Abstract
Periodontal diseases result from inflammation by bacterial infection in plaques, leading to tooth loss. However, regenerative approaches with periodontal tissue regeneration by guided tissue regeneration and enamel matrix derivative are not yet well established. Tissue regeneration requires three factors: cells, scaffold, and growth factors. Dedifferentiated fat cells (DFATs) are pluripotent with the same differentiation capacities as mesenchymal stem cells (MSCs). Access to MSCs is limited, whereas donor cells for DFATs are abundant in adipose tissues and can be non-invasively obtained. Therefore, we tested DFATs as a new source for periodontal tissue regeneration in an experimental periodontal tissue loss model in rats by transplanting DFATs on an atelocollagen scaffold using DFATs isolated from Sprague–Dawley (SD) rats expressing green fluorescent protein (GFP). GFP–DFAT cells were transplanted on the palatal side of the upper left first molar in SD rats and detected by H&E staining, GFP, and proliferating cell nuclear antigen (PCNA) expression. DFAT differentiation was also evaluated in three-dimensional cultures. GFP positive cells were detected in the regenerated tissue by the DFATs/scaffold mixture at 4 weeks after transplantation, and PCNA-positive cells were significantly increased in the periodontal ligament along the new bone in the DFATs/scaffold group more than in the scaffold-only group, suggesting that DFATs differentiate in the same manner as MSCs and regenerate in the defective areas. Consistent with previous reports, DFATs differentiation was slower than that with stem cells. The present study demonstrates that DFATs are pluripotent and an effective new source of cells for periodontal tissue regeneration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Periodontal diseases are inflammatory diseases that are caused mainly by bacteria in the plaque. They involve destruction of the periodontal tissue and, finally, loss of teeth. Scaling, root planing, and periodontal surgical treatment have been performed to remove periodontal pockets and inhibit progression of periodontal diseases, but these treatments do not result in regeneration of the lost periodontal tissue.
Periodontal tissue regeneration methods to regenerate lost periodontal tissue have been widely applied clinically since treatment methods with guided tissue regeneration (GTR) [1] and enamel matrix derivative (EMD) [2] were introduced in the 1980s. In recent years, the application of growth factors, such as bone morphogenetic protein (BMP) and fibroblast growth factor (FGF) which control growth of cells, has been studied [3, 4]. Treatment methods such as GTR and EMD that are currently applied clinically have been reported to show consistent results, but there have also been reports of differences occurring in bone regeneration based on bone loss morphology [5, 6].
Tissue regeneration applies tissue engineering, and it is possible to construct tissues by combining three factors: cells, scaffold, and growth factors. At present, research is in progress in the dental field to apply mesenchymal stem cells (MSCs) in periodontal tissue regeneration, and it has been reported that periodontal tissue regeneration effects are obtained by transplantation of MSCs in alveolar bone defects [7]. However, although such periodontal tissue regeneration effects have been obtained, there are still problems such as the low percentage of MSCs included in the tissue when the cells are harvested and the difficulty in their isolation. Matsumoto et al. [8] focused on adipose tissue that can be easily harvested to solve these problems, and established a method of obtaining dedifferentiated fat cells (DFATs) which have the same pluripotency as MSCs, by isolation, culturing, and dedifferentiation of mature fat cells. These DFATs show about the same cell surface antigen expression pattern as MSCs, and it is already clear that they can be differentiated and converted not only into fat cells but also into other mesodermal cells [8–12]. They are expected to become a new source of cells for regenerative therapy.
In this research, after preparation of DFATs and application of a scaffold were investigated, periodontal tissue regeneration was examined following transplantation in experimental periodontal tissue defects prepared in rats to determine the possibility of periodontal tissue regeneration using DFATs.
Methods
Animals and materials
The laboratory animal used was one male Sprague–Dawley (SD) strain GFP rat at 10 weeks of age for harvesting of fat cells. In the investigation of periodontal tissue regeneration, 8-week old male SD strain rats (n = 20) were used.
For the study of periodontal tissue regeneration, AteloCell® (Koken, Tokyo, Japan) made from atelocollagen was used as a scaffold.
Approval for this research was obtained from the Animal Experimentation Ethics Committee of the School of Life Dentistry at Niigata, Nippon Dental University (Approval No. 103).
