Abstract
Bladder cancer (BC) is one of the most common tumors. Metabolic reprogramming is a feature of neoplasia and tumor growth. Understanding the metabolic alterations in bladder cancer may provide new directions for bladder cancer treatment. Sirtuin 1 (SIRT1) is a lysine deacetylase of multiple targets including metabolic regulators. In pancreatic cancer, the loss of SIRT1 is accompanied by a decreased expression of proteins in the glycolysis pathway, such as GLUT1, and cancer cell proliferation. Thus, we hypothesize that SIRT1 may interact with GLUT1 to modulate the proliferation and glycolysis phenotype in bladder cancer. In the present study, the expression of SIRT1 and GLUT1 was upregulated in BC tissues and cell lines and positively correlated in tissue samples. SIRT1 overexpression or GLUT1 overexpression alone was sufficient to promote cell proliferation and glucose uptake in BC cells. EX527, a specific inhibitor of SIRT1, exerted an opposing effect on bladder cancer proliferation and glucose uptake. The effect of EX527 could be partially reversed by GLUT1 overexpression. More importantly, SIRT1 overexpression significantly promoted the transcriptional activity and expression of GLUT1, indicating that SIRT1 increases the transcription activity and expression of GLUT1, therefore, promoting the cell proliferation and glycolysis in BC cells. Our study first reported that SIRT1/GLUT1 axis promotes bladder cancer progression via regulation of glucose uptake.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bladder cancer (BC) is one of the most commonly-seen malignancies which are considered as one of the top ten leading causes of global cancer-related death [1, 2]. An offensive threat as it is to human health, its underlying mechanism, especially its metabolic alterations, has not been fully clarified yet [3, 4].
In contrast to healthy cells, cancer cells increase aerobic glycolysis to produce energy. This phenomenon is known as “the Warburg effect”, which is one of the key characteristics of [5], and an essential issue during tumorigenesis [6]. The Warburg effect not only promotes rapid uncontrolled proliferation, but also confers a tendency to invade. Recently, it has been found that the epigenetic mechanisms depending on the covalent modifications of DNA and histones play a key role in the glycometabolism regulation. The sirtuin family of histone deacetylases is the key regulatory factors of various physiological and pathological events, including cancer metabolism [7]. Sirtuins 1–7 (SIRT1-7), whose activity depends on NAD+, belong to class III of histone deacetylase enzymes. It has been reported that members of this family of enzymes are promising drug targets for treating cancer.
SIRT1 is a crucial gene in the process of aging [8], energy metabolism, and autophagy [9], as well as the most conserved mammalian protein deacetylase-dependent on NAD+ which has become an important metabolic sensor in almost all the kinds of tissues. Recently, its role in bladder cancer has been reported. SIRT1 possessed an overexpression in human BC tissues compared to non-cancerous or normal bladder tissues, at both transcriptional and protein levels [10]. In an SIRT1-knockdown bladder cancer cell model, cell proliferation and viability were suppressed. Moreover, migration rate was inhibited as well, possibly via reduction of epithelial–mesenchymal transition (EMT) [10]. More importantly, in pancreatic cancer, the loss of SIRT1 could lead to decreased protein expression in the glycolysis pathway, for example, GLUT1 and GAPDH. SIRT1 exerted a stimulatory effect on the proliferation and the expression of glycolysis-related genes of PDAC cells [11]. However, whether SIRT1 plays the same role in bladder cancer remains unclear.
GLUT1 (glucose transporter 1) is a major glucose transporter, one of the 14 members of the mammalian glucose transporter family, and almost all cellular glucose uptake is regulated by GLUTs. Increased expression of GLUT1 in cancer cells is accompanied by increased proliferative capacity, energy expenditure, and cancer cell aggressiveness [12]. The transition from aerobic oxidative metabolism to hypoxic glycolysis is a feature of cancer cells, and overexpression of GLUT1 promotes adaptive upregulation of tumor glycolysis [12]. As we have mentioned, SIRT1 is silenced in PDAC, while the protein expression is reduced in the glycolysis pathway [11]; here, we hypothesize that SIRT1 may interact with GLUT1 to modulate the proliferation and glycolysis phenotype in bladder cancer.
