Abstract
In this study, we aimed to investigate the role of SH2 domain-containing protein tyrosine phosphatase-2 (SHP-2) in cardiac remodeling after myocardial infarction (MI) and explore the underlying molecular mechanism. MI model was established by ligation of the left anterior descending coronary artery. C57/BL6J mice were randomly administered with 3.0 mg/kg/day PHPS1 (PHPS1-treated group) or normal saline (model group) by intraperitoneal injection. After 4 weeks of infusion, the effects of PHPS1 on cardiac remodeling were evaluated. Echocardiography results showed that PHPS1 treatment aggravated the MI-induced deterioration of cardiac function, with worse cardiac function parameters. PHPS1 treatment significantly increased the infarcted area, as well as the fibrotic area and the expression of collagen I and collagen III. Western blots and immunofluorescence staining showed that PHPS1 treatment up-regulated the expression of p-GRK2, p-SMAD2/3 and p-ERK1/2, while U0126 reversed the effect of PHPS1. The present study indicated that PHPS1 treatment contributed to myocardial fibrosis and infarction by activating ERK/SMAD signaling pathway, suggesting that SHP-2 may be a promising treatment target for cardiac remodeling after MI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myocardial infarction (MI), one of the most common cardiovascular diseases, is caused by acute and persistent ischemia and hypoxia of coronary arteries [1]. MI is associated with adverse left ventricular remodeling, involving cardiac fibrosis and cardiomyocyte hypertrophy, which together contribute to ischemic heart failure and alterations in cardiac architecture [2]. When MI occurs, the heart mainly relies on the proliferation of mesenchymal cells and extracellular matrix synthesis to complete remodeling [3, 4]. During acute MI, a large number of cardiomyocytes die, leading the release of damage related molecular patterns. Subsequently, the inflammatory cascade is triggered which promotes the infiltration of neutrophils and macrophages to eliminate the necrotic cardiomyocytes. With the continuous development of MI, the inflammatory cells in the areas of MI transform into a fibroblast phenotype and the fibroblasts also began to transform into cardiac fibroblast. The cardiac fibroblasts further form scar tissue with collagen fibrils as the main component. The above remodeling process can maintain the integrity and function of the heart [5]. Regrettably, continuous cardiac fibrosis after MI will lead to ventricular stiffness and decrease compliance which causes cardiac systolic and diastolic dysfunction [6]. Evidence has proved that all-cause mortality, arrhythmic events and sudden cardiac death in patients with cardiac fibrosis after MI significantly increased [7]. Slowing or reversing cardiac remodeling after MI is becoming a therapeutic strategy to prevent heart failure after MI [8]. Although there are more aggressive approaches to prevent cardiac remodeling after MI, these strategies are often turned out to be disappointed [9]. Therefore, identification of novel therapeutic targets to reduce or reverse cardiac remodeling after MI is a challenge in current cardiovascular disease research.
SH2 domain-containing protein tyrosine phosphatase-2 (SHP-2, encoded by the PTPN11 gene), is a widely expressed cytoplasmic phosphatase that is highly relevant to human health [10]. Previous study has shown that SHP2 is a master regulator of fibroblast homeostasis in idiopathic pulmonary fibrosis [11]. However, the role of SHP-2 in cardiac remodeling after MI remains unclear. Interestingly, research has showed that SHP-2 play a biological role in cancer by regulating ERK1/2 [12]. ERK1/2 signaling pathway contributes to the formation of tissue fibrosis after MI [13, 14]. Other research has showed that the SMAD2/3 signaling pathway in cardiomyocytes is significantly activated after cardiac injury [15]. More importantly, recent studies have shown that ERK1/2 and SMAD2/3 signaling pathway have a synergistic effect in promoting fibrosis production and scar formation [16, 17]. Thus, combining these published evidences, we speculated that SHP-2 may participate in cardiac remodeling after MI through ERK1/2/SMAD2/3 signaling pathways. We utilized PHPS1, a specific SHP-2 inhibitor, to investigate the role of SHP-2 in cardiac remodeling after MI and explore the underlying molecular mechanism.
