Abstract
The aim of this study is to investigate the effect of lncRNA DUXAP8 on proliferation and apoptosis of ovarian cancer cells, and to explore its potential mechanism. DUXAP8 interfering and overexpressing cell lines were constructed and the cell proliferation and apoptosis were tested. Hematoxylin–eosin, TdT-mediated dUTP nick end labeling, and immunohistochemistry were used to detect the effect of DUXAP8 on the ability of tumor formation. Quantitative real-time polymerase chain reaction and western blot were used to detect the mRNA and protein expression of miR-590-5p and YAP1, respectively. Dual luciferase assay was used to determine the target relationship between DUXAP8, miR-590-5p, and YAP1. DUXAP8 interference and miR-590-5p down-regulated cell lines were further constructed. Compared with normal ovarian cells, the expression of DUXAP8 in ovarian cancer cells was significantly increased, while the expression of miR-590-5p was decreased (p < 0.05). After DUXAP8 interference, cell proliferation and colony formation were decreased, and apoptosis was increased. The results of in vivo experiment are consistent with the in vitro experiments. The expression of miR-590-5p was up-regulated and the expression of YAP1 was decreased after DUXAP8 interference. Moreover, miR590-5p inhibitor can attenuate the effect of DUXAP8 interference on ovarian cancer cells. Taken together, lncRNA DUXAP8 can regulate the proliferation and apoptosis of ovarian cancer cells, and its mechanism may be related to the regulation of YAP1 gene by targeting miR-590-5p.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian cancer has become the leading cause of death among gynecological cancers [1]. The incidence and mortality of ovarian cancer increase with age, and most cases of ovarian cancer occur in women over the age of 50 [2]. Due to its asymptomatic development, ovarian cancer is often diagnosed at an advanced stage of incurability [3]. Although chemotherapy based on platinum compounds and taxanes is the standard treatment for ovarian cancer, majority of patients will experience recurrent disease and chemotherapy resistance [4]. Thus, new therapeutic targets are needed to improve outcomes for women with this disease.
Long noncoding RNAs (lncRNAs) are a class of RNAs more than 200 nucleotides in length and do not possess the protein-coding function. Previous studies have shown that the dysregulation of lncRNAs expression plays important role in tumorigenesis [5, 6]. lncRNAs exhibit multiple biological functions in various stages of cancer development, including cell development and immunity, cell proliferation and differentiation, modulation of apoptosis, function as markers of genomic imprinting, and act as competing endogenous RNAs (ceRNAs) that share micro RNAs (miRNA) biding sites [7,8,9]. Accumulating researches of ovarian cancer have focused on nonprotein coding genes, and shown that the dysregulation of lncRNA greatly contributes to malignant phenotypical changes [10, 11]. Therefore, lncRNA may be used as a biomarker for the treatment of ovarian cancer.
DUXAP8, a pseudogene-derived lncRNA, was located on chromosome 20q11 with 2307 bp in length. The gene sequence of lncRNA DUXAP8 was detected in tumor cell lines and has a regulatory role in cell cycle and reproductive development [12]. Numerous studies have revealed that DUXAP8 has participated in the development of many cancers. A study has demonstrated that DUXAP8 could promote gastric cancer cell proliferation and migration [13]. Another study also shown that DUXAP8 promotes growth of pancreatic carcinoma cells by epigenetically silencing CDKN1A and KLF2 [14]. However, the role and potential molecular mechanisms of DUXAP8 in ovarian cancer are still not elucidated. Thus, it is significant to investigate the effects and potential underlying mechanisms of the DUXAP8 on the tumorigenesis and progression of ovarian cancer.
Materials and methods
Cell lines and culture conditions
Ovarian cancer cells, SKOV3 and OVCAR3, were purchased from ATCC (USA) and cultured in RPMI 1640 medium (Gibco, Rockville, MD, USA) at 37 ˚C in a cell culture incubator (Thermo Fisher Scientific, Waltham, USA) with 5% of CO2 and saturated humidity. The RPMI 1640 medium was supplemented with 10% of fetal bovine serum (Sigma-Aldrich, St. Louis, MO, USA), 100 U/ml of penicillin, and 100 mg/ml of streptomycin. Cells in the logarithmic phase of growth were used in the experiments.