Herein, the expression and protein levels of SIRT1 and GLUT1 in BC tissues, as well as cell lines were evaluated. Next, we examined how SIRT1 and GLUT1-affected bladder cancer proliferation and glucose uptake. Restore experiments were performed to validate whether SIRT1 interacts with GLUT1 to modulate cell proliferation and glycolysis phenotype in bladder cancer. Finally, the transcriptional activity and expression of GLUT1 in response to SIRT1 overexpression were examined. In summary, we demonstrate the role of SIRT1 and GLUT1 in glycolysis phenotype and progression in BC.
Materials and methods
Clinical samples
Human bladder cancer tissue samples (n = 12) and adjacent non-cancerous tissues (n = 12) were all collected from patients undergoing surgery in Hunan People’s Hospital with the approval of Ethics Committee of Hunan People’s Hospital. All procedures performed were in accordance with the ethical standards of the Ethics Committee of Hunan People’s Hospital and with the 1964 Helsinki declaration The samples were fixed in formalin or stored at − 80 °C. Written informed consent was obtained from all subjects. Ethical approval: “All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.”
Cell lines, cell culture and cell transfection
Human bladder cancer cell lines, 5637 (ATCC® HTB-9™) and T24 (ATCC® HTB-4™), and a normal cell line, SV-HUC-1 (uroepithelium epithelial SV40 immortalized cell line, ATCC® CRL-9520™), are obtained from ATCC (Manassas, VA, USA). 5637 cells are cultured in RPMI-1640 Medium (ATCC) supplemented with 10% FBS (Invitrogen, Waltham, MA, USA), T24 cells are cultured in McCoy’s 5a Medium Modified and supplemented with 10% FBS (Invitrogen), and SV-HUC-1 cells are cultured in F-12K Medium (ATCC) supplemented with 10% FBS (Invitrogen). All cells are cultured at 37 °C in 5% CO2.
SIRT1 or GLUT1 expression in cells is achieved by transfection of NC (negative control) or SIRT1 or GLUT1 overexpressing vectors (SIRT1 or GLUT1 OE, Genepharma, Shanghai, China). Cell transfection is performed with the help of Lipo2000 (Invitrogen).
RNA extraction and SYBR green quantitative PCR analysis
Total RNA was extracted using Trizol reagent (Invitrogen, CA, USA). mRNA expression was measured by an SYBR green qPCR assay (Takara, Dalian, China). Expression of Tubulin was used as an endogenous control. Data were processed using the 2−ΔΔCT method.
Immunohistochemistry (IHC) staining
Collected tissue specimens were fixed in 10% formalin overnight and then processed by paraffin embedding and sectioning. Sections of 4 µm were deparaffinized and incubated at 4 °C overnight with primary antibodies against SIRT1 or GLUT1. After incubated with secondary antibody, the sections were incubated in freshly prepared DAB reagent (Beyotime, China), and subsequently counterstained with hematoxylin (Beyotime, China). The sections were visualized by light microscopy (Olympus, Tokyo, Japan).
Immunoblotting
Cells were lysed in RIPA buffer with 1% PMSF. Protein was loaded onto an SDS-PAGE minigel and transferred onto PVDF membrane. The blots were probed with the following antibodies: anti-SIRT1 (ab110304, Abcam, Cambridge, CA, USA), anti-GLUT1 (ab115730, Abcam), anti-LDHA (ab101562, Abcam), anti-HK2 (ab104836, Abcam), and anti-Tubulin (ab6160, Abcam) at 4 °C overnight, and the blots were subsequently incubated with HRP-conjugated secondary antibody (1:5000). Signals were visualized using ECL substrates (Millipore, MA, USA). Tubulin was used as an endogenous protein for normalization. The blot density was analyzed by the ImageJ software (NIH, USA).
