Abstract
The essential oil (EO) obtained from the fresh and dried leaves of Cinnamomum tamala was analysed by gas chromatography/mass spectrometry. EO from fresh leaves showed the presence of 21 compounds, whereas, EO from the dried leaves of C. tamala showed the presence of 20 compounds. In vitro assays namely scavenging ability against 1,1-diphenyl-2-picrylhydrazyl, reducing power and chelating ability on Fe2+ ions were used to determine the antioxidant potential of EO of C. tamala. With regard to antifungal activity, EO from dried leaves was more effective against Alternaria alternata and Curvularia lunata than the EO from fresh leaves. Similarly, EO from C. tamala leaves also showed potent antibacterial activity against two Gram negative and two Gram positive bacteria namely, Bacillus subtilis, Micrococcus luteus, Staphylococcus aureus and Pseudomonas aeruginosa. Furthermore, bioactive molecule from C. tamala EO having antifungal and antioxidant activity was isolated and characterized using bioautography, preparative thin layer chromatography and GC/MS analysis and was determined as eugenol. Its minimum inhibitory amount against A. alternata and C. lunata was determined using bioautography assay and was found to be 9.5 and 8.2 µg respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Based on their use in traditional medicine, a good number of modern drugs have been isolated from natural sources. Traditional medicine is a mean for health care for more than 65 % of world population (Nabavi et al. 2014). Different chemical compounds present in medicinal plants may act individually, additively or in synergy to improve health. As per one report published by World Health Organization (WHO), about 80 % of world population use medicinal plants to cure human diseases (Serrentino 1991). Raw materials or preparations containing phyto-chemicals with significant antioxidant activities and health benefits have been produced from some endemic species of medicinal plants (Exarchou et al. 2002). It has been reported that phenolic compounds, such as flavonoids, phenolic acids, tannins and phenolic diterpenes are primarily responsible for the antioxidant effect of plant products (Pietta 2000). The antioxidant properties of plant raw materials are attributed to the presence of polyphenols in them (Hamrouni et al. 2013). Further more, the antioxidant activity of polyphenols is mainly due to their redox properties, which allow them to act as reducing agents, hydrogen donors, singlet oxygen quenchers, metal chelators and reductants (Hamedo 2015).
Similarly, there has been a considerable interest to use plants and spices for the elimination of microorganisms because of increasing antibiotic resistance (Kunin 1993; Rahman 1999). There are reports of the active principles of essential oils from various plants with antibacterial or antifungal activity. The antimicrobial activity of essential oils is assigned to a number of small terpenoids and phenolic compounds (thymol, carvacrol, eugenol) which also in pure form demonstrate high antibacterial activity (Hamedo 2015).
Cinnamomum tamala is one of the species of the genus Cinnamomum and is native to India. It is a moderate sized evergreen tree belonging to family Lauraceae. In northern India, the plants are raised from seeds sown in the nursery beds in March–April. The ease with which essential oils can be obtained from this plant makes it ideal for cash crop farming (Gutteridge and Shelton 1994). Different species of genus Cinnamomum has been reported to possess excellent antibacterial, antitermite, antimite, antifungal and antioxidant properties (Chen et al. 2002).
The objective of this study was to evaluate the antioxidant and antimicrobial activity of C. tamala essential oil in vitro and to isolate and characterize the bioactive compound.
Materials and methods
Collection and identification of plant material
Fresh leaves from C. tamala were collected and identified at Herbal Garden and Herbarium Research Institute in ISM, Joginder Nagar, District Mandi (HP), India.
Sample preparation
Cinnamomum tamala leaves were divided into two parts. One part of the fresh leaves was used for extraction of essential oil and rest was dried in shade. Dried leaves were further used for the extraction of essential oil.
Extraction of essential oil
Essential oil from fresh as well as dried leaves was extracted through hydro-distillation using Clevenger-type apparatus (Guleria et al. 2008). The yield of the EO was determined and expressed in percentage.