Cell transfections
DUXAP8 interference vector 1 and 2 (DUXAP8 siRNA-1#: 5ʹ-GGAACTTCCCAAACCT CCATGATTT-3ʹ; siRNA-2#: 5ʹ-CAGCATACTTCAAATTCACAGCAAA-3ʹ) and control vector, overexpression vector and control vector were provided by Shanghai Gemma Gene Company (GenePharma, Shanghai). DUXAP8 siRNA sequence is UUUAGACCCAUUCUCGUAUGGAGGU and siRNA-NC sequence is UUCUCCGAACGUGUCACGUTT. miR-590-5p mimic (5′-CAGGCCGAUUGCGAUGCAAUA-3′), mimic-NC (5′-GUCCAGUGAAUU CCCAG-3′), miR-590-5p inhibitor (5′-AAAUAUGCUGUAUGUCA UGUGUU-3′), and inhibitor NC (5′-CAGUACUUUUGUGUAGUACAA-3′) were purchased from Guangzhou Ruibo Biotechnology Co., Ltd. (Guangzhou, China). The cells were digested with 0.25% of trypsin (Invitrogen, Carlsbad, CA, USA) and 2 × 105 cells in logarithmic growth phase were seeded in 6-well plates. After 24 h, the cell growth was observed under inverted microscope until the cell density reached 30–50%. DUXAP8 overexpression vector (DUXAP8 mimic group) and empty vector control (mimic-NC group), DUXAP8 interference vector (DUXAP8 siRNA group) and control (siRNA-NC group) were used to analysis the effects of DUXAP8 on ovarian cancer cells. miR-590-5p mimic (miR-590-5p mimic group) and mimic NC (NC group) were used to analysis the effects of miR-590-5p on ovarian cancer cells. DUXAP8 interference vector (DUXAP8 siRNA group) and control (siRNA-NC group), DUXAP8 interference + miR-590-5p inhibitor (DUXAP8 siRNA + miR inhibitor group), and DUXAP8 interference + inhibitor NC (DUXAP8 siRNA + miR inhibitor NC group) were used to analysis whether DUXAP8 works through miR-590-5p. The above plasmid or oligonucleotide was transfected into ovarian cancer cell lines SKOV3 and OVCAR3 using Lipofectamine 2000 (Thermo Fisher Scientific, USA) according to the instructions.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNAs were extracted from tissues or cells using MagMAX™ MiRVana™ Total RNA Isolation Kit (Thermo Fisher Scientific, Waltham, USA). Total RNAs were then reversely transcribed into cDNA using the reverse transcription kit (Roche, Indianapolis, IN, USA). qRT-PCR was performed using the SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA). DUXAP8, miR-590-5p, and YAP1 primers were synthesized by Shanghai Shenggong Bioengineering Co., Ltd. And the cycling conditions are as follows: 95 ˚C for 10 min, and then 40 cycles of 95 ˚C for 15 s, 60 ˚C for 60 s. Relative expression levels were calculated via the 2−ΔΔCt method. U6 is the internal reference of miR-590-5p, and GAPDH is the internal reference of DUXAP8 and YAP1. Primers sequences are as following: miR-590-5p forward: 5′-TCCATTGAAACGCCTAGGAGAATTTGC-3′; miR-590-5p reverse: 5′-GCAAATTCTCCTAGGCGTTTCAATGGA-3′; U6 forward: 5′-GCTTCGGCAGCACATATACTAAAAT-3′; U6 reverse: 5′-CGCTTCAGAATTTGCGTGTCAT-3′; DUXAP8 forward: 5′-AGACGCCATGGAACAT-3′; DUXAP8 reverse: 5′-AAGCGGAGACCTGAGGAG-3′; YAP1 forward: 5′-TAGCCCTGCGTAGCCAGTTA-3′; YAP1 reverse: 5′-TCATGCTTAGTCCACTGTCTGT-3′; GAPDH forward: 5′-CGCTCTCTGCTCCTCCTGTTC-3′; GAPDH reverse: 5′-ATCCGTTGACTCCGACCTTCAC-3'.