Flow cytometer assay
Quantification of apoptotic cells was conducted using Annexin V-FITC apoptosis detection kit (Keygen, Nanjing, China). Briefly, the cell samples were harvested with 0.25% trypsin without EDTA after 48 h of infection and then washed twice with ice-cold PBS and re-suspended in 500 µl binding buffer. Then, cells were incubated with 5 µl Annexin V-FITC specific antibodies and 5 µl propidium iodide (PI) then incubated for 15–20 min in dark and detected by BD Accuri C6 flow cytometer (BD, Franklin Lakes, NJ, USA) with the excitation wavelength of Ex = 488 nm and emission wavelength of Em = 530 nm. Each experiment was repeated three times in triplicate.
MTT assay
MTT assay was performed to evaluate cell viability. 24 h after seeding into 96-well plates (5 × 103 cells/well), cells were transfected and/or treated as described. 48 h after transfection, 20 µl MTT (at a concentration of 5 mg/ml; Sigma-Aldrich), was added, and the cells were incubated for an additional 4 h in a humidified incubator. 200 µl DMSO was added after the supernatant discarded to dissolve the formazan. OD490 nm value was measured. The viability of the non-treated cells (control) was defined as 100%, and the viability of cells from all other groups was calculated separately from that of the control group.
Measurement of glucose and lactate
Glucose levels were determined using a commercial glucose assay kit, Glucose Uptake-Glo™ Assay (Promega, Fitchburg, WI, USA). Glucose uptake was calculated by deducting the detected glucose concentration in the medium from the original glucose concentration. Lactate levels were determined using a lactate assay kit (Biovision, Milpitas, CA, USA) in accordance with the manufacturer’s instruction. All values were normalized by the BCA protein assay (Thermo Fisher Scientific, Waltham, MA, USA).
Statistical analysis
All data from three independent experiments were expressed as mean ± SD and processed using the SPSS17.0statistical software. The differences between two groups were estimated by Student’s t test; the differences among more than two groups were estimated by one-way ANOVA. A P value of < 0.05 was considered to be statistically significant.
Results
Expression and protein levels of SIRT1 and GLUT1 in BC tissues and cell lines
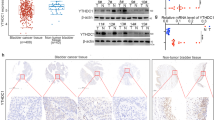
First, the expression and protein levels of SIRT1 and GLUT1 in tissue samples and cell lines were evaluated. Referring to Fig. 1a, b, the expression of SIRT1 and GLUT1 mRNA was remarkably upregulated in BC tissues, in comparison with that in non-cancerous tissues. GLUT1 and SIRT1 were positively related to each other in tissue samples (Fig. 1c). IHC staining and westernblot results further confirmed that SIRT1 and GLUT1 protein expressions were increased in BC tissues (Fig. 1d–g). Similarly, SIRT1 and GLUT1 protein expression were upregulated in two BC cell lines, 5637 and T24 (Fig. 1e, g). These findings that indicate SIRT1 may rescue GLUT1 expression in bladder cancer.
Expression and protein levels of SIRT1 and GLUT1 in bladder cancer tissues and cell lines a SIRT1 expression in bladder cancer tissues examined by qPCR, compared to non-cancerous tissues. b GLUT1 expression in bladder cancer tissues examined by qPCR, compared to non-cancerous tissues. c Correlation between SIRT1 and GLUT1 analyzed by Pearson’s correlation analysis. d, f SIRT1 and GLUT1 protein expression in bladder cancer tissues was examined by IHC staining (400×). e, g SIRT1 and GLUT1 protein levels in bladder cancer tissues and cell lines examined by Immunoblotting, compared to non-cancerous tissues and a normal cell line, SV-HUC-1. The data are presented as mean ± SD of three independent experiments. *P < 0.05, **P < 0.01
SIRT1 overexpression promotes the proliferation and alters the glycolytic phenotype in BC cells
Since SIRT1 expression is upregulated in bladder cancer, next, we evaluated its cellular function. SIRT1 overexpression in BC cells is achieved by transfection of SIRT1 overexpressing vector (SIRT1 OE), as confirmed by qPCR (Fig. 2a). Regarding the cellular functions, SIRT1 overexpression significantly inhibited cell apoptosis, while promoted cell viability of 5637 and T24 cells (Fig. 2b, c).