Method
Animals
C57/BL6J mice (8–12 weeks, weighing 20–25 g) were purchased from Shanghai National Center for Laboratory Animals (Shanghai, China). They were housed in a pathogen-free environment (12–12 h/light–dark cycle, 55 ± 10% humidity and 22 ± 2 °C) with free access to a standard laboratory diet and water. All animal experiments were performed in accordance with the guidelines for animal care and the experimental protocols were approved by the Institutional Animal Care and Use Committee of our hospital.
Model of MI and treatment
The model of MI was induced as previously described [18]. The mice were anesthetized with isoflurane (1.5–2%) and received the thoracotomy. Ligatures were then placed around the left anterior descending coronary arteries. The successful establishment of AMI models was assessed by ST elevation. All mice were randomly assigned into 2 groups: model group (n = 9) and PHPS1-treated group (n = 9). Before surgery, the mice in PHPS1-treated group were administered with 3.0 mg/kg PHPS1 (dissolved in saline with 0.5% dimethyl sulfoxide) every day by intraperitoneal injection for 4 weeks and mice in model group were injected with an equal volume of saline with 0.5% dimethyl sulfoxide on the same days. PHPS1 was purchased from Sigma-Aldrich (No: P0039).
Triphenyltetrazolium chloride (TTC) staining
TTC staining was used to define the infarcted area. After 4 weeks of infusion, the brains were rapidly removed on ice, frozen at − 80 °C and cut into five equally thick sections in semi-frozen state. Slices were then incubated in 1% TTC in 0.1 M sodium phosphate-buffered saline (pH 7.4) at 37 °C for 25 min and fixed in 10% formalin. In the slices, red area represented normal brain tissue and the pale area was infarct tissue. The slices were measured using DPX View-Pro micro color image processing system. The infarcted area was calculated as a percentage of the area of the infarcted area to the left ventricular area.
Echocardiographic
The cardiac functions of the mice were evaluated by echocardiography. Mice were anesthetized with 4.5% vaporize isoflurane, maintained in the decubitus position, placed on a thermostatic hot plate (37 °C), and were allowed to breathe spontaneously during the procedure. Echocardiography was performed using Vevo2100 VisualSonics equipped with a 40 MHz transducer (VisualSonics Inc., Ontario, Canada). Then, mice were kept anaesthetized with 1.5% isoflurane during the echocardiographic examination. M-mode tracings of the left ventricles (LVs) were recorded at the papillary muscle level.
Masson's Trichrome staining
Masson's Trichrome staining was used to identify the fibrotic areas. Masson's trichrome staining was performed following the procedure as described previously [19]. The collagen fibers were dyed blue and the muscle fibers were dyed red. The IPP6.0 software was used to measure the fibrotic area. The fibrotic area was calculated as a percentage of the area of collagen to the total myocardial area.
Immunofluorescence (IF)
The cells cultured on a coverslip were collected and washed twice by PBS after the cells nearly grew at a logarithmic phase which were then fixed in 4% paraformaldehyde overnight. Subsequently, they were treated in Triton X-100 solution for 10 min and rinsed 3–5 times by PBS. Add 3 mL blocking solution and incubate at room temperature for 1 h. The coverslip was transferred into the incubation solution containing primary antibody for 1–2 h at room temperature or overnight at 4 °C. Secondary antibody was diluted and incubated onto coverslip for 1–2 h at room temperature. Finally, the coverslip was blocked by a cover slip with nail enamel and images were captured with Leica confocal microscope.