Cell counting assay kit-8 (CCK-8) assay
Cells were planted into 96-well plates at a density of 1 × 105 cells/ml with 100 ul per well, and set three parallel holes. Then, 10 ul CCK-8 solution (PA137267, Pierce) was added to each well after 24 h, 48 h, 72 h, and 96 h. After cultured for another 4 h, the absorbance (OD) value of each well was measured at 450 nm by enzyme-linked immunosorbent assay.
Clone formation
The logarithmic growth phase cells were digested with 0.25% trypsin and blown into individual cells. Then, the cells were suspended in complete medium and diluted by a gradient. Each group of cells was inoculated separately into a 37 °C pre-warmed dish at a density of 200 cells. The cells were cultured in a 37 °C, 5% CO2 environment for 2 weeks. The cells were fixed with 4% paraformaldehyde for 15 min and stained by crystal violet solution for 30 min (RBG1019-1, Roles-Bio), then washed twice in ultrapure water. After air drying, the picture was taken by a camera.
Cell apoptosis assay
The cell apoptosis was detected using the Annexin V-FITC/PI kit (70-AP101-60, Multi Sciences, Hangzhou Lianke Biotechnology Co., Ltd.). After centrifuged at 1000 rpm for 5 min, the cells were resuspended in 1 × binding buffer. Among them, 400 ul of 1 × binding buffer was used for the control, and 100 ul of 1 × binding buffer was used for other groups. 100 ul above solution was taken to the flow tube, then incubated with Annexin V-FITC and propidium iodide (PI) at 37 °C. The control cells were divided into: non-stained group, single-stained Annexin V group, single-stained PI group, and double-stained Annexin V PI group. After incubation in the dark for 15 min, the cells were diluted in 400 ul 1 × binding buffer and analyzed using a flow cytometry (BD FACSCanto II, Beijing Delica Biotechnology Co., Ltd.).
Experimental animals
Thirty male and female BALB/C nude mice (7–8 weeks, 20 ± 2 g) were purchased from Jinan Pengyue Experimental Animal Breeding Co., Ltd., license number SCXK (Lu) 2014-0007. The mice were kept in a specific pathogen-free (SPF) laboratory animal facility under room temperature with a relative humidity of 50–60%. Animal experiments were conducted following the National Institute of Health (NIH) guidelines (NIH Pub. No. 85-23, revised 1996). The experiments have been reviewed and approved by the Animal Protection and Use Committee of Jinan Maternity and Child Care Hospital.
Construction of nude mouse xenograft model
The mice were randomly divided into Model group, DUXAP8 mimic group, mimic-NC group, DUXAP8 siRNA group, and siRNA-NC group. Cells (SKOV3 and OVCAR3) in the logarithmic phase of growth were collected and trypsinized into cell suspension with the concentration adjusted to 1.0 × 107 cells/ml. To establish a subcutaneous nude mouse xenograft model, 100 ul of cell suspension was injected subcutaneously into the right flank of each mouse.
The tumor size was measured every week with vernier caliper. The tumor volume (V) was calculated using the following equation: V = a*b2/2 (a: tumor longest diameter; b: tumor shortest diameter). Tumor was measured on a weekly basis for 5 weeks and the tumor growth curve was drawn. Six weeks later, nude mice were anesthetized with 2% sodium pentobarbital (50 mg/kg, New Asiatic Pharmaceutical, China) and sacrificed by cervical dislocation.
Immunohistochemistry
The ovarian cancer tissues were deparaffinized with xylene, and then hydrated in an ethanol gradient solution. Following incubate in 3% H2O2 at room temperature for 10 min to block endogenous peroxidase activity. The slices were blocked with 5% goat serum (Gibco, USA) for 20 min, and then incubated with rabbit anti-human anti-Ki67 antibody (1:1000, ab15580, Abcam, UK) overnight at 4 °C. Samples were then incubated with horseradish peroxidase-labeled goat anti-rabbit IgG antibody (11:1000, ab6721, Abcam, UK) at 37 °C for 40 min. 3,3′-Diaminobenzidine staining, hematoxylin counterstaining for 3 min, dehydration and sealed. The expression of Ki67 + in each group was observed under microscope (Olympus, Japan).