SIRT1 overexpression promotes the proliferation and alters the glycolytic phenotype in BC cells a SIRT1 overexpression achieved in 5637 and T24 cells by transfection of SIRT1 overexpressing vector (SIRT1 OE), as confirmed by qPCR. b Cell apoptosis of SIRT1 OE-transfected 5637 and T24 cells examined by Flow cytometry assay. c Cell viability of SIRT1 OE-transfected 5637 and T24 cells examined by MTT assay. d Lactate production SIRT1 OE-transfected 5637 and T24 cells examined by a lactate assay kit. e Glucose levels in medium of SIRT1 OE-transfected 5637 and T24 cells examined by a glucose assay kit. f Protein levels of SIRT1, GLUT1, LDHA, and HK2 in SIRT1 OE-transfected 5637 and T24 cells examined by Immunoblotting. The data are presented as mean ± SD of three independent experiments. *P < 0.05, **P < 0.01
As for the glycolytic phenotype and glucose uptake, we monitored the production of lactate, the levels of glucose in medium, and the protein levels of Warburg signaling key factors, including GLUT1, LDHA, and HK2 in SITR1-overexpressing bladder cancer cell lines. In both cell lines, SIRT1 overexpression significantly increased lactate production (Fig. 2d), decreased the glucose levels in medium (Fig. 2e), and increased the protein levels of GLUT1, LDHA, and HK2 (Fig. 2f), indicating that SIRT1 overexpression promotes glucose uptake in BC cells.
Dynamic effect of SIRT1 and GLUT1 on BC cells
After confirming the promotive effect of SIRT1 and GLUT1 overexpression on glucose uptake and proliferation in BC cells, the dynamic effect of SIRT1 and GLUT1 on BC cells was examined. 5637 and T24 cells were transfected with GLUT1 OE with or without EX527, a specific inhibitor of SIRT1, and then examined for the mRNA expression of GLUT1, bladder cancer cell proliferation, apoptosis, glycolytic phenotype, and protein levels of SIRT1 and GLUT1. GLUT1 mRNA expression was significantly decreased by EX527, while increased by GLUT1 overexpression, EX527-suppressed GLUT1 expression could be partially rescued by GLUT1 overexpression (Fig. 3a). Moreover, we also determined the protein stability of GLUT1. EX527 reduced the half-life of GLUT1 protein (Fig.S1C and D).
Dynamic effect of SIRT1 and GLUT1 on BC cells 5637 and T24 cells was transfected with GLUT1 OE in the presence or the absence of EX527, a specific inhibitor of SIRT1, and examined for the expression of SIRT1 and GLUT1 by qPCR (a), cell viability by MTT assay (b), cell apoptosis by flow cytometry (c), lactate production by a lactate assay kit (d), and glucose levels by a glucose assay kit (e), the protein levels of GLUT1, SIRT1, LDHA, and HK2 by immunoblotting. The data are presented as mean ± SD of three independent experiments. **P < 0.01, compared to control group; #P < 0.05, compared to DMSO + GLUT1 OE group
As shown in Fig. 3, GLUT1 overexpression promoted the cell proliferation, inhibited the cell apoptosis, and promoted the glucose uptake and glycolysis marker protein levels; the effect of EX527 on the cell viability, apoptosis, glucose uptake, as well as glycolysis markers in BC cells was opposing to that of GLUT1 overexpression (Fig. 3b–f). Above all, GLUT1 overexpression may partially reduce the effect of EX527, indicating that EX527-induced SIRT1 inhibition could suppress GLUT1 overexpression-induced cell proliferation and glucose uptake in BC cells.