Western blot
The total protein was extracted from cells or tissue using RIPA lysis buffer (Thermo Fisher Scientific, United States) and quantified by bicinchoninic acid method. It was separated on a 12.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel and transferred to a polyvinylidene fluoride (PVDF) membrane (Sigma-Aldrich, United States) in turn which was blocked by 1% bovine serum albumin (BSA). Then it was immersed in an incubation solution containing-specific primary antibody and then with corresponding secondary antibody (Abcam, United Kingdom). Finally, NBT/BCIP Reagent Kit (Thermo Fisher Scientific, United States) was used to stain the PVDF membrane and ImageJ software was used to quantify the protein.
Cell culture
Human cardiac fibroblasts were purchased from Procell Life Science & Technology Co., Ltd (Wuhan, China) and maintained in CM-H078 complete culture medium (Procell Life Science & Technology Co., Ltd, china) which contained 10% fetal bovine serum (Gibco, Life Technologies), 100 U/mL of penicillin (Gibco, Life Technologies) and 100 μg/mL of streptomycin (Gibco, Life Technologies). Human cardiac fibroblasts were treated with 10 mM PHPS1. The cell lines grown in the logarithmic phase were selected for the subsequent experiments.
Statistical analysis
Results from three separate experiments were expressed as the mean ± standard deviation (mean ± SD). Students’ t test was used for comparison between the two groups. SPSS (version 22.0, SPSS Institute, IL, USA) was used to perform the statistical analysis. A p < 0.05 was considered significant.
Results
PHPS1 treatment aggravated the MI-induced deterioration of cardiac function
To investigate the role of SHP-2 in cardiac remodeling after MI, C57/BL6J mice were used to construct MI models. PHPS1, a specific SHP-2 inhibitor, was utilized to treat MI model mice. All mice were randomly assigned into a model group or PHPS1-treated group. After treatment for 4 weeks, TTC staining was used to define the infarcted area. As shown in Fig. 1a, the infarcted area (pale area) in PHPS1-treated group was obviously bigger than that in model group. In addition, the infarcted area in PHPS1-treated group was about 1.5 times that in model group (Fig. 1b). Echocardiography was performed to assess cardiac functions (Fig. 2a). Compared with the model group, ejection fraction (EF) and fraction shortening (FS), the LV anterior wall end-diastolic thickness (LVAWd), the LV anterior wall thickness (LVAWs) were significantly decreased, while the LV internal dimension at end-systole (LVIDs) and LV volume at end systole (VolS) were increased in the PHPS1-treated group (all p < 0.05, Fig. 2b). These results showed that PHPS1 treatment aggravated the MI-induced deterioration of cardiac function, which indicated the role of SHP-2 in cardiac remodeling.
PHPS1 treatment increased infarcted area after MI. a Triphenyltetrazolium chloride staining was performed to detect the infarcted area of the heart after MI in the model group (n = 9) and PHPS1-treated group (n = 9) after treatment for 4 weeks. The infarcted area appeared pale while non-infarcted area was red. b The quantification of the infarcted area in the model group and PHPS1-treated group. MI myocardial infarction; **p < 0.01
PHPS1 treatment aggravated the MI-induced deterioration of cardiac function. a left ventricle (LV) M-mode echocardiograms from MI model mice and infarcted mice treated with PHPS1. b Compared with the model group, ejection fraction (EF) and fraction shortening (FS), the LV anterior wall end-diastolic thickness (LVAWd), the LV anterior wall thickness (LVAWs) were significantly decreased, while the LV internal dimension at end-systole (LVIDs) and LV volume at end systole (VolS) were increased in the PHPS1-treated group (n = 9 pre-group). MI myocardial infarction; *p < 0.05, **p < 0.01
PHPS1 treatment promoted myocardial fibrosis after MI
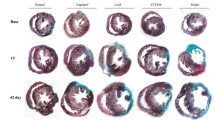
Considering the possible role of SHP-2 in cardiac remodeling, we further investigated the effect of PHPS1 treatment on cardiac fibrosis by Masson's Trichrome staining. As shown in the Fig. 3a, the fibrotic area in PHPS1-treated group was significantly bigger than that in model group (p < 0.01). It was consistent with the increase in collagen deposition that had manifested as higher expression levels of collagen I and collagen III (p < 0.01) in the PHPS1-treated group (Fig. 3b, c). These results indicated that SHP-2 may be involved in cardiac remodeling after MI by regulating cardiac fibrosis.