TUNEL staining
TUNEL staining was performed using In Situ Cell Death Detection Kit (Roche). Paraffin sections were dewaxed with xylene, dehydration with gradient ethanol, and treated with proteinase K for 15–30 min. Then, TUNEL reaction mixed droplets were prepared and added to the specimen, reacted in a dark humid chamber at 37 °C for 1 h. After stained with 3,3′-diaminobenzidine and hematoxylin, dehydration and sealed, the apoptotic cells were observed under light microscope (Olympus, Japan) and photographed.
Dual luciferase reporter assay
Target genes for DUXAP8 and miR-590-5p were predicted using the TargetScan, miRanda, and miRDB databases. Full-length amplification of DUXAP8 and YAP1 wild type and mutant using overlapPCR. pmirGIO vector was cleaved with XhoI and Xbal double enzymes. The DUXAP8 and YAP1 target fragments and the pmirGIO double-adhesive vector were constructed into pmirGIO-DUXAP8 and pmirGIO-YAP1 recombinant vectors. SKOV3 cells were seeded at 2 × 105 cells/well in 24-well plates, the recombinant reporter vector was transfected into cells with LipofectamineTM2000 at cells grown to about 70–80%. Luciferase activity was measured 24 h after transfection using the dual luciferase reporter system (Promega, Madison, WI, USA).
Western blot
The total proteins were extracted and protein concentration was measured using bicinchoninic acid (BCA) protein assay kit (23,225, Pierce™ BCA Protein Assay Kit, Thermo Fisher Scientific, Waltham, USA). 40 μg protein sample was separated on SDS-PAGE 10% gel (Mini-Protean-3 type, Bio-Rad, Hercules, CA, USA) and then transferred to a PVDF membrane (Millipore, Massachusetts, USA). Membranes were blocked with 5% not-fat milk for 1 h and followed an incubation with primary antibodies overnight at 4 °C, including rabbit anti-human anti-YAP1 (1:1000, ab56701, Abcam) and anti-β-actin (1:2000, orb178392, Biorbyt, Cambridge, UK). Subsequently, the membranes were incubated with horseradish peroxidase-labeled goat anti-rabbit secondary antibody (1:2000, ab6721, Abcam) at room temperature for 2 h. The blots were detected use the ECL system (Thermo Fisher Scientific, Inc.), and the expression levels were quantified by ImageJ software (NIH). β-actin was used as an internal control.
Statistical analysis
Data processing was performed using SPSS 19.0 statistical analysis software, and the results of data analysis were expressed as mean ± standard deviation (mean ± SD). Data analysis was performed between the two groups by t test. One-way analysis of variance (ANOVA) was used for data analysis among multiple groups. Tukey’s test was used for subsequent analysis. The difference was statistically significant at p < 0.05.
Results
The expression of DUXAP8 in different ovarian cancer cell lines
The results in (Fig. 1) clearly showed that the expression of DUXAP8 was clearly decreased after transfection of siRNA-1# and siRNA-2# (p < 0.05). Further, the interference effect of siRNA-2# on DUXAP8 in SKOV3 and OVCAR3 cells was significantly stronger than that of siRNA-1# (p < 0.05).
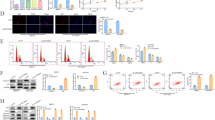
Effects of DUXAP8 on the proliferation and apoptosis of ovarian cancer cells
Figure 2a showed that the expression of DUXAP8 was significantly down-regulated in DUXAP8 siRNA-1# and siRNA-2#group, and the expression of DUXAP8 was significantly up-regulated in DUXAP8 mimic group (p < 0.05). The cell proliferation ability and colony formation were significantly decreased after DUXAP8 interference in SKOV3 and OVCAR3 cells (Fig. 2b, c, p < 0.05). The flow cytometry results showed that DUXAP8 interference promoted apoptosis (Fig. 2d, p < 0.05). By contrast, the DUXAP8 mimic group showed diametrically opposite results. These results indicated that DUXAP8 could regulate the proliferation and apoptosis of ovarian cancer cells.