SIRT1 promotes transcriptional factor-mediated GLUT1 transcription
Since SIRT1 and GLUT1 are positively correlated with each other and dynamically modulate the cell proliferation and glucose uptake in BC cells, next, we examined if SIRT1 could promote GLUT1 transcription and increase its expression. A luciferase reporter assay was performed via constructing psiCHECK 2-GLUT1 reporter vector. We co-transfected HEK293 cells with pcDNA3.1/SIRT1 and evaluated these cells for luciferase activity in the presence or the absence of EX527. As shown in Fig. 4a, SIRT1 overexpression significantly promoted, while EX527 treatment suppressed the luciferase activity of psiCHECK 2-GLUT1 vector; EX527 treatment might partially reduce the promotive effect of SIRT1 overexpression. Moreover, in 5637 and T24 cells, SIRT1 overexpression significantly increased GLUT1 expression (Fig. 4b). As shown in Fig. 4c, SIRT1 could promote the transcription and subsequent expression of GLUT1, therefore, increasing the glucose uptake in BC cells.
SIRT1 promotes transcriptional factors-mediated GLUT1 transcription. a Luciferase reporter assay was performed with psiCHECK 2-GLUT1 vector. HEK293 cells were co-transfected with psiCHECK 2-GLUT1 vector and pcDNA3.1/SIRT1 and examined for the luciferase activity. b GLUT1 expression in response to SIRT1 overexpression was examined in 5637 and T24 cells. c Schematic diagram representing SIRT1 cooperation with GLUT1 to promote cell proliferation and glycolysis in bladder cancer. The data are presented as mean ± SD of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.005, compared to control group; ##P < 0.01, compared to psiCHECK 2-GLUT1 vector + pcDNA3.1/SIRT1 group in the absence of EX527
Discussion
Herein, the mRNA expression and protein levels of SIRT1 and GLUT1 were significantly increased in BC tissues and cell lines. SIRT1 positively related to GLUT1 in tissue samples. SIRT1 overexpression or GLUT1 overexpression inhibited cell apoptosis, while promoted cell proliferation and glucose uptake in BC cells. EX527, a specific inhibitor of SIRT1, acted the opposite way on BC cells; GLUT1 overexpression could partially reverse the effect of EX527. SIRT1 overexpression increased the transcription and the expression of GLUT1. Via promoting GLUT1 transcription and expression, SIRT1 increases cell proliferation and glucose uptake in BC cells.
SIRT1 is a key member of Sirtuins, which has been reported to be involved in metabolic diseases and tumors. In bladder cancer, knockdown SIRT1 could suppress cell growth and migration via FOXO3a-mediated pathway [10]. Previously, it has been reported that SIRT1 regulates glucose uptake in non-cancerous tissues and adipocytes [13]. In the human placenta, as an activator of SIRT1 [14, 15], resveratrol could inhibit lipopolysaccharide (LPS)-induced inflammation through SIRT1 [16]. Moreover, as reported by Lappas et al. [17], resveratrol could remarkably reduce not only the decreased glucose uptake in placenta due to oxidative stress, but also GLUT1 mRNA expression and protein level. As we mentioned, in an SIRT1-deficient mouse model, a weaker IHC staining of GLUT1 and HK2 was observed in pancreatic lesions [11]. In the present study, SIRT1 and GLUT1 mRNA expression and protein levels are both significantly upregulated in BC tissues and cell lines, suggesting an increased glucose uptake in bladder cancer. More importantly, SIRT1 and GLUT1 expression was positively correlated in tissue samples, suggesting they may cooperate to modulate the glycolysis in bladder cancer.