PHPS1 treatment promoted cardiac fibrosis after MI. a Masson's Trichrome staining was used to identify the fibrotic areas of the heart after MI in the model group (n = 9) and PHPS1-treated group (n = 9) after treatment for 4 weeks. The collagen fibers were dyed blue and the muscle fibers were dyed red. The fibrotic area was calculated as a percentage of the area of collagen to the total myocardial area. b The expression of collagen I (red fluorescence) in the fibrotic area was measured by Immunofluorescence. c The expression of collagen III (green fluorescence) in the fibrotic area was measured by Immunofluorescence. MI myocardial infarction; **p < 0.01
PHPS1 treatment activated the phosphorylation of GRK2 and SMAD2/3
Previous study showed that TGF-β plays a leading role in the process of fibrosis mainly through SMAD-dependent and SMAD-independent pathways [20,21,22]. Therefore, the expression level of related factors involved in both pathways was visualized and quantified by IF and western blot respectively. Our results showed that PHPS1 treatment promoted the fluorescence intensity of phosphorylated-GRK2 (p-GRK2) in the fibrotic area (Fig. 4a), as well as the expression of p-SMAD2/3 (Fig. 4b). In addition, the expression of p-GRK2 and p-SMAD2/3 were overlapped with Vimention (the marker of cardiac fibroblast), which indicated that the expression of p-GRK2 and p-SMAD2/3 increased in cardiac fibroblast. Corresponding to the above results, western blot (Fig. 4c) showed that PHPS1 treatment significantly enhanced the expressions of collagen I, collagen III, p-SMAD2/3 and p-GRK2 (Ser 670). These results demonstrated that PHPS1 treatment promoting cardiac fibrosis and subsequent myocardial infarction may be by regulating the expression of p-SMAD2/3 and p-GRK2 in the cardiac fibroblasts.
PHPS1 treatment activated the p-GRK2 and p-SMAD2/3 expression. a Digital images of mice cardiac fibroblast after p-GRK2 (red fluorescence) and p-SMAD2/3 (green fluorescence) immunofluorescence staining and the quantification of the expression of p-GRK2 and p-SMAD2/3. b Western blot analysis of p-GRK2 and p-SMAD2/3 expression in each group. MI myocardial infarction; *p < 0.05, **p < 0.01
PHPS1 promoted myocardial fibrosis after MI through ERK/SMAD signaling pathway
It was reported that ERK protected GRK2 from proteosomal degradation by phosphorylating GRK2 on serine 670 [23]. In the present study, we also found that the expression level of p-GRK2 and p-ERK1/2 in PHPS1-treated group was significantly higher than that in model group (p < 0.05) (Fig. 5a). To further illuminate the effects of PHPS1 on ERK1/2 signaling pathway, the cardiac fibroblasts of neonatal rat were isolated and cultured in CM-H078 complete culture medium containing transforming growth factor–β (TGF-β) or TGF-β (5 ng/mL) plus U0126 (10 μmol/L). As shown in Fig. 5b, PHPS1 treatment significantly increased the expression levels of p-GRK2 and p-ERK1/2 by IF after TGF-β induction, while decreased the expression level of t-GRK2 (Fig. 5c). In vitro experiment, we also found that PHPS1 treatment significantly increased the expression levels of p-GRK2, p-ERK1/2 and p-SMAD2/3 after TGF-β induction in human cardiac fibroblasts (Fig. 5d). U0126, the specific inhibitor of ERK1/2, was used to block the ERK1/2 signaling pathway. The results showed that U0126 reversed the effect of PHPS1 on the increase of p-GRK2 (Ser 670) and p-ERK1/2 (Fig. 5c) in mice cardiac fibroblasts. In addition, the protein level of p-SMAD2/3 significantly increased after co-treatment by PHPS1 and TGF-β which was reversed by adding U0126 (Fig. 5c). These results indicated that SHP-2 may prevent cardiac remodeling after MI through TGF-β/ERK and subsequent SMAD signaling pathways.