GS/MS analysis
The mass spectrometer used for GC–MS analysis was GC–MS 4000 (Varian, USA) under the following conditions: capillary column, HP-5 MS (30 m × 0.25 mm; film thickness 0.25 µm); temperature program, 50 °C (held for 5 min) raised to 280 °C at a rate of 3 °C/min; injector temperature, 280 °C; carrier gas, helium, at flow rate of 1.0 ml/min. The MS scan parameters included electron impact ionization voltage of 70 eV and a mass range of 40–500 m/z. The identification of the components was based on the comparison of their mass spectra with those stored in NIST05 library or with mass spectra from literature (Adams 2007). Furthermore, in addition to MS, retention times (Rts) of compounds present in EO were authenticated by running standards.
1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay
This method was performed as described by Bozin et al. (2006). 1 ml of various dilutions of test material were mixed with 1 ml of a 90 µM DPPH methanolic solution and final volume was made to 4 ml with methanol. After an incubation of 1 h in the dark at 25 °C, the absorbances at 517 nm were recorded as Asample, using a UV/VIS spectrophotometer (Labomed, USA). A blank experiment was also performed using the same procedure to a solution without the test material and the absorbance was recorded as Ablank. The free radical scavenging activity of each solution was then calculated as percent inhibition according to the following equation:
Antioxidant activities of test compounds were expressed as IC50, defined as the concentration of test material required to produce a 50 % decrease in initial DPPH concentration. BHT was used as standard antioxidant compound.
Reducing power assay
The reducing power was determined according to the method of Oyaizu (1986). Each test sample was mixed with 2.5 ml of 200 mM sodium phosphate buffer (pH 6.6) and 2.5 ml of 1 % potassium ferricyanide and the mixture was incubated for 30 min at room temperature. Then, 2.5 ml of 10 % trichloroacetic acid was added, and the mixture was centrifuged at 1036 g for 10 min. The upper layer (2.5 ml) was mixed with 2.5 ml of 0.1 % ferric chloride. Finally the absorbance was measured at 700 nm against a blank. BHT was used as a control. The test sample concentration providing 0.5 of absorbance (IC50) was calculated from the graph of absorbance versus concentration.
Chelation power on metal ions
Chelating effect was determined according to the method of Dinis et al. 1994. The essential oil (100 µl) was mixed with 2.9 ml of methanol and 60 µl of 2 mM FeCl2 and the reaction was allowed to proceed for 30 s. After 120 µl of 5 mM ferrozine was added, the solution was mixed, left to stand for 10 min at room temperature, and then the absorbance of the mixture was determined at 562 nm in UV/VIS spectrophotometer (Labomed, USA). The ratio of inhibition of ferrozine Fe2+ complex formation was calculated as follows:
Quercetin was used as control.
Determination of antifungal activity
Antifungal activity was studied by using contact assay in vitro, which produces hyphal growth inhibition. Briefly, potato dextrose agar (PDA) plates were prepared, using 9 cm diameter glass petri dishes. Test sample was added to the sterilized potato dextrose agar containing 0.001 % Tween 20 v/v and the mixture was poured in 9 cm glass petri plates. 5 mm bit of test fungus was placed in the centre of the dishes. The plates were then incubated in the dark at 26 °C. The extension diameter (mm) of hyphae from the centres to the sides of dishes was measured every 24 h till the growth of fungus in the plate without test component (control) reached the edge of the plate. Growth inhibition of treatment against control was calculated as percentage, using the formula [(C − T/C) × 100] where C is the hyphal extension (mm) of control and T is the hyphal extension (mm) of plate treated with essential oil solution.
Determination of antibacterial activity
Antimicrobial activity of essential oils was determined by agar–well diffusion method (Feyza et al. 2009). Four test bacteria namely Bacillus subtilis MTCCCC 2389, Staphylococcus aureus MTCC7443), Micrococcus luteus (MTCC 4821) and Pseudomonas aeruginosa MTCC2642) were used in the study. 20 µl of the essential oil (diluted with DMSO, 1:1) was added to the well in the agar plate previously seeded with test organism. The plates were incubated at 37 °C for 24 h and the net zone of inhibition (mm) was determined. Chloramphenicol was used as positive control.