Preparation of DFATs
Dedifferentiated fat cel preparation was based on the method of Matsumoto et al. [8]. First, after euthanization of SD strain GFP rats by an overdose of pentobarbital sodium (Nembutal®; Dainippon Sumitomo Pharmaceutical, Osaka, Japan), inguinal adipose tissue was harvested and was washed with phosphate-buffered saline (PBS) adjusted to pH 7.4. The adipose tissue was cut into thin slices and treated for 20 min at 37 °C with collagenase. Thereafter, the undigested tissue was filtered with a filter (250 μm) and separated by centrifugation for 2 min at 600 rpm. The floating top layer containing adipocytes was collected. Then, Dulbecco’s modified Eagle’s medium (DMEM; Gibco Life Technologies, Carlsbad, CA, USA) with 20 % fetal bovine serum (FBS; JR Scientific, Woodland, CA, USA) added was placed in a cell culture flask, the isolated mature fat cells were seeded and ceiling cultured under 5 % CO2 flow with the cell adhesion layer at the top. After 7 days of culture, the flask was inverted so that the cell adhesion layer was at the bottom and an ordinary culture was performed. The culture medium was replaced every 4 days. After confluence was reached, subcultures were performed.
Application of DFATs to the scaffold
For observation of attachment of DFATs to the scaffold, DFATs from GFP rats were cultured on an atelocollagen scaffold (Koken) and the adherence of DFATs on days 1 and 7 after seeding was observed with a confocal laser microscope LSM710 (Carl Zeiss, Oberkochen, Germany). Nuclear staining was performed using 4′,6-diamidine-2-phenylindole dihydrochloride (DAPI; Kirkegaard & Perry Laboratories, Gaithersburg, MD, USA). Growth of DFATs on the three-dimensional scaffold was examined by counting the number of cells using a 96-well plate. The cells were seeded at a 5.0 × 103 cells/well in each well and evaluated. DFATs cultured three-dimensionally using a monolayer culture and the scaffold were counted using Countess (Invitrogen Life Technologies, Carlsbad, CA, USA) at days 1, 5, 10, and 15. Cell growth was compared between the groups. Statistical analysis was performed using Student’s t test.
Evaluation of induced differentiation of DFATs was performed with a three-dimensional culture using the scaffold. Differentiation was induced by culturing for 3 weeks each using an osteoinduction medium [10 % FBS + DMEM, 100 nM Dexamethasone (Sigma-Aldrich, St. Louis, MO, USA), 10 mM β-glycerophosphate (Sigma-Aldrich), 50 μM L-ascorbic acid-2-phsphate (Sigma-Aldrich)] and an adipogenic induction medium [10 % FBS + DMEM, 1 μM dexamethasone, 1× insulin-transferrin-selenium-X (Gibco), 0.5 mM isobutylmethylxanthine (Wako Pure Chemical Industries, Osaka, Japan)]. Induction of osteogenic differentiation was evaluated using Alizarin Red (Sigma-Aldrich) stain and induction of adipogenic differentiation using Oil Red O (Sigma-Aldrich) stain.
Preparation of alveolar bone defects in rats
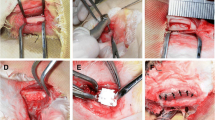
Alveolar bone defects were prepared under intraperitoneal anesthesia of the rats with pentobarbital sodium (0.8 ml/kg). The mouths were opened with the animals restrained in a reclining position. Then an intracrevicular incision was made with a No. 12 substitute scalpel (Feather Safety Razor, Osaka, Japan) from the palatal mesial angle of the upper left second molar (M2) to the mesial center of the upper left first molar (M1), and the incision was extended along the alveolar crest in the mesial direction. After the incision, flap reflection with a full-thickness flap was performed using a dental excavator, and the alveolar bone, periodontal ligament, and cementum were excised using a dental round bur (ISO Standard 010) under physiological saline irrigation to prepare the alveolar bone defect.
Dedifferentiated fat cel transplantation in the alveolar bone defect prepared was performed by seeding DFATs on the scaffold at 5.0 × 105 cells/scaffold and dividing the rats into two groups, a group with transplantation only on the scaffold (scaffold group) and a group with transplantation after 2 weeks of culture in a osteogenic differentiation induction medium from the day after seeding (DFATs/scaffold group). Observations were performed immediately after and at 4 weeks after transplantation.
Preparation of tissue specimens and histological investigation
The tissue specimens were fixed by perfusion with a 4 % paraformaldehyde solution in each group immediately after and at 4 weeks after transplantation. Then, the maxilla including the periodontal tissue was excised and fixed by immersion in 4 % paraformaldehyde solution for 1 week. After completion of fixation, decalcification was performed for 2 weeks using 10 % EDTA. Dehydration was performed with ethanol and, after replacement with xylene, the specimens were embedded in paraffin by the usual method.