PHPS1 promoted myocardial fibrosis after MI through ERK/SMAD signaling pathway. a PHPS1 treatment significantly increased the expression levels of p-ERK1/2 and p-GRK2 in the mice cardiac fibroblast cells by western-blot. b PHPS1 treatment significantly increased the expression levels of p-ERK1/2 and p-GRK2 in the mice cardiac fibroblast cells by immunofluorescence. Red fluorescence represented p-ERK1/2 and green fluorescence represented p-GRK2. c U0126 could reverse the effect of PHPS1 on the increase of p-GRK2 and p-ERK1/2 and p-SMAD2/3 in the mice cardiac fibroblast cells. d PHPS1 treatment significantly increased the expression levels of p-GRK2, p-ERK1/2 and p-SMAD2/3 after TGF-β induction in human cardiac fibroblasts. MI myocardial infarction; *p < 0.05, **p < 0.01
Discussion
The adult vertebrate heart almost has no regeneration ability, so the heart mainly relies on the proliferation of mesenchymal cells and extracellular matrix synthesis to complete remodeling when MI occurs [3, 4]. However, it is inevitable that cardiac fibrosis after MI will appear. At present, clinical drugs can’t inhibit or even reverse the cardiac fibrosis which results in poor prognosis, so it is urgent to find new drug targets. In this study, we found that PHPS1, the specific inhibitors of SHP2, increased cardiac fibrosis and infarcted area after MI, aggravated the MI-induced deterioration of cardiac function. These results demonstrated that SHP-2 played a positively role in promoting cardiac remodeling after MI which provides a new molecular target for early prevention of cardiac fibrosis after MI and drug development.
Next, we investigated the underlying molecular mechanism of SHP-2 in preventing cardiac remodeling after MI. Previous study showed that genetic or pharmacologic inactivation of SHP2 inhibits TGF-β induced fibroblast activation and ameliorates dermal and pulmonary fibrosis [24]. The TGF-β family played an important role in regulating cellular processes such as cell proliferation, differentiation or cell death, which was critical to the homeostasis of organs and tissues [25]. Other studies showed that TGF-β played a leading role in the process of fibrosis mainly through SMAD-dependent and SMAD-independent pathways [20,21,22]. In this study, we found that PHPS1 treatment up-regulated the expression of p-GRK2 and p-SMAD2/3, which indicated that PHPS1 treatment promoting cardiac fibrosis and subsequent MI may be by regulating the expression of p-SMAD2/3.
ERK was reported to protect GRK2 from proteosomal degradation by phosphorylating GRK2 on serine 670 [23]. In addition, evidences showed that SHP-2 played an important role in a variety of solid tumors and Leukemia through RAS-ERK signaling pathway [12, 26, 27]. Ivins et al. found that the deletion of SHP-2 in mice fibroblasts reduced phosphorylation of Akt and ERK1/2 expression [28]. In addition, Chen et al. found that PHPS1 treatment suppressed ERK phosphorylation in vascular smooth muscle cells and showed anti-atherosclerotic effects [29]. Contrary to the above reports, the results in the present study showed that PHPS1 treatment up-regulated the expression of p-GRK2, p-SMAD2/3 and p-ERK1/2, while U0126 reversed the effect of PHPS1. Interestingly, the study had found that ERK1/2 played an important role in the proliferation of fibrotic cells and excessive deposition of collagen to promote scar formation, which reminded us the potential relation between SHP-2 and ERK1/2 in cardiac remodeling after MI [13, 14]. More importantly, recent studies had shown that ERK1/2 and SMAD2/3 signaling pathway had a synergistic effect in promoting fibrosis production and scar formation [16, 17]. These results indicated that SHP-2 may prevent cardiac remodeling after MI through TGF-β/ERK and subsequent SMAD signaling pathways.