Effects of DUXAP8 on the proliferation and apoptosis of ovarian cancer cells in SKOV3 and OCVAR3 cells (n = 3). a qRT-PCR was used to detect the expression of DUXAP8; (b) CCK8 was used to detect the cell proliferation; (c) Cloning formation experiment; (d) Flow cytometry was used to detect apoptosis. *p < 0.05 compared with control group; #p < 0.05 compared with mimic-NC group; ^p < 0.05 compared with siRNA-NC group; &p < 0.05 compared with DUXAP8 mimic group
Effect of DUXAP8 on the proliferation and apoptosis of ovarian cancer cells in nude mice transplanted tumor model
Compared with the siRNA-NC group, the expression of miR-590-5p was significantly increased in the siRNA-1# and siRNA-2# group, while the expression of YAP1 has the opposite trend (p < 0.05, Fig. 3a, b). As shown in (Fig. 3c, d), compared with the siRNA-NC group, the growth rate, volume, and the weight of tumor were significantly reduced in siRNA-1# and siRNA-2#group (p < 0.05). Furthermore, TUNEL assay showed that DUXAP8 interference promoted apoptosis in cells (Fig. 3e). On the contrary, the DUXAP8 mimic group showed diametrically opposite results. Ki-67 detection showed that compared with the siRNA-NC group, the pathological damage was reduced and the ovarian cancer proliferation ability was diametrically decreased in the siRNA-1# and siRNA-2# group (Fig. 3f). These results indicated that DUXAP8 could regulate the proliferation and apoptosis of ovarian cancer cells in nude mice transplanted tumor model.
Effects of DUXAP8 on the proliferation and apoptosis of ovarian cancer cells in nude mice transplanted tumor model (n = 3). qRT-PCR was used to detect the expression of miR-590-5p (a) and YAP1 (b) in tumor (n = 3); Tumor weight (c), volume (d); (e) TUNEL staining was used to observe the apoptosis (n = 3, 400 ×); F Immunohistochemistry was used to detect Ki-67 positive cells (n = 3, 400 ×). *p < 0.05 compared with control group; #p < 0.05 compared with mimic-NC group; ^p < 0.05 compared with siRNA-NC group; &p < 0.05 compared with DUXAP8 mimic group
Effect of DUXAP8 on miR-590-5p
The ovarian cancer cell lines, SKOV3 and OVCAR3, with high expression of DUXAP8 were selected to construct a DUXAP8 interfering cell line. qRT-PCR showed that compared with the siRNA-NC group, the expression of miR-590-5p was significantly increased in the DUXAP8 siRNA group (p < 0.05), indicating that DUXAP8 negatively regulates miR-590-5p expression (Fig. 4a). Targetscan, miRanda, miRDB software showed that miR-590-5p was a potential target of DUXAP8 (Fig. 4b). To further verify whether DUXAP8 targeted miR-590-5p, a dual luciferase reporter system was used. The results showed that miR-590-5p reduced luciferase activity of DUXAP8 containing WT 3′UTR (p < 0.05), but did not decrease luciferase activity of DUXAP8 containing Mut 3′UTR (p > 0.05), which demonstrating that miR-590-5p is a target gene of DUXAP8 in cells (Fig. 4b).
DUXAP8 regulates the proliferation and apoptosis of ovarian cancer cells via regulating miR-590-5p
DUXAP8 siRNA group, siRNA-NC group, DUXAP8 siRNA + miR inhibitor group, and DUXAP8 siRNA + miR inhibitor NC group were constructed by siRNA2#, and the biological behaviors such as proliferation ability (Fig. 5a), colony formation (Fig. 5b) and apoptosis (Fig. 5c) were detected. The results showed that after DUXAP interference, the cell proliferation ability and colony formation were inhibited, and apoptosis rate was increased. Those biological behaviors in DUXAP8 + miR inhibitor NC group were similar with DUXAP8 siRNA group. However, the treatment of miR inhibitor can attenuate the above effects of DUXAP8 on cells. All these findings suggested that DUXAP8 regulates the proliferation and apoptosis of ovarian cancer cells by regulating miR-590-5p.