Thereafter, serial specimens 3 μm thick were prepared in the buccal-palatal plane. The specimens were stained with hematoxylin-eosin stain (H&E stain) and examined by optical microscopy.
Immunohistochemical study
Immune staining was performed using anti-GFP antibody (Aves Labs, OR, USA) to detect the location of cells on the transplantation side and with a proliferating cell nuclear antigen (PCNA) staining kit (Invitrogen) for detection of cell growth activity at the transplantation site.
Green fluorescent protein detection was performed by incubating the specimens after deparaffination at room temperature for 30 min with 0.3 % hydrogen peroxide solution and by blocking with endogenous peroxidase. After washing with PBS, the specimens were incubated with 0.1 % trypsin for 30 min at 37 °C for antigen activation and then incubated for 30 min at room temperature with 1 % bovine serum albumin (BSA) to suppress non-specific protein binding. The anti-GFP antibody was diluted 1:1,000 and incubated for 2 h at room temperature. For the secondary antibody, LSAB2 Kit (Dako, Glostrup, Denmark) was used. It was visualized by treatment for 10 min using DAB (3,3′-diaminobenzidine) substrate and counter-stained with hematoxylin.
Measurement of PCNA-positive cells
For measurement of PCNA-positive cells, the number of PCNA-positive cells (100 μm × 100 μm each) of periodontal ligament adjacent to the native bone, periodontal ligament adjacent to the new bone- and connective tissue at the alveolar crest were counted in specimens in the scaffold group and DFATs/scaffold group at 4 weeks after transplantation (Fig. 1).
Determination sites of PCNA positive cells. (1) Periodontal ligament adjacent to native bone. (2) Periodontal ligament adjacent to new bone. (3) These transplanted cells were positively stained with PCNA antibody on the alveolar crest. D dentin, E enamel, C cementum, B native bone, NB new bone, P periodontal ligament
Statistical analysis was performed using Wilcoxon’s signed-rank test for periodontal membrane adjacent to native bone and Student’s t test for periodontal membrane adjacent to new bone and alveolar crest connective tissue.
Results
Preparation of DFATs
The morphology of DFATs was observed as changes from mature adipocyte to fibroblast-like cells at 1 week after the start of culture (Fig. 2a). DFAT culture became confluent in 2 weeks (Fig. 2b).
Application of DFATs to the scaffold
Dedifferentiated fat cells seeded on the scaffold were observed for adherence to the scaffold on day 1 after seeding (Fig. 3a) and DFATs showed adherence and proliferation on the scaffold on day 7 after seeding (Fig. 3b).
The cell count of DFATs in a monolayer culture and cultured three-dimensionally using a scaffold tended to be higher in the three-dimensional culture than in the monolayer culture on day 1 after seeding, and significantly higher in the three-dimensional culture than in the monolayer culture on days 5, 10, and 15 after seeding (Fig. 4, p < 0.01).
In evaluation of induced differentiation of DFATs aggregation of calcified substrate was observed using Alizarin Red stain (Fig. 5a, c) and fat droplets were observed using Oil Red O stain (Fig. 5b, d) in both the three-dimensional culture and the monolayer culture.
Evaluation of differentiation in a three-dimensional culture. a DFATs inducing osteogenic differentiation in monolayer culture. b DFATs inducing adipogenic differentiation in monolayer culture. c DFATs inducing osteogenic differentiation in three-dimensional culture. d DFATs inducing adipogenic differentiation in three-dimensional culture. DFATs that induced osteogenic differentiation in monolayer culture and three-dimensional cultures were stained positive with Alizarin Red stain and those that induced adipogenic differentiation were stained positive with Oil Red O stain
Morphological observations
In defects incised in the cementum, periodontal ligament and alveolar bone immediately after transplantation, the transplanted scaffold was observed in both the scaffold and DFATs/scaffold groups, and the scaffold together with attached cells were observed in the DFATs/scaffold group (Fig. 6a, b).