Multiple genes may mediate the regulation of expression of glycolysis-related genes in a SIRT1-dependent manner [18]. It has been reported that SIRT1 could protect HIF1α from acetylation and degradation, thus increasing the expression of GLUT1 [19]. The histone deacetylase inhibitors are not specific to SIRT1, but they have been found to suppress the expression of GLUT1 and to reduce the enzymatic activity of hexokinase 1 in various myeloma cells [20]. In addition to being the rate-limiting step of glycolysis, glucose transmembrane transport is also considered as the first step of glucose metabolism. Many studies indicate that GLUT1 could promote cell proliferation and metastasis as well as inhibit apoptosis, thus playing a key role in various types of cancer, including hepatocellular carcinoma, breast cancer, and kidney cancer [21,22,23]. In breast cancer, GLUT1 promotes cancer cell migration and invasion by regulating EGFR and integrin signaling [22]. In bladder cancer cells, GLUT1 overexpression promoted cell proliferation and chemoresistant to cisplatin [24]. In the present study, SIRT1 overexpression or GLUT1 overexpression promotes proliferation, inhibits apoptosis, and promotes glucose uptake in BC cells. These findings are consistent with the previous studies that SIRT1 could upregulate the glycolytic phenotype in tissues and cells, possibly via cooperating with GLUT1.
To further confirm these above findings, we used EX527, a specific SIRT1 inhibitor, for the restore experiments. Opposing to SIRT1 or GLUT1 overexpression, EX527 treatment significantly promotes apoptosis, inhibits proliferation, and suppresses glucose uptake in BC cells. Above all, GLUT1 overexpression could partially reverse the effect of EX527, further confirming the hypothesis that SIRT1 may cooperate with GLUT1 to promote bladder cancer cell proliferation and glucose uptake. Furthermore, we examined the transcription activity of GLUT1 in response to SIRT1 overexpression. Consistent with the expression pattern, SIRT1 overexpression significantly promoted the transcriptional activity of GLUT1, which was partially attenuated by EX527, indicating that SIRT1 increases GLUT1 transcription and subsequent GLUT1 expression. SIRTs possess NAD+-dependent histone deacetylase activity [25]. As previously reported, at least to a certain extent, changes in deacetylation could modulate the expression of GLUT1 and glucose uptake. In myeloma cells, for instance, a few different histone deacetylase inhibitors could lead to a reduction in both glucose uptake and the expression of GLUT1 [20]. Similarly, in colorectal cancer cells (HT29) treated with the above inhibitors, glucose uptake was also inhibited [26]. As for the molecular mechanism of SIRT1 cooperation with GLUT1, future in vivo and in vitro studies are required to determine whether histone acetylation is involved.
Taken together, SIRT1 increases the transcription activity and expression of GLUT1, therefore, promoting the cell proliferation and glycolysis in BC cells. Our study revealed a novel mechanism of bladder cancer metastasis from the perspective of metabolic reprogramming.
References
Burger M, Catto JW, Dalbagni G, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63:234–41.
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-86.
von Rundstedt FC, Rajapakshe K, Ma J, et al. Integrative pathway analysis of metabolic signature in bladder cancer: a linkage to the cancer genome atlas project and prediction of survival. J Urol. 2016;195:1911–9.
Deng SP, Zhu L, Huang DS. Mining the bladder cancer-associated genes by an integrated strategy for the construction and analysis of differential co-expression networks. BMC Genom. 2015;16(Suppl 3):4.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Vazquez A, Liu J, Zhou Y, Oltvai ZN. Catabolic efficiency of aerobic glycolysis: the Warburg effect revisited. BMC Syst Biol. 2010;4:58.
Frye RA. Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem Biophys Res Commun. 1999;260:273–9.
Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–80.
Preyat N, Leo O. Sirtuin deacylases: a molecular link between metabolism and immunity. J Leukoc Biol. 2013;93:669–80.