DUXAP8 regulates the proliferation and apoptosis of ovarian cancer cells via regulating miR-590-5p (n = 3). a CCK8 was used to detect the cell proliferation; (b) Cloning formation experiment; (c) Flow cytometry was used to detect apoptosis. ^p < 0.05 compared with siRNA-NC group; %p < 0.05 compared with DUXAP8 siRNA + miR inhibitor NC group
DUXAP8 promotes the expression of YAP1 via regulating miR-590-5p in ovarian cancer cells
Targetscan, miRanda, and miRDB software showed that YAP1 was a target of miR-590-5p (Fig. 6a). Dual luciferase reported that miR-590-5p reduced luciferase activity of YAP1 containing WT 3′UTR, but did not reduce the luciferase activity of YAP1 containing Mut 3′UTR (p < 0.05), which demonstrating that YAP1 is a target gene of miR-590-5p in cells (Fig. 6a). qRT-PCR result showed that the expression of miR-590-5p was significantly up-regulated after miR-590-5p mimic transfection (p < 0.05, Fig. 6b). Meanwhile, the mRNA and protein expression of the YAP1 were significantly down-regulated after miR-590-5p mimic transfection (p < 0.05, Fig. 6c, d). Further studies showed that DUXAP8 interference reduced the expression of YAP1, while the treatment of miR inhibitor could attenuate the effects of DUXAP8 on the expression of YAP1 (p < 0.05, Fig. 6e, f). All these results indicated that DUXAP8 promotes the expression of the YAP1 via inhibiting the expression of miR-590-5p in ovarian cancer cells.
DUXAP8 promotes the expression of YAP1 via regulating miR-590-5p in ovarian cancer cells (n = 3). a DUXAP8 and miR-590-5p binding sites; (b) qRT-PCR was used to detect the mRNA expression of miR-590-5p; (c) qRT-PCR was used to detect the mRNA expression of YAP1 after miR-590-5p overexpression treatment; (d) Western blot was used to detect the protein expression of YAP1; (e) qRT-PCR was used to detect the mRNA expression of YAP1 after DUXAP8 and miR-590-5p inhibitor treatment; (f) Western blot was used to detect the protein expression of YAP1 after DUXAP8 and miR-590-5p inhibitor treatment. *p < 0.05 compared with NC group; ^p < 0.05 compared with siRNA-NC group; %p < 0.05 compared with DUXAP8 siRNA + miR inhibitor NC group
Discussion
The signaling network of lncRNAs regulated cancer development is complex and needs thorough investigations. Previous studies have shown that lncRNAs can regulate gene expression by complementary binding with miRNAs [15,16,17,18]. In this study, through predicted by databases and dual luciferase assay, we found that DUXAP8 could complement binding with miR-590-5p, and YAP1 is a miR-590-5p target gene. Previous study shown that YAP1 can promote the occurrence and development of ovarian cancer [19]. In view of this, we examined the expression of DUXAP8 in different ovarian cancer cells and analyzed whether DUXAP8 involved in ovarian cancer cell proliferation and apoptosis by in vivo and in vitro experiments, and further explored the molecular mechanism of DUXAP8 exerting its biological function.
lncRNA DUXAP8 is a newly discovered cancer-promoting lncRNA, which have participated in the development of lots cancers [13, 14, 20]. We focused on several fundamental characteristics of ovarian cancer and found that DUXAP8 could regulate the proliferation and apoptosis of ovarian cancer cells in vivo and in vitro. MiR-590-5p is belongs to the miR-590 family and used as a tumor suppressor gene in some cancers such as lung cancer and gastric cancer [21,22,23]. Databases analysis and dual luciferase assay shown that DUXAP8 could complement binding with miR-590-5p. In vitro and in vivo experiments further shown that DUXAP8 regulates the proliferation and apoptosis of ovarian cancer cells by regulating miR-590-5p.
YAP1 gene was also investigated in this study to further understand the mechanisms of regulations between DUXAP8 and miR-590-5p in suppression of ovarian cancer. YAP1 gene encodes a downstream nuclear effector of the Hippo signaling pathway which is involved in development, growth, repair, and homeostasis [24]. Previous studies have reported that YAP1 acts as a transcriptional regulator of this signaling pathway in the development and progression of many cancers and may function as a potential target for cancer therapy [25,26,27]. miR-590-5p could inhibit tumor cell invasion and migration by targeting YAP1 [28, 29]. In the present study, the mRNA and protein expression of the YAP1 were significantly down-regulated after miR-590-5p mimic transfection, and expression of YAP1 was reduced after the treatment of DUXAP8 siRNA, while miR inhibitor could attenuate the effects of DUXAP8 on the expression of YAP1. The results demonstrated that DUXAP8 promotes the expression of the YAP1 via inhibiting the expression of miR-590-5p in ovarian cancer.