H&E stained specimens. a Scaffold group immediately after transplantation. b DFATs/scaffold group immediately after transplantation (dashed line is transplantation area). c Scaffold group at 4 weeks after transplantation. d DFATs/scaffold group at 4 weeks after transplantation. e Higher magnification of square area of (d). Immediately after transplantation, the scaffold was observed in the defect in both groups. At 4 weeks after transplantation, newly formed alveolar bone (arrowhead of c, d) and periodontal ligament (arrow of c, d) were present in both groups. In the DFATs/scaffold group, cementum-like structure was also observed (arrow of e)
At 4 weeks after transplantation, no transplanted scaffold was observed in either the scaffold or DFATs/scaffold groups and osteogenesis was found to be present (Fig. 6c, d). Infiltration was not found in the apical direction in the epithelium. Continuous osteogenesis was seen in the DFATs/scaffold group, but in the scaffold group, only non-continuous sporadic osteogenesis was observed. In the vicinity of the osteogenesis in both groups, accumulations of osteoblasts and osteoclasts were present and a continuous periodontal ligament-like structure in the direction of the root surface from the new bone was also found. New cementum formation was not observed in the scaffold group, but, in the DFATs/scaffold group, formation of a small amount of a cementum-like structure was confirmed on the exposed root surface.
Immunohistochemical analysis
In the DFATs/scaffold group at 4 weeks after transplantation, GFP-positive cells were present in newly formed bone, new cementum and the periodontal ligament (Fig. 7).
Immune stained specimens using anti-GFP antibody. a DFATs/scaffold group immediately after transplantation. b DFATs/scaffold group at 4 weeks after transplantation. c Higher magnification of (b). Positive cells were found in the scaffold immediately after transplantation. At 4 weeks after transplantation, positive cells were present in the new periodontal ligament, new alveolar bone, and connective tissue (arrow GFP-positive, **new alveolar bone)
In both the scaffold and DFATs/scaffold groups at 4 weeks after transplantation, PCNA-positive cells were present in the periodontal ligament (Fig. 8).
Immune stained specimens using PCNA. a Scaffold group immediately after transplantation. b DFATs/scaffold group immediately after transplantation. c Scaffold group at 4 weeks after transplantation. d DFATs/scaffold group at 4 weeks after transplantation. e Higher magnification of (b). f Higher magnification of (d). A few PCNA positive cells were found in the scaffold group at 4 weeks after transplantation. Many PCNA-positive cells were present in the periodontal membrane in the DFATs/scaffold group immediately after transplantation and at 4 weeks after transplantation (dashed square PCNA-positive area, arrowhead PCNA-positive)
Measurement of PCNA positive cells
Proliferating cell nuclear antigen-positive cells were present in significantly higher numbers at all sites in periodontal ligament adjacent to the native bone, periodontal ligament adjacent to the new bone, and the alveolar crest in the DFATs/scaffold group than in the scaffold group (Table 1; p < 0.01). PCNA-positive cells in the DFATs/scaffold group tended to show higher numbers in the periodontal ligament than in the alveolar crest.
Discussion
The properties of DFATs include conversion to pluripotent cells by dedifferentiation of terminally differentiated fat cells. Adipose tissue is present subcutaneously and can be collected with minimum invasion. Adipose-derived stem cells (ASC) are pluripotent cells obtained from the same adipose tissue by mincing it, separating in a centrifuge, and culturing the stromal vascular fraction (SVF) in the bottom layer. Since the SVF is not a pure cell line, including cells other than ASC, further isolation is necessary to obtain ASC with high purity. However, DFATs can be obtained as a pure cell line since they are obtained by ceiling cultures of fat cells suspended in the upper layer after centrifugation. Since DFATs have a high proliferation capacity and maintain differentiation capacity even after repeated subcultures, it is possible to obtained large numbers of DFATs from a small amount of adipose tissue [8].
Dedifferentiated fat cells were reported as new cells by Yagi et al. [13], and their efficacy in regenerative medicine was reported by Matsumoto et al. [8]. Research on DFATs has been performed in many fields of medicine and their application to tissue regeneration has been attempted using their pluripotency.
Research on DFATs has not been reported in the dental field. The present research was intended to investigate the role of DFATs in periodontal tissue regeneration by transplantation of DFATs of GFP rat origin in normal rats and detecting their localization in an attempt to apply DFATs in periodontal tissue regeneration.
The DFATs prepared in this study originated from the adipose tissue of GFP rats and had a fibroblast-like morphology similar to the DFATs of Jumabay et al. [11] using adipose tissue from the same strain of rats. It appeared that DFATs derived from adipose tissue of GFP rats with the morphology changed from round fat cells to fibroblast-like cells. The DFATs that we prepared were found to induce differentiation in fat-inducing media and in bone-inducing media and showed pluripotency.