SHP-2 could activate or inhibit ERK1/2 activity in different biological processes or under different stimulation, suggesting that SHP-2 had various functions. However, the underlying molecular mechanism of SHP-2 directly or indirectly regulating ERK1/2 was not included in this study, and we attempt to explain this. SHP-2 had been considered an important mediator of the inhibitory receptor (IR) signalling. These IRs contained intracellular SHP-2-binding immunoreceptor tyrosine-based inhibitory motifs (ITIMs) which could recruit SHP-2 to the intracellular tail of IRs to antagonize activating cascades [30]. It was reported that SHP-2 could interact with the cytoplasmic tail of programmed cell death 1 and thereby inhibit T cell activation pathways by dephosphorylates TCR signaling molecules, including ERK1/2 [31]. It may be explained by that IRs reduced the availability of SHP-2, thus preventing its contribution in activating ERK cascade according to Yokosuka [10]. Additionally, SHP-2 could combine to the ITIMs of PZR protein, promoting SHP2-dependent migration over a fibronectin [32]. Therefore, these seemed to explain the negative effect of SHP-2 on ERK1/2 and SMAD signaling pathways in cardiac remodeling after MI. However, the detailed regulation mechanism remains to be further explored.
Conclusions
In conclusion, the present study showed that PHPS1 treatment significantly increased cardiac fibrosis and infarcted area after MI, aggravated the MI-induced deterioration of cardiac function. In addition, PHPS1 treatment up-regulated the expression of p-GRK2, p-SMAD2/3 and p-ERK1/2, while U0126 reversed the effect of PHPS1. Taken together, these results demonstrated that PHPS1 treatment contributed to cardiac remodeling after MI by activating ERK/SMAD signaling pathway, suggesting that SHP-2 may be a promising treatment target for cardiac remodeling after MI.
Data availability statement
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Khan M, Kwiatkowski P, Rivera BK, Kuppusamy P. Oxygen and oxygenation in stem-cell therapy for myocardial infarction. Life Sci. 2010;87(9–10):269–74.
Martínez-Martínez E, Buonafine M, Boukhalfa I, Ibarrola J, Fernández-Celis A, Kolkhof P, et al. Aldosterone target NGAL (Neutrophil Gelatinase-Associated Lipocalin) is involved in cardiac remodeling after myocardial infarction through NFκB pathway. Hypertension (Dallas, Tex: 1979). 2017;70(6):1148–56. https://doi.org/10.1161/hypertensionaha.117.09791.
Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. 2014;11(5):255–65. https://doi.org/10.1038/nrcardio.2014.28.
Huang S, Frangogiannis NG. Anti-inflammatory therapies in myocardial infarction: failures, hopes and challenges. Br J Pharmacol. 2018;175(9):1377–400. https://doi.org/10.1111/bph.14155.
Frangogiannis NG. The role of transforming growth factor (TGF)-β in the infarcted myocardium. J Thorac Dis. 2017;9(Suppl 1):S52–s63. https://doi.org/10.21037/jtd.2016.11.19.
Kanisicak O, Khalil H, Ivey MJ, Karch J, Maliken BD, Correll RN, et al. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat Commun. 2016;7(1):1–14.
Moore-Morris T, Guimarães-Camboa N, Banerjee I, Zambon AC, Kisseleva T, Velayoudon A, et al. Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. J Clin Investig. 2014;124(7):2921–34. https://doi.org/10.1172/jci74783.
Liu Y, Baumgardt SL, Fang J, Shi Y, Qiao S, Bosnjak ZJ, et al. Transgenic overexpression of GTP cyclohydrolase 1 in cardiomyocytes ameliorates post-infarction cardiac remodeling. Sci Rep. 2017;7(1):3093. https://doi.org/10.1038/s41598-017-03234-6.