Determination of MIC by broth dilution method
Minimum inhibitory concentration of the essential oils was determined using micro dilution method (NCCLS 2002). Essential oil diluted with DMSO (1:1) was dissolved in nutrient broth to achieve the required concentrations (62.5–2000 μg/ml). The test bacterial suspension was added into each tube to yield bacterial density of 106 cfu/ml and the inoculated tubes were incubated at 37 °C for 24 h. 50 μl of 0.2 mg/ml p-iodonitrotetrazolium violet (INT) was then added in each tube and incubated for 30 min at 37 °C. Development of pink colour in the tube indicated the bacterial growth. MIC corresponded to the lowest concentration at which no bacterial growth was observed.
Qualitative antifungal and antioxidant activity assay by bioautography
Bioautographic evaluation was conducted using pre-coated aluminium silica gel TLC plates (5 × 20 cm, 0.25 mm thickness, silica gel G 60 F254, Merck, Darmstadt, Germany). A 5 μl of 1:10 dilution of test essential oil in ethyl acetate was spotted on two plates (one plate was used for antibacterial and another for antioxidant activity screening) and allowed to dry for a few minutes. The plates were developed with hexane: ethyl acetate (9:1 v/v) solvent mixture in a pre-saturated glass chamber and air-dried. For determining the antifungal activity against A. alternata and C. lunata, aliquots of 25–50 ml of inoculum spray solution (ca. 3 × 105 conidia/ml) were prepared for test fungi with liquid potato dextrose. Plates were sprayed lightly to a damp appearance three times with spore suspension and incubated for 4 days in a dark moist chamber at 25 °C. Fungal growth inhibition appeared as clear zones against a dark background (Sridhar et al. 2003). For determining the antioxidant activity, the developed air-dried plate was sprayed with 0.2 % DPPH in methanol and was further air-dried for 30 min. Appearance of yellow spots on a purple background on the TLC plate showed the presence of antioxidant compound (Mimica-dukic et al. 2003).
Isolation of antifungal/antioxidant constituent from C. tamala essential oil
A streak of test essential oil was applied manually on a PTLC glass plate (20 × 20 cm, 1 mm thickness) and allowed to dry for a few minutes. After air drying the plate was developed using the same solvent mixture as described earlier and two sets of plates were used in parallel. One of the plates from each set was used to perform direct bioautography with test pathogens or sprayed with DPPH as described previously to visualize the antifungal and antioxidant compounds. Bands that showed antifungal or antioxidant activity were scrapped off from the second plate of each set of experiment. The bioactive molecule was then set free from silica gel by elution with ethyl acetate and then centrifuged (12000 × g, 15 min) to remove the silica gel and the supernatants were collected.
Antifungal activity of bioactive molecule isolated from C. tamala
Different amounts of bioactive molecule Ct1 were loaded onto the TLC plate and bioautography was performed as described earlier using A. alternata and C. lunata as test pathogens. Antifungal activity was determined as MIA of active compound required for the inhibition of fungal growth on TLC plate.
Identification of bioactive compound
The isolated bioactive compound corresponding to Ct1 having antifungal and antioxidant activity was determined using GC/MS analysis as described earlier.
Statistical analysis
All experiments were carried out in triplicate. Data are expressed as mean ± standard deviation.
Results and discussion
Essential oils are valuable natural products consisting of many compounds that are used as raw materials in spices, food, perfumes, cosmetics, aromatherapy and phytotherapy. This has attracted the attention of many scientists and encouraged screening of plants in order to study the biological activities of their oils or chemical constituents. Each of the constituents contributes to the benefit or adverse effects of these oils (Lahlou 2004). The recent upsurge in antibiotic-resistant reports has increased the popularity of the essential oils as naturally occurring antimicrobial agents and therefore, plant essential oils may offer a great potential alternative for the treatment of many infectious diseases that could not be cured by current drugs (Lahlou 2004; Cosentino et al. 1999). A number of small terpenoids and phenolic compounds such as thymol, carvacrol, and eugenol are responsible for the antimicrobial activity of essential oils (Karapinar and Aktung 1987; Conner 1993). In the present study, yield of the yellow coloured oil obtained from the dried and fresh leaves of C. tamala was 0.6 and 0.15 percent respectively.