In the present study, when DFATs were transplanted into rats, atelocollagen was used as a scaffold. Atelocollagen has low antigenicity and cells can proliferate on the inner surface of the pores. In transplantation aimed at periodontal tissue regeneration, it has been reported that absorption takes place in 4 weeks and good periodontal tissue regeneration has been reported [14]. We applied DFATs to atelocollagen and investigated their function.
In research applying fibroblasts and endothelial cells to atelocollagen, call adhesion and good cell proliferation have been reported [15]. In research applying ASC or bone marrow-derived stem cells (BMSC) that have the same pluripotency as DFATs, it has been reported that it is possible to induce adhesion of cells and differentiation on atelocollagen [16, 17]. From these reports, it was evident that atelocollagen shows good results irrespective of the type of cells. The scaffold is one element of tissue engineering and it is necessary that cells show normal function on the scaffold. In the present research, adhesion and proliferation on atelocollagen and induction of differentiation of DFATs on atelocollagen were shown to be possible. Cells appear to show normal functions on the scaffold and it is suggested that atelocollagen is effective as a scaffold for DFATs.
In the present research, positive cells were observed not only in the alveolar bone but also in the periodontal ligament by immunohistochemical analysis using anti-GFP antibodies at 4 weeks after transplantation, and it was clear that the transplanted DFATs remained at 4 weeks after transplantation. In the present research, undifferentiated DFATs before transplantation were transplanted into rats after they were induced to form osteoblasts in osteogenic-inducing medium. It was noteworthy that GFP-positive cells were observed in the periodontal ligament.
Dedifferentiated fat cells were reported by Heijl et al. [2] to show osteogenesis, and it is considered that DFATs induced in osteoblasts are directly involved in attachment of bone as positive cells in alveolar bone. However, as positive cells in the periodontal ligament, it is unlikely that DFATs induced in osteoblasts, terminally differentiated cells, are converted to periodontal ligament cells. Oki et al. [10] reported the MSC have periodontal ligament-like properties when periodontal ligament cells and MSC are co-cultured. In the present research, it was possible that undifferentiated DFATs not induced in differentiation-inducing medium were differentiated to periodontal ligament cells. Osteogenesis was observed in the scaffold group at 4 weeks, but the scaffold has a space-making role and restored bone appears to be formed to prevent infiltration of the epithelium in the apical direction.
The DFATs/scaffold group at 4 weeks after transplantation showed a newly formed cementum-like structure but the presence of the periodontal ligament is important for new formation of cementum. This newly formed cementum-like structure might be affected by the growth activity of the periodontal ligament. The new cementum-like structure observed at 4 weeks was not large, and it was not clear if it was regenerated cementum. In counting the PCNA-positive cells, the DFATs/scaffold group showed a significantly higher number of positive cells than the scaffold group and high cell proliferation activity was found in the periodontal ligament. In the future, it should be possible to observe new cementum formation by long-term observations. Future studies are also necessary on the effects of cell proliferation activity of the periodontal ligament on transplantation.
In the present study, partial formation of cementum-like structure was observed on the exposed root surface in addition to that on the alveolar bone and periodontal ligament, and newly formed tissue and surrounding anti-GFP antibody-positive cells were found. The localization of DFATs was clear. In immune staining using PCNA, positive cells were found around the newly formed tissue and it appeared that DFATs have some role in periodontal ligament regeneration.
Hasegawa et al. [14] prepared bone defects in the furcation area in dogs and transplanted bone marrow stem cells with the GFP gene inserted. The results showed regeneration of cementum, periodontal ligament and alveolar bone at 4 weeks after transplantation. GFP-positive cells as PCNA-positive cells were found in the regenerated tissue and they reported that bone marrow stem cells are involved in and promote periodontal tissue regeneration.
Tobita et al. [18] studied transplantation of ASC with the GFP gene introduced in rats with alveolar bone defects, the same as in this study, with the objective of periodontal tissue regeneration. They found regeneration of alveolar bone, periodontal ligament, and cementum and also GFP-positive cells in the regenerated alveolar bone and periodontal ligament by immunohistochemical observations. They reported the efficacy of the application of ASC in periodontal tissue regeneration.
