lai CT (2000) Modeling assimilation and intercellular CO2 from measured conductance: a synthesis of approaches. Plant Cell Environ 23:1313–1328. https://doi.org/10.1046/j.1365-3040.2000.00641.x
Kim JH, Lee JW, Ahn TI, Shin JH, Park KS, Son JE (2016) Sweet pepper (Capsicum annuum L.) canopy photosynthesis modeling using 3D plant architecture and light ray-tracing. Front Plant Sci 7:1321. https://doi.org/10.3389/fpls.2016.01321
Larbi A, Vázquez S, El-Jendoubi H, Msallem M, Abadía J, Abadía A, Morales F (2015) Canopy light heterogeneity drives leaf anatomical, eco-physiological, and photosynthetic changes in olive trees grown in a high-density plantation. Photosyn Res 123:141–155. https://doi.org/10.1007/s11120-014-0052-2
Le Roux X, Grand S, Dreyer E, Daudet FA (1999a) Parameterization and testing of a biochemically based photosynthesis model for walnut (Juglans regia) trees and seedlings. Tree Physiol 19:481–492. https://doi.org/10.1093/treephys/19.8.481
Le Roux X, Sinoquet H, Vandame M (1999b) Spatial distribution of leaf dry weight per area and leaf nitrogen concentration in relation to local radiation regime within an isolated tree crown. Tree Physiol 19:181–188. https://doi.org/10.1093/treephys/19.3.181
Leuning R (1995) A critical appraisal of a combined stomatal-photosynthesis model for C3 plants. Plant Cell Environ 18:339–355. https://doi.org/10.1111/j.1365-3040.1995.tb00370.x
Léchaudel M, Lopez-Lauri F, Vidal V, Sallanon H, Joas J (2013) Response of the physiological parameters of mango fruit (transpiration, water relations, and antioxidant system) to its light and temperature environment. J Plant Physiol 170:567–576. https://doi.org/10.1016/j.jplph.2012.11.009
Marshall B, Biscoe PV (1980) A model for C3 leaves describing the dependence of net photosynthesis on irradiance. J Exp Bot 31:29–39. https://doi.org/10.1093/jxb/31.1.29
Mavengahama S, Ogunlela VB, Mariga IK (2006) Response of paprika (Capsicum annuum L.) to basal fertilizer application and ammonium nitrate topdressing in semi-arid Zimbabwe. Crop Res 32:421–429
Medina-Ruíz CA, Mercado-Luna IA, Soto-Zarazúa GM, Torres-Pacheco I, Rico-García E (2011) Mathematical modeling on tomato plants: a review. Afr J Agric Res 6:6745–6749. https://doi.org/10.5897/ajarx11.001
Noe SM, Giersch C (2004) A simple dynamic model of photosynthesis in oak leaves: coupling leaf conductance and photosynthetic carbon fixation by a variable intercellular CO2 pool. Funct Plant Biol 31:1195–1204. https://doi.org/10.1071/fp03251
Osborne CP, la Roche J, Garcia RL, Kimball BA, Wall GW, Pinter PJ, la Morte RL, Hendrey GR, Long SP (1998) Does leaf position within a canopy affect acclimation of photosynthesis to elevated CO2? Plant Physiol 117:1037–1045. https://doi.org/10.1104/pp.117.3.1037
Park KS, Bekhzod K, Kwon JK, Son JE (2016) Development of a coupled photosynthetic model of basil hydroponically grown in plant factories. Hortic Environ Biotechnol 57:20–26. https://doi.org/10.1007/s13580-016-0019-7
Pestana M, David M, de Varennes A, Abadía J, Faria EA (2001) Responses of “Newhall” orange trees to iron deficiency in hydroponics: effects on leaf chlorophyll, photosynthetic efficiency, and root ferric chelate reductase activity. J Plant Nutr 24:1609–1620. https://doi.org/10.1081/pln-100106024
Qian T, Elings A, Dieleman JA, Gort G, Marcelis LFM (2012) Estimation of photosynthesis parameters for a modified Farquhar-von Caemmerer-Berry model using simultaneous estimation method and nonlinear mixed effects model. Environ Exp Bot 82:66–73. https://doi.org/10.1016/j.envexpbot.2016.05.016