GC/MS analysis of the EO extracted from the fresh leaves of C. tamala revealed the presence of 21 compounds. In totality 93.82 percent compounds were identified in EO from fresh leaves consisting of a complex mixture of monoterpenes (25.04 %), sesquiterpenes (0.14 %) and phenylpropanoids (68.64 %) (Table 1). Similarly, EO from the dried leaves of C. tamala showed the presence of 20 compounds. In this case, total 94.42 percent compounds were identified in EO from dried leaves consisting of a complex mixture of monoterpenes (23.9 %), sesquiterpenes (0.20 %) and phenylpropanoids (70.32 %) (Table 2).
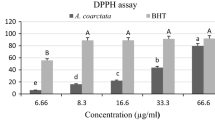
The DPPH radical scavenging activity of the EO extracted from fresh and dried leaves of C. tamala is shown in Table 3. The results showed higher DPPH radical scavenging activity in the essential oil from the dried leaves (IC50 value of 1.6 ± 0.1 mg/ml) than that obtained from the fresh leaves of C. tamala (IC50 value of 1.7 ± 0.1 mg/ml). Neutralization of DPPH free radical either by transfer of an electron or hydrogen atom may be responsible for observed antioxidant activity of the essential oils (Naik et al. 2003; Guleria et al. 2013). Furthermore, most natural antioxidant compounds often work synergistically with each other to produce a broad spectrum antioxidant effect that creates an effective defence system against free radical attack (Madsen and Bertelsen 1995). DPPH is a free radical compound that has been widely used to determine the free radical scavenging capacity of various samples (Dandapat et al. 2014). Hydrogen donating ability of the antioxidants is responsible for their DPPH radical scavenging activity and when an antioxidant scavenges the free radical the DPPH solution becomes light yellow (Pieme et al. 2014).
The reducing power of the EO obtained from fresh and dried leaves of C. tamala is shown in Table 3. Higher reducing power was observed in the EO extracted from the dried leaves (IC50 of value 2.75 ± 0.098 mg/ml) as compared to the fresh leaves (IC50 of value 2.8 ± 0.1 mg/ml) of C. tamala. In this assay, the yellow colour of the test solution changes to green depending on the reducing power of test specimen. The reductants present in the solution leads to the reduction of the Fe3+ ferricyanide complex to the ferrous form. The ability of essential oils to reduce Fe(III) may be attributed to hydrogen donation from phenolic compounds (Shimada et al. 1992; Duh 1998). The reducing power of a compound may serve as a significant indicator of its potential antioxidant activity (Meir et al. 1995).
Metal chelating capacity is considered as one of the important mechanism of antioxidant activity. The ferrous ions are the most powerful pro-oxidants among various species of transition metals present in food system (Yomauchi et al. 1998). The chelating power on metal ions of the EO obtained from fresh and dried leaves of C. tamala is depicted in Table 3. It is evident from the results that EO derived from dried leaves of C. tamala showed higher metal ion chelating activity (88.2 ± 1.2 % at 4.95 mg/ml) as compared to EO obtained from fresh leaves (85.0 ± 1.4 % at 4.95 mg/ml). Furthermore, decrease in the absorbance of ferrozine Fe2+ complex was dose dependent. This study is in conformity with the observation made in the literature that iron binding to phenolic antioxidants can cause reduced interaction of iron with oxygen molecules by changing the redox potential, thus converting Fe2+ ion to Fe3+ and thereby slowing down oxidative damage (Singh et al. 2007a, b).