Our research using DFATs showed the same results as those of Hasegawa et al. [14] and Tobita et al. [18] using stem cells. We confirmed the localization of transplanted DFATs in newly formed tissue and their affect on the cell proliferation activity of the periodontal ligament, suggesting the possibility that DFATs are involved in periodontal tissue regeneration. Since a uniform population of DFATs can easily be obtained, and the same results were obtained as in research on periodontal tissue regeneration using other stem cells, the possibility that DFATs will be effective as a new source of cells for periodontal tissue regeneration treatment using cell transplantation was suggested.
References
Nyman S, Lindhe J, Karring T, Rylander H. New attachment following surgical treatment of human periodontal disease. J Clin Periodontol. 1982;9:290–6.
Heijl L, Heden G, Svärdström G, Ostgren A. Enamel matrix derivative (EMDOGAIN®) in the treatment of intrabony periodontal defect. J Clin Periodontol. 1997;24(9 Pt 2):705–14.
Ripamonti U, Heliotis M, van den Heever B, Reddi AH. Bone morphogenetic proteins induce periodontal regeneration in the baboon (Papio ursinus). J Periodont Res. 1994;29:439–45.
Kitamura M, Nakashima K, Kowashi Y, et al. Periodontal tissue regeneration using fibroblast growth factor-2: randomized controlled phase II clinical trial. PLoS ONE. 2008;3(7):e2611.
Lekovic V, Kenney EB, Kovacevic K, Carranza FA Jr. Evaluation of guide tissue regeneration in class II furcation defects. A clinical re-entry study. J Periodontol. 1989;60:694–8.
Tonetti MS, Lang NP, Cortellini P, et al. Enamel matrix proteins in the regenerative therapy of deep intrabony defect. J Clin Periodontol. 2002;29:317–25.
Yamada Y, Ueda M, Hibi H, Baba S. A novel approach to periodontal tissue regeneration with mesenchymal stem cells and plateletrich plasma using tissue engineering technology. A clinical case report. Int J Periodont Rest. 2006;26:363–9.
Matsumoto T, Kano K, Kondo D, et al. Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential. J Cell Physiol. 2008;215:210–22.
Kazama T, Fujie M, Endo T, Kano K. Mature adipocyte-derived dedifferentiated fat cells can transdifferentiate into skeletal myocytes in vitro. Biochem Biophys Res Commun. 2008;377:780–5.
Oki Y, Watanabe S, Endo T, Kano K. Mature adipocyte-derived dedifferentiated fat cells can trans-differentiate into osteoblasts in vitro and in vivo only by all-trans retinoic acid. Cell Struct Funct. 2008;33:211–22.
Jumabay M, Matsumoto T, Yokoyama S, et al. Dedifferentiated fat cells convert to cardiomyocyte phenotype and repair infarcted cardiac tissue in rats. J Mol Cell Cardiol. 2009;47:565–75.
Tanaka N, Matsumoto T, Takahata S, Kano K, Ryu J, Mugishima H. Mature adipocyte-derived dedifferentiated fat (DFAT) cells show osteogenic potential. J Nihon Univ Med Ass. 2006;65:400–5.
Yagi K, Kondo D, Okazaki Y, Kano K. A novel preadipocyte cell line established from mouse adult mature adipocytes. Biochem Biophys Res Commun. 2004;321:967–74.
Hasegawa N, Kawaguchi H, Hirachi A, et al. Behavior of transplanted bone marrow-derived mesenchymal stem cells in periodontal defects. J Periodontol. 2006;77:1003–7.
Itoh H, Aso Y, Furuse M, Noishiki Y, Miyata T. A honeycomb collagen carrier for cell culture as a tissue engineering scaffold. Artif Organs. 2001;25:213–7.
Kakudo N, Shimotsuma A, Miyake S, Kushida S, Kusumoto K. Bone tissue engineering using human adipo-derived stem cells and honeycomb collagen scaffold. J Biomed Mater Res A. 2008;84:191–7.
George J, Kuboki Y, Miyata T. Differentiation of mesenchymal stem cells into osteoblasts on honeycomb collagen scaffolds. Biotechnol Bioeng. 2006;95:404–11.
Tobita M, Uysal AC, Ogawa R, Hyakusoku H, Mizuno H. Periodontal tissue regeneration with adipose-derived stem cells. Tissue Eng Part A. 2008;14:945–53.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sugawara, A., Sato, S. Application of dedifferentiated fat cells for periodontal tissue regeneration. Human Cell 27, 12–21 (2014). https://doi.org/10.1007/s13577-013-0075-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-013-0075-6