Hu J, Zhang L, Zhao Z, Zhang M, Lin J, Wang J, et al. OSM mitigates post-infarction cardiac remodeling and dysfunction by up-regulating autophagy through Mst1 suppression. Biochim Biophys Acta. 2017;1863(8):1951–61. https://doi.org/10.1016/j.bbadis.2016.11.004.
Niogret C, Birchmeier W, Guarda G. SHP-2 in lymphocytes' cytokine and inhibitory receptor signaling. Front Immunol. 2019;10:2468. https://doi.org/10.3389/fimmu.2019.02468.
Tzouvelekis A, Yu G, Herazo-maya J, Xylourgidis N, Herzog E, Bennett A, et al. SH2 domain-containing phosphatase-SHP-2 is a novel regulator of fibroblast homeostasis in Pulmonary Fibrosis. QJM. 2016;109(suppl_1):S20-S.
Liu X, Li Y, Zhang Y, Lu Y, Guo W, Liu P, et al. SHP-2 promotes the maturation of oligodendrocyte precursor cells through Akt and ERK1/2 signaling in vitro. PLoS ONE. 2011;6(6):e21058. https://doi.org/10.1371/journal.pone.0021058.
Bandyopadhyay B, Han A, Dai J, Fan J, Li Y, Chen M, et al. TbetaRI/Alk5-independent TbetaRII signaling to ERK1/2 in human skin cells according to distinct levels of TbetaRII expression. J Cell Sci. 2011;124(Pt 1):19–24. https://doi.org/10.1242/jcs.076505.
Otsuka M, Goto K, Tsuchiya S, Aramaki Y. Phosphatidylserine-specific receptor contributes to TGF-beta production in macrophages through a MAP kinase. ERK Biol Pharm Bull. 2005;28(9):1707–10. https://doi.org/10.1248/bpb.28.1707.
Hao J, Ju H, Zhao S, Junaid A, Scammell-La Fleur T, Dixon IM. Elevation of expression of Smads 2, 3, and 4, decorin and TGF-beta in the chronic phase of myocardial infarct scar healing. J Mol Cell Cardiol. 1999;31(3):667–78. https://doi.org/10.1006/jmcc.1998.0902.
Odekerken JC, Walenkamp GH, Brans BT, Welting TJ, Arts JJ. The longitudinal assessment of osteomyelitis development by molecular imaging in a rabbit model. Biomed Res Int. 2014;2014:424652. https://doi.org/10.1155/2014/424652.
Patel M, Rojavin Y, Jamali AA, Wasielewski SJ, Salgado CJ. Animal models for the study of osteomyelitis. Semin Plast Surg. 2009;23(2):148–54. https://doi.org/10.1055/s-0029-1214167.
Nishiya D, Omura T, Shimada K, Matsumoto R, Kusuyama T, Enomoto S, et al. Effects of erythropoietin on cardiac remodeling after myocardial infarction. J Pharmacol Sci. 2006;101(1):31–9. https://doi.org/10.1254/jphs.fp0050966.
Shen S, Jiang H, Bei Y, Zhang J, Zhang H, Zhu H, et al. Qiliqiangxin attenuates adverse cardiac remodeling after myocardial infarction in ovariectomized mice via activation of PPARγ. Cell Physiol Biochem. 2017;42(3):876–88. https://doi.org/10.1159/000478641.
Biernacka A, Dobaczewski M, Frangogiannis NG. TGF-β signaling in fibrosis. Growth Fact (Chur, Switzerland). 2011;29(5):196–202. https://doi.org/10.3109/08977194.2011.595714.
Yu L, Hébert MC, Zhang YE. TGF-beta receptor-activated p38 MAP kinase mediates Smad-independent TGF-beta responses. EMBO J. 2002;21(14):3749–59. https://doi.org/10.1093/emboj/cdf366.