Also it has been reported that non-flavonoids polyphenolics can reduce iron and then form Fe2+-polyphenol complexes that are inert (Laughton et al. 1987). Antioxidants causes the chelation of transition metal ions which possesses the ability to catalyse H2O2 decomposition, is an important mechanism of antioxidant activity (Manian et al. 2008). Higher metal ion chelating activity of EO derived from dried leaves as compared to EO obtained from fresh leaves of C. tamala may be attributed to the presence of certain phenolic compounds with properly oriented functional groups.
In the present study, antifungal activity of EO from C. tamala leaves was evaluated using two agriculturally important phytopathogenic fungi namely A. alternata and C. lunata. Comparison of antifungal indices and IC50 values of EO obtained from C. tamala revealed that C. lunata was more sensitive to EO of C. tamala as compared to A. alternata. Antifungal activity of EO from fresh and dried leaves of C. tamala is depicted in Table 4. On the basis of the results of the antifungal test, it is evident that the antifungal index of EO from fresh and dried leaves of C. tamala against A. alternata was 25 ± 1.15 % and 28.42 ± 1.2 % and IC50 values were 2932 ± 72.27 μg/ml and 2403.7 ± 19 μg/ml respectively. Antifungal index of EO from fresh and dried leaves of C. tamala against C. lunata was 29.49 ± 1.5 % and 34.86 ± 1.5 % respectively. Similarly, IC50 values of EO from fresh and dried leaves of C. tamala against C. lunata were 2417.3 ± 23 μg/ml and 2117.7 ± 19.8 μg/ml respectively. Results obtained from antibacterial activity assays of the EO from fresh leaves of C. tamala are presented in Table 5. The oil was tested for antibacterial activity against two Gram-negative and two Gram-positive bacteria and was found effective against all the tested bacterial strains. The oil was highly inhibitory against Bacillus subtilis (MTCC 2389) followed by Micrococcus luteus (MTCC4821), Staphylococcus aureus (MTCC7443) and Pseudomonas aeruginosa (MTCC 2642). Minimum inhibitory concentration (MIC) of EO from C. tamala leaves was 550 µg/ml against Bacillus subtilis MTCC2389. Furthermore, antifungal compound of the EO from C. tamala leaves was observed on the TLC plate using A. alternata and C. lunata as test organisms in bioautography (Fig. 1b, c). It was further isolated using repeated preparative TLC. Similarly, for the isolation of antioxidant compound, DPPH· was used in direct bioautography assay. When bioautography was performed for observing antifungal activity one inhibition zone at R f value 0.40 was observed. Intriguingly, this compound also showed antioxidant activity in direct bioautography test (Fig. 1d). The compound corresponding to R f value 0.40 was marked as Ct1. Pure Ct1 was further subjected to direct bioautography for determination of MIA against A. alternata and C. lunata. The results showed that Ct1 possessed stronger fungitoxic activity against C. lunata (MIA = 8.2 ± 0.02 µg) than A. alternata with MIA value of 9.5 ± 0.01 µg. The bioactive constituent of the C. tamala EO corresponding to Ct1 was identified as eugenol using GC/MS analysis (Fig. 2).
TLC of essential oil from Cinnamomum tamala leaves. a Detection with vaniline/sulphuric acid reagent; b bioautography with Alternaria alternata spores; c bioautography with Curvularia lunata spores; d detection of free radical scavenging activity using 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical (0.5 mM). Ct1 corresponds to the component of the essential oil having antifungal and free radical scavenging activity
In the present study we investigated the antimicrobial and antioxidant activity of EO from fresh and dried leaves of C. tamala. To the best of our knowledge this may be assumed as first report on isolation and identification of the bioactive compound responsible for antifungal and antioxidant activity of C. tamala grown in north-western Himalaya. The results demonstrate the antifungal potential of bioactive molecule namely eugenol from C. tamala EO against A. alternata and C. lunata. In addition, EO from fresh and dried leaves of C. tamala showed significant antioxidant and antifungal activity which was higher in EO from dried leaves as compared to EO from fresh leaves. Seasonal variation in EO content of C. tamala and level of bioactive molecule may be investigated in future for ascertaining its commercial value.