Hu Q, Wang G, Peng J, et al. Knockdown of SIRT1 suppresses bladder cancer cell proliferation and migration and induces cell cycle arrest and antioxidant response through FOXO3a-mediated pathways. Biomed Res Int. 2017; 2017:3781904.
Pinho AV, Mawson A, Gill A, et al. Sirtuin 1 stimulates the proliferation and the expression of glycolysis genes in pancreatic neoplastic lesions. Oncotarget. 2016;7:74768–78.
Ancey PB, Contat C, Meylan E. Glucose transporters in cancer—from tumor cells to the tumor microenvironment. FEBS J. 2018.
Yoshizaki T, Milne JC, Imamura T, et al. SIRT1 exerts anti-inflammatory effects and improves insulin sensitivity in adipocytes. Mol Cell Biol. 2009;29:1363–74.
Bai L, Pang WJ, Yang YJ, Yang GS. Modulation of Sirt1 by resveratrol and nicotinamide alters proliferation and differentiation of pig preadipocytes. Mol Cell Biochem. 2008;307:129–40.
Backesjo CM, Li Y, Lindgren U, Haldosen LA. Activation of Sirt1 decreases adipocyte formation during osteoblast differentiation of mesenchymal stem cells. Cells Tissues Organs. 2009;189:93–7.
Lappas M, Mitton A, Lim R, Barker G, Riley C, Permezel M. SIRT1 is a novel regulator of key pathways of human labor. Biol Reprod. 2011;84:167–78.
Lappas M, Andrikopoulos S, Permezel M. Hypoxanthine-xanthine oxidase down-regulates GLUT1 transcription via SIRT1 resulting in decreased glucose uptake in human placenta. J Endocrinol. 2012;213:49–57.
Zhang C, Liu J, Wu R, et al. Tumor suppressor p53 negatively regulates glycolysis stimulated by hypoxia through its target RRAD. Oncotarget. 2014;5:5535–46.
Joo HY, Yun M, Jeong J, et al. SIRT1 deacetylates and stabilizes hypoxia-inducible factor-1alpha (HIF-1alpha) via direct interactions during hypoxia. Biochem Biophys Res Commun. 2015;462:294–300.
Wardell SE, Ilkayeva OR, Wieman HL, et al. Glucose metabolism as a target of histone deacetylase inhibitors. Mol Endocrinol. 2009;23:388–401.
Amann T, Maegdefrau U, Hartmann A, et al. GLUT1 expression is increased in hepatocellular carcinoma and promotes tumorigenesis. Am J Pathol. 2009;174:1544–52.
Oh S, Kim H, Nam K, Shin I. Glut1 promotes cell proliferation, migration and invasion by regulating epidermal growth factor receptor and integrin signaling in triple-negative breast cancer cells. BMB Rep. 2017;50:132–37.
Chan DA, Sutphin PD, Nguyen P, et al. Targeting GLUT1 and the Warburg effect in renal cell carcinoma by chemical synthetic lethality. Sci Transl Med. 2011;3:94ra70.
Li P, Yang X, Cheng Y, et al. MicroRNA-218 increases the sensitivity of bladder cancer to Cisplatin by targeting Glut1. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol. 2017;41:921.
North BJ, Verdin E. Sirtuins. Sir2-related NAD-dependent protein deacetylases. Genome Biol. 2004;5:224.
Boren J, Lee WN, Bassilian S, et al. The stable isotope-based dynamic metabolic profile of butyrate-induced HT29 cell differentiation. J Biol Chem. 2003;278:28395–402.
Acknowledgements
This study was supported by Hunan Provincial Natural Science Fund (2017JJ3108).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, J., Cao, L., Li, Z. et al. SIRT1 promotes GLUT1 expression and bladder cancer progression via regulation of glucose uptake. Human Cell 32, 193–201 (2019). https://doi.org/10.1007/s13577-019-00237-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-019-00237-5