Massagué J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–91. https://doi.org/10.1146/annurev.biochem.67.1.753.
Im YN, Lee YD, Park JS, Kim HK, Im SY, Song HR, et al. GPCR Kinase (GRK)-2 is a key negative regulator of itch: l-glutamine attenuates itch via a rapid induction of GRK2 in an ERK-dependent way. J Invest Dermatol. 2018;138(8):1834–42. https://doi.org/10.1016/j.jid.2018.02.036.
Zehender A, Huang J, Györfi AH, Matei AE, Trinh-Minh T, Xu X, et al. The tyrosine phosphatase SHP2 controls TGFβ-induced STAT3 signaling to regulate fibroblast activation and fibrosis. Nat Commun. 2018;9(1):3259. https://doi.org/10.1038/s41467-018-05768-3.
Fabregat I, Moreno-Càceres J, Sánchez A, Dooley S, Dewidar B, Giannelli G, et al. TGF-β signalling and liver disease. FEBS J. 2016;283(12):2219–32. https://doi.org/10.1111/febs.13665.
Fiorentini C, Savoia P, Savoldi D, Barbon A, Missale C. Persistent activation of the D1R/Shp-2/Erk1/2 pathway in l-DOPA-induced dyskinesia in the 6-hydroxy-dopamine rat model of Parkinson's disease. Neurobiol Dise. 2013;54:339–48. https://doi.org/10.1016/j.nbd.2013.01.005.
Rosário M, Birchmeier W. How to make tubes: signaling by the Met receptor tyrosine kinase. Trends Cell Biol. 2003;13(6):328–35. https://doi.org/10.1016/s0962-8924(03)00104-1.
Ivins Zito C, Kontaridis MI, Fornaro M, Feng GS, Bennett AM. SHP-2 regulates the phosphatidylinositide 3'-kinase/Akt pathway and suppresses caspase 3-mediated apoptosis. J Cell Physiol. 2004;199(2):227–36. https://doi.org/10.1002/jcp.10446.
Chen J, Cao Z, Guan J. SHP2 inhibitor PHPS1 protects against atherosclerosis by inhibiting smooth muscle cell proliferation. BMC Cardiovasc Disord. 2018;18(1):72. https://doi.org/10.1186/s12872-018-0816-2.
Salmond RJ, Alexander DR. SHP2 forecast for the immune system: fog gradually clearing. Trends Immunol. 2006;27(3):154–60. https://doi.org/10.1016/j.it.2006.01.007.
Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, Hashimoto-Tane A, Azuma M, Saito T. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med. 2012;209(6):1201–17. https://doi.org/10.1084/jem.20112741.
Zannettino AC, Roubelakis M, Welldon KJ, Jackson DE, Simmons PJ, Bendall LJ, et al. Novel mesenchymal and haematopoietic cell isoforms of the SHP-2 docking receptor, PZR: identification, molecular cloning and effects on cell migration. Biochem J. 2003;370(Pt 2):537–49. https://doi.org/10.1042/bj20020935.
Funding
This work was supported by Prevention and Control of Geriatric Diseases in 2018 [number 2018135809-2].
Author information
Authors and Affiliations
Contributions
Conception and design, YT and YL; Data collection, YL, HT, QM and XL; Data analysis and interpretation, JC and XZ; Drafting article, YL, XL and YT; Administrative support, YT. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All animal experiments were performed in accordance with the guidelines for animal care and the experimental protocols were approved by the Institutional Animal Care and Use Committee of our hospital.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lu, YG., Tan, H., Ma, Q. et al. SH2 domain-containing protein tyrosine phosphatase-2 (SHP-2) prevents cardiac remodeling after myocardial infarction through ERK/SMAD signaling pathway. Human Cell 34, 325–334 (2021). https://doi.org/10.1007/s13577-020-00430-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-020-00430-x