Abbreviations
- BHT:
-
Butylated hydroxytoluene
- GC/MS:
-
Gas chromatography/mass spectrometry
- TLC:
-
Thin layer chromatography
- PTLC:
-
Preparative thin layer chromatography
- MIA:
-
Minimum inhibitory amount
- DPPH:
-
1,1-diphenyl-2-picrylhydrazyl
- EO:
-
Essential oil
- MIC:
-
Minimum inhibitory concentration
References
Adams RP (2007) Identification of essential oil components by gas chromatography/mass spectrometry, 4th edn. Allured Publishing Corporation, Illinois, USA
Bozin B, Mimica-Dukic M, Simin N, Anackov G (2006) Characterization of the volatile composition of essential oils of some lamiaceae species and the antimicrobial and antioxidant activities of the entire oils. J Agric Food Chem 54:1822–1828
Chen PF, Chang ST, Wu HH (2002) Antimite activity of essential oils and their components from Cinnamomum osmophloeum. China Q35:69–74
Conner DE (1993) Naturally occurring compounds. In: Davidson P, Branen AL (eds) Antimicrobials in Foods. Marcel Dekker, New York, pp 441–468
Cosentino S, Tuberosa CIG, Pisano B, Satta M, Arzedi E, Palmas F (1999) In vitro antimicrobial activity and chemical composition of sardinian Thymus essential oils. Lett Appl Microbiol 29:130–135
Dandapat S, Kumar M, Sinha MP (2014) Pharmacological and phytochemical screening of Aegle marmelos (L.) and Cinnamomum tamala (Buch.-ham.) leaves for therapeutic efficacy. Middle East J Sci Res 22:626–632
Dinis TCP, Madeira VMC, Almeida LM (1994) Action of phenolic derivatives (acetoaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys 315:161–169
Duh PD (1998) Antioxidant activity of burdock (Arctium lappa Linne): its scavenging effect on free radical and active oxygen. J Am Oil Chem Soc 75:455–465
Exarchou V, Nenadis N, Tsimidou MO, Gerothanassis IP, Troganis A, Boskou D (2002) Antioxidant activities and phenolic composition of extracts from Greek oregano, Greek sage, and summer savory. J Agric Food Chem 50:5294–5299
Feyza O, Belma A, Sahlan O, Senol A (2009) Essential oil composition of antimicrobial and antioxidant activity of Satureja cuneifolia Ten. Food Chem 112:874–879
Guleria S, Kumar A, Tiku AK (2008) Chemical composition and fungitoxic activity of essential oil of Thuja orietalis L. grown in north-western Himalaya. Z Naturforsch C 63:211–214
Guleria S, Tiku A K, Koul A, Gupta S, Singh G A, Razdan V K (2013) Antioxidant and antimicrobial properties of the essential oil and extracts of Zanthoxylum alatum grown in north-western Himalaya. Sci World J. doi:10.1155/2013/790580
Gutteridge RC, Shelton HM (1994) Forage tree legumes in tropical agriculture. CAB International, Wallingford
Hamedo HA (2015) Activity of Cinnamomum zeylanicum essential oil and ethanolic extract against extended-spectrum β-lactamase-producing bacteria. Afr J Biotechnol 4:292–297
Hamrouni SI, Rahali FZ, Rebey IB, Bourgou S, Limam F, Marzouk B (2013) Total phenolics, flavonoids, and antioxidant activity of sage (Salvia officinalis L.) plants as affected by different drying methods. Food Bioprocess Technol 6:806–817
Karapinar M, Aktung SE (1987) Inhibition of food borne pathogens by thymol, eugenol, menthol and anethole. Int J Food Microbiol 4:161–166
Kunin CM (1993) Resistance to antimicrobial drugs-a world-wide calamity. Ann Intern Med 118:557–561
Lahlou M (2004) Methods to study the phytochemistry and bioactivity of essential oils. Phytother Res 18:435–448
Laughton MJ, Halliwell B, Evans PJ, Holult JRS (1987) Antioxidant and pro-oxidant actions of the plant phenolics quercetin, gossypol and myricetin. Biochem Pharmacol 36:717–720