In conclusion, the interference of DUXAP8 could inhibit the proliferation of ovarian cancer cells and promote apoptosis of ovarian cancer cells in vivo and in vitro. Further study shown that the mechanism of DUXAP8 on ovarian cancer cells is related to the regulation of YAP1 gene by targeting miR-590-5p. The regulation of DUXAP8 maybe an effective potential therapeutic and preventive approach for ovarian cancer.
References
Kossaï M, Leary A, Scoazec JY, Genestie C. Ovarian cancer: a heterogeneous disease. Pathobiology. 2018;85:41–9.
Roett MA, Evans P. Ovarian cancer: an overview. Am Fam Physician. 2009;80:609–16.
Kujawa KA, Lisowska KM. Ovarian cancer - from biology to clinic. Postepy Hig Med Dosw (Online). 2015;69:1275–90.
Adams SF, Benencia F. Immunotherapy for ovarian cancer: what are the targets of the future? Future Oncol. 2015;11:1293–6.
Qi P, Du X. The long non-coding RNAs, a new cancer diagnostic and therapeutic gold mine. Mod Pathol. 2013;26:155–65.
Kogo R, Shimamura T, Mimori K, Kawahara K, Imoto S, Sudo T, Tanaka F, Shibata K, Suzuki A, Komune S, Miyano S, Mori M. Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 2011;71:6320–6.
Struhl K. Transcriptional noise and the fidelity of initiation by RNA polymerase II. Nat Struct Mol Biol. 2007;14:103–5.
Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, Wang Y, Brzoska P, Kong B, Li R, West RB, van de Vijver MJ, Sukumar S, Chang HY. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–6.
Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146:353–8.
Mitra R, Chen X, Greenawalt EJ, Maulik U, Jiang W, Zhao Z, Eischen CM. Decoding critical long non-coding RNA in ovarian cancer epithelial-to-mesenchymal transition. Nat Commun. 2017;8:1604.
Tripathi MK, Doxtater K, Keramatnia F, Zacheaus C, Yallapu MM, Jaggi M, Chauhan SC. Role of lncRNAs in ovarian cancer: defining new biomarkers for therapeutic purposes. Drug Discov Today. 2018;23:1635–43.
Nunes FD, de Almeida FC, Tucci R, de Sousa SC. Homeobox genes: a molecular link between development and cancer. Pesqui Odontol Bras. 2003;17:94–8.
Ma HW, Xie M, Sun M, Chen TY, Jin RR, Ma TS, Chen QN, Zhang EB, He XZ, De W, Zhang ZH. The pseudogene derived long noncoding RNA DUXAP8 promotes gastric cancer cell proliferation and migration via epigenetically silencing PLEKHO1 expression. Oncotarget. 2016;8:52211–24.
Lian Y, Yang J, Lian Y, Xiao C, Hu X, Xu H. DUXAP8, a pseudogene derived lncRNA, promotes growth of pancreatic carcinoma cells by epigenetically silencing CDKN1A and KLF2. Cancer Commun (Lond). 2018;38:64.
Lian Y, Xiao C, Yan C, Chen D, Huang Q, Fan Y, Li Z, Xu H. Knockdown of pseudogene derived from lncRNA DUXAP10 inhibits cell proliferation, migration, invasion, and promotes apoptosis in pancreatic cancer. J Cell Biochem. 2018;119:3671–82.
Jiang B, Hailong S, Yuan J, Zhao H, Xia W, Zha Z, Bin W, Liu Z. Identification of oncogenic long noncoding RNA SNHG12 and DUXAP8 in human bladder cancer through a comprehensive profiling analysis. Biomed Pharmacother. 2018;108:500–7.
Huang T, Wang X, Yang X, Ji J, Wang Q, Yue X, Dong Z. Long Non-coding RNA DUXAP8 enhances Renal cell carcinoma progression via downregulating miR-126. Med Sci Monit. 2018;24:7340–7.