Schaffer B, Whiley AW, Searle C, Nissen RJ (1997) Leaf gas exchange, dry matter partitioning, and mineral element concentrations in mangos as influenced by elevated atmospheric carbon dioxide and root restriction. J Am Soc Hortic Sci 122:849–855. https://doi.org/10.21273/jashs.122.6.849
Shin JH, Ahn TI, Son JE (2011) Quantitative measurement of carbon dioxide consumption of a whole paprika plant (Capsicum annumm L.) using a large sealed chamber. Kor J Hortic Sci Technol 29:211–214
Sinoquet H, le Roux X, Adam B, Ameglio T, Daudet FA (2001) RATP: a model for simulating the spatial distribution of radiation absorption, transpiration and photosynthesis within canopies: application to an isolated tree crown. Plant Cell Environ 24:395–406. https://doi.org/10.1046/j.1365-3040.2001.00694.x
Sinoquet H, Thanisawanyangkura S, Mabrouk H, Kasemsap P (1998) Characterization of the light environment in canopies using 3D digitising and image processing. Ann Bot 82:203–212. https://doi.org/10.1006/anbo.1998.0665
Suharja S, Sutarno S (2009) Biomass, chlorophyll and nitrogen content of leaves of two chili pepper varieties (Capsicum annuum) in different fertilization treatments. Nusantara Biosci 1:9–16. https://doi.org/10.13057/nusbiosci/n010102
Sun G, Wang X, Sun Y, Ding Y, Lu W (2019) Measurement method based on multispectral three-dimensional imaging for the chlorophyll contents of greenhouse tomato plants. Sensors 19:3345. https://doi.org/10.3390/s19153345
Thornley JHM (1974) Light fluctuations and photosynthesis. Ann Bot 38:363–373. https://doi.org/10.1093/oxfordjournals.aob.a084820
Thornley JHM (2004) Acclimation of photosynthesis to light and canopy nitrogen distribution: an interpretation. Ann Bot 93:473–475. https://doi.org/10.1093/aob/mch051
Valladares F, Allen MT, Pearcy RW (1997) Photosynthetic responses to dynamic light under field conditions in six tropical rainforest shrubs occurring along a light gradient. Oecologia 111:505–514. https://doi.org/10.1007/s004420050264
Yin X, Struik PC, Romero P, Harbinson J, Evers JB, van der Putten PEL, Vos J (2009) Using combined measurements of gas exchange and chlorophyll fluorescence to estimate parameters of a biochemical C3 photosynthesis model: a critical appraisal and a new integrated approach applied to leaves in a wheat (Triticum aestivum) canopy. Plant Cell Environ 32:448–464. https://doi.org/10.1111/j.1365-3040.2009.01934.x
Yu KQ, Zhao YR, Zhu FL, Li XL, He Y (2016) Mapping of chlorophyll and SPAD distribution in pepper leaves during leaf senescence using visible and near-infrared hyperspectral imaging. Trans ASABE 59:13–24. https://doi.org/10.13031/trans.59.10536
Acknowledgements
This research was supported by the MSIT (Ministry of Science and ICT), Korea, under the Grand Information Technology Research Center support program (IITP-2020-0-01489) supervised by the IITP (Institute for Information & communications Technology Planning & Evaluation).
Author information
Authors and Affiliations
Contributions
Conceptualization, methodology, and investigation, DHJ, IH, JYS and JES; resources and data curation, DHJ, IH and JYS; writing and editing, DHJ and JES; funding acquisition, JES.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Young Yeol Cho, Ph.D.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jung, D.H., Hwang, I., Shin, J. et al. Analysis of leaf photosynthetic rates of hydroponically-grown paprika (Capsicum annuum L.) plants according to vertical position with multivariable photosynthesis models. Hortic. Environ. Biotechnol. 62, 41–51 (2021). https://doi.org/10.1007/s13580-020-00295-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13580-020-00295-x