Madsen HL, Bertelsen G (1995) Spices as antioxidant. Trends Food Sci Technol 6:271–277
Manian R, Anusuya N, Siddhuraju P, Manian S (2008) The antioxidant activity and free radical scavenging potential of two different solvents extracts of Camellia sinessis (L.) O. Kuntz. Ficus bengalensis L. and Ficus racemosa L. Food Chem 107:1000–1007
Meir S, Kanner J, Akiri B, Philosoph-Hadas S (1995) Determination and involvement of aqueous reducing compounds in oxidative defense systems of various senescing leaves. J Agric Food Chem 43:1813–1819
Mimica-Dukic N, Bozin B, Sokovic M, Mihajlovic B, Matavulji B (2003) Antimicrobial and antioxidant activities of three mentha species essential oils. Planta Med 69:410–419
Nabavi SF, Daglia M, Moghaddam AH, Habtemariam S, Nabavi SM (2014) Curcumin and liver disease: from chemistry to medicine. Compr Rev Food Sci Food Saf 13:62–77
Naik GH, Priyadarsini KI, Satav JG, Banavalikar MM, Sohoni PP, Biyani MK (2003) Comparative antioxidant activity of individual herbal components used in Ayurvedic medicine. Phytochemistry 63:97–104
NCCLS (National Committee of Clinical Laboratory Standards) (2002) National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts: proposed standard, M27-A2
Oyaizu M (1986) Studies on product of browning reaction prepared from glucose amine. Jpn J Nutr 44:307–315
Pieme CA, Ngoupayo J, Nkoulou CHK-K, Moukette BM, Nono BLN, Moor VJA, Minkande JZ, Ngogang JY (2014) Syzyguim guineense extracts show antioxidant activities and beneficial activities on oxidative stress induced by ferric chloride in the liver homogenate. Antioxidants 3:618–635
Pietta PG (2000) Flavonoids as antioxidants. J Nat Prod 63:1035–1042
Rahman MS (1999) Handbook of food preservation. Marcel Dekker, New York, pp 285–308
Serrentino J (1991) How natural remedies work Point Robert. Harley and Marks Publishers, Vancouver, pp 20–22
Shimada K, Fujikawa K, Yahara K, Nakamura T (1992) Antioxidative properties of xanthan on the autoxidation of soyabean oil in cyclodextrin emulsion. J Agric Food Chem 40:945–948
Singh G, Maurya S, Lampasona DMP, Catalan CAN (2007a) A Comparison of chemical, antioxidant and antimicrobial studies of cinnamon leaf and bark volatile oils, oleoresins and their constituents. Food Chem Toxicol 45:1650–1661
Singh G, Maurya S, Marimuthu P, Murali HS, Bawa AS (2007b) Antioxidant and antibacterial investigations on essential oils and acetone extracts of some spices. Nat Prod Rad 6:114–121
Sridhar SR, Rajagopal RV, Rajavel R, Masilamani S, Narasimahan S (2003) Antifungal activity of some essential oils. J Agric Food Chem 51:7596–7599
Yomauchi R, Tatsumi Y, Asano M, Kato K, Ueno Y (1998) Effect of metal salts and fructose on the antioxidant of methyl linoleate emulsions. Agric Biol Chem 52:849–850
Acknowledgments
The authors thank Incharge, Herbal Garden and Herbarium Research Institute in ISM, Joginder Nagar, District Mandi, H.P., India for the identification of plant material.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Heer, A., Guleria, S. & Razdan, V.K. Chemical composition, antioxidant and antimicrobial activities and characterization of bioactive compounds from essential oil of Cinnamomum tamala grown in north-western Himalaya. J. Plant Biochem. Biotechnol. 26, 191–198 (2017). https://doi.org/10.1007/s13562-016-0381-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13562-016-0381-7