Xu LJ, Yu XJ, Wei B, Hui HX, Sun Y, Dai J, Chen XF. Long non-coding RNA DUXAP8 regulates proliferation and invasion of esophageal squamous cell cancer. Eur Rev Med Pharmacol Sci. 2018;22:2646–52.
Yan H, Li H, Li P, Li X, Lin J, Zhu L, Silva MA, Wang X, Wang P, Zhang Z. Long noncoding RNA MLK7-AS1 promotes ovarian cancer cells progression by modulating miR-375/YAP1 axis. J Exp Clin Cancer Res. 2018;37:237.
Lin MG, Hong YK, Zhang Y, Lin BB, He XJ. Mechanism of lncRNA DUXAP8 in promoting proliferation of bladder cancer cells by regulating PTEN. Eur Rev Med Pharmacol Sci. 2018;22:3370–7.
Ekhteraei-Tousi S, Mohammad-Soltani B, Sadeghizadeh M, Mowla SJ, Parsi S, Soleimani M. Inhibitory effect of hsa-miR-590-5p on cardiosphere-derived stem cells differentiation through downregulation of TGFB signaling. J Cell Biochem. 2015;116:179–91.
Wang J, Xu W, He Y, Xia Q, Liu S. LncRNA DUXAP8 impacts proliferation, invasion, and migration of ovarian cancer cells through regulating PTEN. Inflamm Res. 2018;67:927–36.
Zhang J, Zhou Y, Huang T, Wu F, Pan Y, Dong Y, Wang Y, Chan A, Liu L, Kwan J, Cheung A, Wong C, Lo A, Cheng A, Yu J, Lo K, Kang W, To K. FGF18, a prominent player in FGF signaling, promotes gastric tumorigenesis through autocrine manner and is negatively regulated by miR-590-5p. Oncogene. 2019;38:33–46.
Hauri S, Wepf A, van Drogen A, Varjosalo M, Tapon N, Aebersold R, Gstaiger M. Interaction proteome of human Hippo signaling: modular control of the co-activator YAP1. Mol Syst Biol. 2013;9:713.
Rosenbluh J, Nijhawan D, Cox AG, Li X, Neal JT, Schafer EJ, Zack TI, Wang X, Tsherniak A, Schinzel AC, Shao DD, Schumacher SE, Weir BA, Vazquez F, Cowley GS, Root DE, Mesirov JP, Beroukhim R, Kuo CJ, Goessling W, Hahn WC. β-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell. 2012;151:1457–73.
Lehmann W, Mossmann D, Kleemann J, Mock K, Meisinger C, Brummer T, Herr R, Brabletz S, Stemmler MP, Brabletz T. ZEB1 turns into a transcriptional activator by interacting with YAP1 in aggressive cancer types. Nat Commun. 2016;7:10498.
Gruber R, Panayiotou R, Nye E, Spencer-Dene B, Stamp G, Behrens A. YAP1 and TAZ Control pancreatic cancer initiation in mice by direct up-regulation of JAK-STAT3 signaling. Gastroenterology. 2016;151:526–39.
Ou C, Sun Z, Li X, Li X, Ren W, Qin Z, Zhang X, Yuan W, Wang J, Yu W, Zhang S, Peng Q, Yan Q. MiR-590-5p, a density-sensitive microRNA, inhibits tumorigenesis by targeting YAP1 in colorectal cancer. Cancer Lett. 2017;399:53–63.
Mou K, Ding M, Han D, Zhou Y, Mu X, Liu W, Wang L. miR-590-5p inhibits tumor growth in malignant melanoma by suppressing YAP1 expression. Oncol Rep. 2018;40:2056–66.
Acknowledgments
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there is no conflict of interests regarding the publication of this paper.
Human and animal rights
Animal experiments were conducted following the National Institute of Health (NIH) guidelines (NIH Pub. No. 85-23, revised 1996). The experiments have been reviewed and approved by the Animal Protection and Use Committee of Jinan Maternity and Child Care Hospital.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Meng, Q., Li, Z., Pan, J. et al. Long noncoding RNA DUXAP8 regulates proliferation and apoptosis of ovarian cancer cells via targeting miR-590-5p. Human Cell 33, 1240–1251 (2020). https://doi.org/10.1007/s13577-020-00398-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-020-00398-8